
Special Article - Diagnosis and Usefulness of Dermoscopy
Austin J Dermatolog. 2017; 4(3): 1080.
Dermoscopy for the Diagnosis of Melanoma: An Overview
Togawa Y*
Department of Dermatology, Chiba University of Graduate School of Medicine, Japan
*Corresponding author: Yaei Togawa, Department of Dermatology, Chiba University of Graduate School of Medicine, 1-8-1, Inohana, Chuo-ku, Chiba, 260-8670, Japan
Received: September 11, 2017; Accepted: November 21, 2017; Published: December 05, 2017
Abstract
Dermoscope is a noninvasive and useful tool in the diagnosis or differentiation of melanoma. Contact polarized-light dermoscopes with photography equipment are better to obtain and record fine images constantly. After capturing the dermoscopic images, they should be projected onto a largesized display device for detailed inspection and review. The revised two-step algorithm is a popular and reliable approach to differentiate melanoma from benign nevus. In the first-step, one can identify if the lesion is melanocytic or not. If it is, then the second-step should be followed for the differentiation of the melanoma. Six methods are available in the second-step and pattern analysis is the most specific method, although it needs experience to master. It is better to choose an easy method for daily use.
Keywords: Melanoma; Melanocytic nevus; Pattern analysis; Polarized-light dermoscope; Revised two-step algorithm
Abbreviations
ALM: Acral Lentiginous Melanoma; BWV: Blue-Whitish Veil or Blue White Vail; CNMD 2000: Consensus Net Meeting on Dermosocopy; NM: Nodular Melanoma; TDS: Total Dermoscopic Score
Introduction
Dermoscope is a noninvasive and useful tool for detecting the color and structural details of superficial skin lesions [1]. Primarily, non-polarized dermoscopes were the standard instruments for capturing images at approximately 10x magnification, especially for inspection of structures in pigmented lesions [2]. In the last 10 years, polarized-light dermoscopes have become common because non-pigmented lesions or vascular structures in the skin can be easily detected and viewed using them [1,3,4]. To obtain and record fine images constantly, contact dermoscopes with a photography equipment are recommended. When using the contact dermoscopes, the contact glass plate must be set carefully on the skin lesion without excessive downward pressure [5]. It is also important to apply a sufficient amount of nonirritant and translucent ultrasound gel to the lesion, which prevents diffuse reflection of the illuminant caused by the rough and scaly surface of the lesion. After capturing the dermoscopic images, they should be projected onto a large-sized display device for detailed inspection and review [6].
Differentiation of Melanoma
Revised two-step algorithm
A two-step algorithm for a dermoscopic diagnosis, which resulted from the Consensus Net Meeting on Dermoscopy (CNMD 2000), was established after the first World Congress of Dermoscopy in 2001 [7]. It was, however, mainly for pigmented skin lesions. Subsequently, some studies reported the usefulness of polarized dermoscopy to evaluate amelanotic/hypomelanotic neoplasms, including their vascular structures [3,8,9]. Furthermore, in 2010, Marghoob and Braun proposed a revised two-step algorithm that includes polarized dermoscopy and blood vessel morphology (Figure 1) [10,11]. It showed a very high sensitivity (98%) for both, non-nodular invasive melanoma and Nodular Melanoma (NM), and had a high sensitivity for benign nodular melanocytic lesions (95%); meanwhile, the prototype form of this algorithm did not achieve good sensitivity (84% NM, 88% benign lesions) [12,13]. However, the diagnostic specificity may differ depending on the race of the patient or amount of exposure to ultraviolet rays in general.
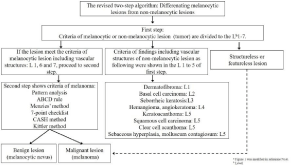
Figure 1: Revised two-step algorithm for dermoscopya
In the first-step, the findings are evaluated to identify whether the lesion is
melanocytic or not. If the melanocytic features can be recognized, one can
skip to the second step for judging if it is a benign lesion or melanoma.
In the first-step of this algorithm, the findings of a melanocytic lesion (Level 1) are evaluated to identify whether the lesion is melanocytic or not (Figure 2) [7,10]. If the following six melanocytic features can be recognized in the lesion (Figure 3), one can skip to the second step for judging if it is a benign lesion or melanoma: a) pigment network, b) streaks, c) aggregated globules, d) homogenous blue pigmentation, e) parallel patterns in palms and soles, and f) pseudonetwork in the face. In this process, the pigment network or pseudonetwork can be seen in solar lentigo and seborrheic keratosis as well. Delicate pigment network at the periphery in dermatofibroma has been added to the exclusion features of melanocytic lesions [10]. However, most dermatofibromas show whitish central scar-like area or central white patch, which is more important to exclude the diagnosis of melanoma than the delicate pigment network at the periphery (Figure 3). These central whitish areas can be observed as white stellate structures or chrysalis-like structures with polarized dermoscopy. Furthermore, these central white findings sometimes form white networks because of thickened and widened rete ridge in the epidermis. It helps differentiate from melanomas, which show blue-white veil only in the thick part of the lesion, although blue nevi usually have uniform blue-white veil on the surface that is called homogenous blue pigmentation (Figure 3). However, it is better to use both, non-polarized and polarized types of dermoscopy together, since it is often difficult to distinguish the uniformity of blue pigmentation or blue-white veil with polarized type dermoscopy alone. In a lesion composed of only pink structureless area or nonspecific features, that is “featureless lesion,” the lesion could also be a risk factor for melanoma. If there is no evidence of distinctive melanocyte features, proceed to differentiation of seborrheic keratosis as level 2, and the following subsequent levels in order: level 3, basal cell carcinoma; level 4, vascular disease; level 5, vasculature of non-melanocytic lesions; level 6, vasculature of melanocytic lesions; and finally level 7, structureless lesions, which are the rare signs of featureless melanoma. The vasculature related to levels 6 and 7 is shown in figure 4. If the evaluation of the lesion in levels 1, 6, and 7 matches the findings of the melanocytic lesion, the second-step for differentiation of the melanoma should be followed.

Figure 2: Levels 1 to 7 of the first step of the revised two-step algorithma.
All of these findings in each levels are basic for diagnosis.
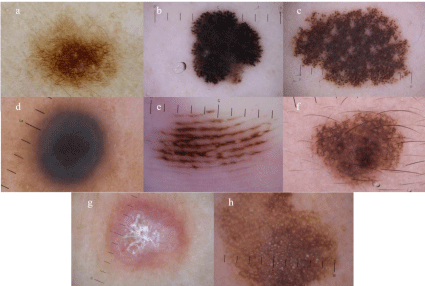
Figure 3: Six features of melanocytic lesions at level 1 of first step.
a) pigment network (melanocytic nevus), b) streaks (Reed/Spitz nevus), c) aggregated globules (congenital melanocytic nevus), d) homogenous blue pigmentation
(Blue nevus), e) parallel pattern (acral nevus which shows parallel furrow pattern), f) pseudonetwork (melanocytic nevus on the face). Exceptions are shown in g)
delicate pigment network (dermatofibroma), and h) pseudonetwork of non-melanocytic lesion (seborrheic keratosis on the face).
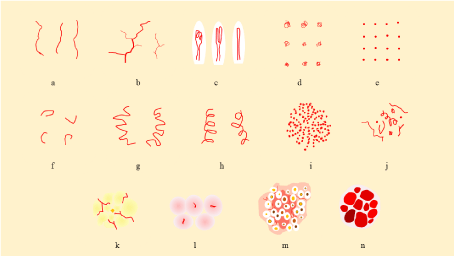
Figure 4: Vessel morphology related with two-step algorithm [1].
a-f six main morphologic categories of vascular patterns. a) linear irregular (linear or serpentine), b) arborizing (serpentine, branched), c) hairpin (looped), d)
glomerular (coiled), e) dotted, f) comma (curved), g) tortuous (serpentine), h) corkscrew (helical), i) pearls on a string or serpiginous pattern (serpiginous), j)
polymorphous (polymorphous), k) crown (radial branched and serpentine), l) milky-red globules (structureless zone, pink), m) strawberry pattern (structureless,
red, interrupted by follicular openings), n) red-purple lacunas (clods, various reddish colors). a,e,f and l are associated with amelanotic/hypomelanotic melanoma,
Spitz nevi, and dermal nevi, respectively. b,c, and d are generally indicative of non-melanocytic tumors such as basal cell carcinoma, seborrheic keratosis or
squamous cell carcinoma including keratoacanthoma, Bowen disease or intra epidermal carcinoma, respectively. g and h are indicative of melanoma collaborated
with dotted, linear irregular, and atypical hairpin vessel on a pink background. i is the hallmark of clear cell acanthoma, j. polymorphous vessels such as an atypical
linear vessels in combination with any other vascular pattern should be ruled out for malignant skin tumors, k. crown vessels surrounding a white-yellow polylobular
center are diagnostic for sebaceous hyperplasia, l. diagnostic finding for thick amelanotic/hypomelanotic melanoma, m. specific feature of facial actinic keratosis,
n. hallmark of hemangioma.
In the second-step, the following six methods for detecting melanoma are available: pattern analysis (Figure 5) [7,14], ABCD rule [15], 7-point check list [16], the Menzies method [17], the CASH method [18,19], and the Kittler method [20], which is a new concept of pattern analysis (Figure 6). Although the sensitivity of detecting melanoma in the ABCD method, 7-point check list, and Menzies method (82.6 to 85.7%) is comparable that of pattern analysis (83.7%) in CNMD2000, the specificity of pattern analysis was 83.4% and it was most excellent in these three (70 to 71.5%) [21].
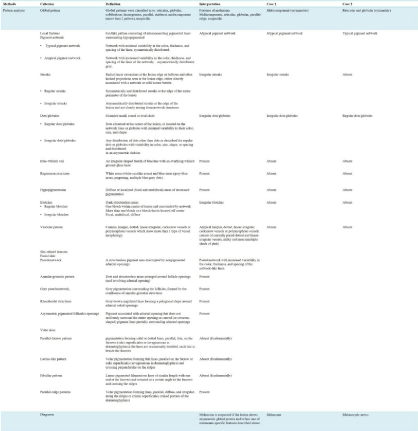
Figure 5: Summary of pattern analysis.
The definition and interpretation of global pattern and local features are shown with two diagnostic examples (the cases 1 and 2 correspond to Figure 7A and B,
respectively).
Pattern analysis
Currently pattern analysis is the most reliable method for detecting melanoma, although it can be influenced by subjective factors and requires a more trained skill compared with other diagnostic methods [7,14]. The terms used in the pattern analysis, which are recommended by consensus meeting of 1990, were improved and redefined in CNMD2000 [14]. First, the global pattern of the whole image of the lesion should be evaluated and then the local features should be identified as follows (Figure 4).
a) Global pattern: The whole image of the lesion is classified into the following eight patterns: reticular (pigment network or pseudonetwork), globular, cobblestone, homogeneous (structureless), parallel (palm and sole in acral), starburst, multicomponent (>2 components above), and unspecific (nonspecific).
Asymmetric pattern including multicomponent pattern is suggestive melanoma (Figure 7A), although symmetric pattern insists usually benign lesion (Figure 7B). However one should keep in mind that some melanoma show symmetric pattern or nonspecific pattern.
b) Local features: The pigment network is simply classified as typical or atypical. A typical pigment network shows symmetry of colors and structures. If the sizes and colors of the holes and grids in a pigment network lack uniformity and the distribution of pigment network itself shows asymmetric distribution, the pigment network is regarded as atypical. Brown globules and black dots are categorized as regular or irregular dots/globules depending on the symmetry of distribution. Blue-Whitish Veil or Blue White Veil (BWV) is a translucent blue-white veil-like area, which is detected on the surface of dark structureless areas. If BWV is detected in a part of the lesion, melanoma is suspected. However, blue nevi usually show BWV existing on the entire surface of the lesion. Black or dark colored structureless area that covers over 10% of the lesion (spotslike pigmentation) are defined as blotches. If it is located in the center of the lesion or distributed symmetrically, it is sign of benign melanocytic lesion. However, if it is recognized as off center blotches, melanoma is highly suspected. Streaks are the linear structures that are composed of radial streaming and/or pseudopods, which are seen in the periphery of the lesion. Radial streaming includes simple lines with dark color, while pseudopods are lines with bulbs at their ends. If it is distributed evenly, it is defined as regular, but if it shows segmental distribution, it is called irregular. Regression structure is composed of white scar-like areas and blue-gray dots. Hypopigmentation includes hypopigmented areas and reticular depigmentation and is an important factor in deciding the asymmetry depending on the distribution.
c) Vascular features: Comma shaped, hairpin, dotted (the shape of a small point), and linear-irregular vessels (line irregularity), and vessels within regression structures are generally seen in melanocytic lesions (Figure 4). Of them, only comma-shaped vessels are specific for banal nevus, while the others are findings sometimes seen in melanoma. Polymorphous vessels are a combination of more than two such patterns. They are sometimes seen in melanoma, especially dotted and linear-irregular vessels (Figure 7C).
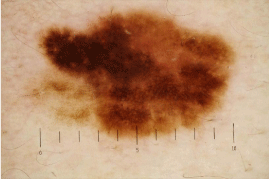
Figure 7A: Dermoscopic image of melanoma on the trunk.
This case is represented in Figure 5 and 6 as case 1. Asymmetric
multicomponent pattern, which is composed of atypical pigment network,
Irregular streaks and dots/globules can be found.
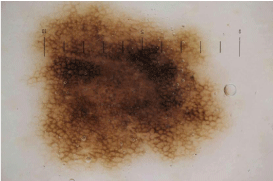
Figure 7b: Dermoscopic image of melanocytic nevus on the trunk
This case is represented in Figure 5 and 6 as case 2. It shows symmetric
reticular and globular pattern. Typical pigment network and regular dots/
globules can be seen.
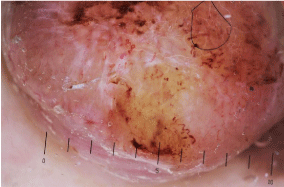
Figure 7c: Dermoscopic vasculature of melanoma.
In this amelanotic nodular part of melanoma, polymorphous vessels
composed of comma, irregular hairpin, dotted, linear-irregular, and corkscrew
vessels can be seen. In the center part of the lesion, white lines that are
distributed perpendicularly are usually regarded as one of the clues for
melanoma in the kittler method, although this is an artifact of polarized-light
dermoscope.
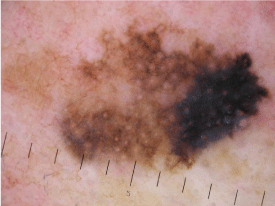
Figure 7D: Dermoscopic image of melanoma on the face.
Annular-granular pattern (in the upper and lower sides of the lesion),
asymmetric pigmented follicular openings and angulated lines (in the upper
side) can be detected. Rhomboidal structures with blue-whitish veil are also
seen in the right side of the lesion.
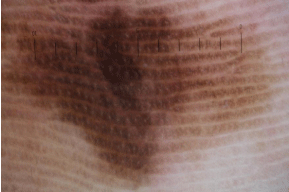
Figure 7e: Dermoscopic image of melanoma on the sole.
This lesion was mainly composed of parallel-ridge patterns. Color variation
was also important clues for melanoma.
d) The findings in a special part (site-related features):
1) Face
Structureless pattern, which is a pattern interrupted by hair follicles, is a common finding of melanocytic lesions and solar lentigo. Asymmetric pigmented follicular openings (asymmetric pigmentation of hair follicle) are findings of early melanoma that infiltrate the asymmetry of hair follicles (Figure 7D). There are other 3 features of facial melanoma. Annular-granular structures are those that consist of gray dots irregularly scattered around the follicles. Rhomboidal structures are dark pigmented areas that avoid the perifollicular space in polygonal residual non-pigmented fashion. In addition, regression structures are detected as burry whitish gray areas with blue-gray dots because of regression of the lesion.
2) Palm and sole
From the early stages, melanomas of the palms and soles (acral lentiginous melanoma, ALM) show a parallel-ridge pattern, because melanoma cells usually proliferate around crista profunda intermedia (Figure 7E). In growing melanomas, irregular diffuse pigmentation can be seen, which are composed of structureless areas with multiple shades of brown color, and some part of the lesion corresponds to a blotch. A multicomponent pattern is also sometimes seen in acral melanomas. In contrast, acral nevi usually show parallel furrow pattern since basal nevicells tend to be located in crista superficial is limitans. Parallel furrow pattern has four variants of lines: single line, single dotted line, double line, and double dotted line. Furthermore, there are two subtypes related to the parallel furrow pattern. The first is fibrillar pattern, and it results from the lateral pressure of the sole. The transverse pressures on the sole encourage slanted keratinization of the cornified layer of the epidermis and it causes slanted melanin column in the stratum corneum resulting in fibrillar appearance. The benign fibrillar pattern presents as a fine brown fibrillary pattern in which the lines show little variation in thickness and color, although atypical “malignant” fibrillar pattern shows variations in thickness and colors. This pattern is commonly seen on the weight bearing parts of the sole such as heel. The second is lattice-like pattern, which can be seen on the palms and arches of the sole. The pattern has a ladder-like appearance, which is composed of parallel lines on the furrow and parallel lines crossing over the ridges from one furrow to the next.
e) How to manage: While differentiating benign melanocytic lesion from melanoma, attention should be paid to both, global pattern and local features [7]. When melanoma is suspected with a global pattern, local features suggestive of malignancy should also be checked. Checking the local features alone will lead to a misdiagnosis. The important three points for diagnosis are 1) the irregularity of the outline of a lesion is not related to malignancy in dermoscopic diagnosis, 2) mixed global pattern (multicomponent) is not related to malignancy when symmetry of the pattern is observed, and 3) the asymmetric structures in the periphery (dots/globules, pigment network, blotches etc.) show a high possibility of malignancy. Non specific or structureless lesions without any findings always show the possibility of melanoma. A special diagnostic approach is better for melanoma on the face or palms and soles because melanoma in such parts is usually recognized as lentiginous type and specific findings mentioned above are quite helpful for such differentiation. The “beauty and the beast sign” reported by Marghoob et al with the revised two-step algorithm is useful for differentiating between benign and malignant lesions based on images [22].
Other methods
The five methods of others which are described below are quite simple compared with pattern analysis.
What one need is basically to find out whether some items fulfill the criteria or not. For the purpose of referring and comparing to these diagnostic criteria quickly, examples of diagnosis using 6 methods including pattern analysis and 3-point checklist are shown (Figure 6).
Figure 6: Other methods of the second-step and 3-point checklist.
Six methods are summarized in this figure. The cases 1 and 2, shown as diagnostic examples, correspond to Figure 7A and B, respectively.
a) ABCD rule: ABCD rule was reported by Stoltz et al. in 1994 as an easy-to-learn checklist [15]. The four initial letters of asymmetry, border sharpness, color, dermoscopic structures are the origin of this method. These only 4 features are required to analysis whether the lesion is melanoma or not. However, identification of a melanoma is not so easy. After asymmetry is judged by two 90% axis, border sharpnes is evaluated with dividing the lesion into eight parts equally. One needs to count the number of following six color in the lesion: white, red, light brown, dark brown, blue-gray, and black. The dermoscopic structures which are five features should be evaluated as flowing: strcutureless areas, pigment network, branched streaks, dots, and globules. The sum of total points of these 4 features is named Total Dermoscopic Score (TDS). A melanoma is strongly suspected if TDS is 5.45 or more. The lesions with 4.75-5.45 TDS in which melanoma cannot be ruled out, excision is recommended and if TDS is lower than 4.75, it is considered to be a benign lesion [21]. In this rule, the reported data was 92.8% sensitivity for melanoma and 91.2% of sensitivity, although 82.6% and 70%, respectively, were reported in CNMD2000 [21].
b) 7-point check list: The 7-point check list was reported by Argenziano et al. in 1998 [16]. They have seven specific structures of the melanoma as following, atypical pigment network, BWV, atypical vascular pattern, irregular streaks, irregular dots/globules/ irregular blotches, regression structures. These are common in pattern analysis, and the scores are calculated depending on the presence of those structures. The sensitivity and specificity of the 7-point checklist in CNMD2000 were 83.6% and 71.5%, respectively [21]. In order to improve the sensitivity of detecting early melanoma while screening pigmented lesions, the assessment was modified that each score was counted by one point in 2011[23]. Although the sensitivity reached as high as 87.8% from the77.9% of the original methods, the degree of singularity decreased from 87.8% to 74.5%.
c) The Menzies method: The Menzies method is method to identify advanced melanomas and was reported in 1996 [17]. This method is very simple and clear because if both the negative findings cannot be detected and at least 1 of 9 positive findings can be found, the lesion is suspected to be a melanoma. Two negative findings are symmetry of pigment pattern and single color. Nine positive findings are BWV, multiple brown dots, radial streaming, pseudopods, scarlike depigmentation, peripheral black dots/ globules, multiple (5 or 6) colors, multiple blue-gray dots, broadened network. The sensitivity and specificity of this test in CNMD2000 were 85.7% and 71.1%, respectively [21].
d) The CASH method: The CASH method was advocated by Braun et al. in 2002 [24]. It was named by considering the initial letters of color, architecture (structure), symmetry, and homogeneity (heterogeneity). After that in 2007, Henning et al. reported the scoring method with a total of 8 points is suggestive of melanoma and includes calculating the score of each of the 4 structures above [19]. One should evaluate the colors same as 6 colors as ABCD rules. Architecture and symmetry are classified into three step, mild, moderate and marked, and none, monoaxial and biaxial respectively. Homogeneity are evaluated by the numbers of the following 7 structures: network, dots/globules/ blotches, regression, streaks, veil (blue), polymorphous vessels. The sum of the each score is called as total CASH score (range 2-17) and the cutoff point is defined more than 7. The sensitivity and specificity were reported as 68% and 98%, respectively. Furthermore, they reported that although the CASH method was similar to the ABCD method and there was almost no difference in their diagnostic accuracies, the specificity was higher than with the Menzies method or 7-point checklist.
e) The Kittler method (of modified pattern analysis): The Kittler method was presented online (DERMA101.COM) in 2007 [20], and the text book was published in 2011with the second edition published recently [25,26]. As a diagnostic procedure, the method is very simple because it requires only five base elements-lines, pseudopods, circles, clods, and dots, in addition to structureless patterns, which are needed to express the structure of a lesion. A report on the standardization of terminology between these simple terms and metaphoric terms was also published [27]. The diagnosis is made by combining three elements-pattern, color, and clues (pattern+color+clues=diagnosis). In the procedure, the pattern of a lesion is decided by the combination of these five elements with structureless pattern, and the color of the structures including the number of colors and clues, which are specific structures for the lesion, is also evaluated. The possibility of a diagnosis is expanded with the classification of the pattern of the non-pigmented lesion by the findings of blood vessels and clues that could not be detected in the conventional pattern analysis, such as melanoma, seborrheic keratosis, and Bowen disease. However, since the diagnostic algorithm is rather complicated in itself, experience and skill training are required. As a simpler version of the Kittler method, the Chaos and Clues method was reported by Rosendahlet al [28]. In this method, we find the color of a lesion and the asymmetry of the structure named “chaos” at first, followed by checking for the eight clues for melanoma. The sensitivity is 90.6% and the specificity is 62.7%. Recently, angulated lines including “polygons” reported by Keir [29] were added and the methods now consists of a total of 9 clues. Unique findings such as chaos, which is asymmetric distribution of the structures or colors and eccentric structureless area i.e., a peripherally located dark pigmented homogenous area, are the practical findings in medical examination. In addition, the podcast about the Kittler method can be accessed for free on the homepage of the International Dermoscopy Society.
f) 3-point checklist: Although it was not contained in the diagnostic method of the second-step mentioned above, the 3-point checklist, which was reported by Soyer et al. in 2004 [30], is the simplest diagnostic method that can be used for differentiating a melanoma and basal cell carcinoma from benign tumor. It has good diagnostic accuracy with 91% sensitivity (86.7% in non-experienced clinicians) and 71.9% specificity [31]. When using a dermoscopy, at least this 3-point checklist should be kept in mind.
Conclusion
It is better to use and master one’s favorite diagnostic method. The best way to master dermoscopy is learn to use a dermoscope without preconceived notions. In the most cases of melanocytic lesion, symmetry in the distribution of colors and structures is a sign of benign, although the irregularity of the outline of a lesion is not related to malignancy in dermoscopy. On the other hand, melanoma tends to show asymmetric pattern with many colors and structures. However, one should always keep in mind that some melanoma show symmetric pattern or nonspecific/featureless pattern.
References
- Arrazola P, Mullani NA, Abramovits W. DermLite II: an innovative portable instrument for dermoscopy without the need of immersion fluids. Skinmed. 2005; 4: 78-83.
- Blum A, Jaworski S. Clear differences in hand-held dermoscopes. J Dtsch Dermatol Ges. 2006; 4: 1054-1057.
- Benvenuto-Andrade C, Dusza SW, Agero AL, Scope A, Rajadhyaksha M, Halpern AC, et al. Differences between polarized light dermoscopy and immersion contact dermoscopy for the evaluation of skin lesions. Arch Dermatol. [Comparative Study Research Support, Non-U.S. Gov’t]. 2007; 143: 329-338.
- Argenziano G, Zalaudek I, Corona R, Sera F, Cicale L, Petrillo G, et al. Vascular structures in skin tumors: a dermoscopy study. Arch Dermatol. 2004; 140: 1485-1489.
- Zalaudek I, Kreusch J, Giacomel J, Ferrara G, Catricala C, Argenziano G. How to diagnose nonpigmented skin tumors: a review of vascular structures seen with dermoscopy: part II. Nonmelanocytic skin tumors. J Am Acad Dermatol. [Research Support, Non-U.S. Gov’t Review]. 2010; 63: 377-386.
- Togawa Y. Review of vasculature visualized on dermoscopy. J Dermatol. 2017; 44: 525-532.
- Argenziano G, Soyer HP, Chimenti S, Talamini R, Corona R, Sera F, et al. Dermoscopy of pigmented skin lesions: results of a consensus meeting via the Internet. J Am Acad Dermatol. [Consensus Development Conference Research Support, Non-U.S. Gov’t Review]. 2003; 48: 679-693.
- Pan Y, Gareau DS, Scope A, Rajadhyaksha M, Mullani NA, Marghoob AA. Polarized and nonpolarized dermoscopy: the explanation for the observed differences. Arch Dermatol. [Review]. 2008; 144: 828-829.
- Wang SQ, Dusza SW, Scope A, Braun RP, Kopf AW, Marghoob AA. Differences in dermoscopic images from nonpolarized dermoscope and polarized dermoscope influence the diagnostic accuracy and confidence level: a pilot study. Dermatol Surg. 2008; 34: 1389-1395.
- Marghoob AA, Braun R. Proposal for a revised 2-step algorithm for the classification of lesions of the skin using dermoscopy. Arch Dermatol. 2010; 146: 426-428.
- Ashfaq A, Marghoob JM, Braun RP. An Atlas of Dermoscopy, Second Edition. 2012: 384.
- Menzies SW, Moloney FJ, Byth K, Avramidis M, Argenziano G, Zalaudek I, et al. Dermoscopic evaluation of nodular melanoma. JAMA Dermatol. [Multicenter Study]. 2013; 149: 699-709.
- Menzies SW, Kreusch J, Byth K, Pizzichetta MA, Marghoob A, Braun R, et al. Dermoscopic evaluation of amelanotic and hypomelanotic melanoma. Arch Dermatol. [Comment Multicenter Study]. 2008; 144: 1120-1127.
- Pehamberger H, Steiner A, Wolff K. In vivo epiluminescence microscopy of pigmented skin lesions. I. Pattern analysis of pigmented skin lesions. J Am Acad Dermatol. [Research Support, Non-U.S. Gov’t]. 1987; 17: 571-583.
- Stolz WRA, Armand B, Cognetta, Pillet L, Abmayr W. ABCD rule of dermatoscopy: a new practical method for early recognition of malignant melanoma. Eur J Dermatol. 1994; 4: 521-527.
- Argenziano G, Fabbrocini G, Carli P, De Giorgi V, Sammarco E, Delfino M. Epiluminescence microscopy for the diagnosis of doubtful melanocytic skin lesions. Comparison of the ABCD rule of dermatoscopy and a new 7-point checklist based on pattern analysis. Arch Dermatol. [Comparative Study]. 1998; 134: 1563-1570.
- Menzies SW, Ingvar C, Crotty KA, McCarthy WH. Frequency and morphologic characteristics of invasive melanomas lacking specific surface microscopic features. Arch Dermatol. 1996; 132: 1178-1182.
- Braun RP, Rabinovitz HS, Oliviero M, Kopf AW, Saurat JH. Pattern analysis: a two-step procedure for the dermoscopic diagnosis of melanoma. Clin Dermatol. [Comparative Study Review]. 2002; 20: 236-239.
- Henning JS, Dusza SW, Wang SQ, Marghoob AA, Rabinovitz HS, Polsky D, et al. The CASH (color, architecture, symmetry, and homogeneity) algorithm for dermoscopy. J Am Acad Dermatol. [Comparative Study Research Support, Non-U.S. Gov’t Validation Studies]. 2007; 56: 45-52.
- Kittler H. Dermatoscopy: introduction of a new algorithmic method based on pattern analysis for diagnosis of pigmented skin lesions. Dermatopathology: Practical & Conceptual, 2007. 2007; 13: 1.
- Malvehy J, Puig S, Argenziano G, Marghoob A, Soyer H. Dermoscopy report: Proposal for standardization Results of a consensus meeting of the International Dermoscopy Society. J Am Acad Dermatol. 2007; 57: 84-95.
- Marghoob AA, Korzenko AJ, Changchien L, Scope A, Braun RP, Rabinovitz H. The beauty and the beast sign in dermoscopy. Dermatol Surg. 2007; 33: 1388-1391.
- Argenziano G, Catricala C, Ardigo M, Buccini P, De Simone P, Eibenschutz L, et al. Seven-point checklist of dermoscopy revisited. Br J Dermatol. 2011; 164: 785-790.
- Braun RP, Rabinovitz HS, Krischer J, Kreusch J, Oliviero M, Naldi L, et al. Dermoscopy of pigmented seborrheic keratosis: a morphological study. Arch Dermatol. 2002; 138: 1556-1560.
- Kittler HRC, Cameron A, Tschandl P. Dermatoscopy: An algorithmic method based on pattern analysis. [Book]. 2011.
- Kittler HRC, Cameron A, Tschandl P. Dermatoscopy: An algorithmic method based on pattern analysis 2nd edition. [Book]. 2016.
- Kittler H, Marghoob AA, Argenziano G, Carrera C, Curiel-Lewandrowski C, Hofmann-Wellenhof R, et al. Standardization of terminology in dermoscopy/ dermatoscopy: Results of the third consensus conference of the International Society of Dermoscopy. J Am Acad Dermatol. 2016; 74: 1093-1096.
- Rosendahl C, Cameron A, McColl I, Wilkinson D. Dermatoscopy in routine practice - ‘chaos and clues’. Aust Fam Physician. 2012; 41: 482-487.
- Keir J. Dermatoscopic features of cutaneous non-facial non-acral lentiginous growth pattern melanomas. Dermatol Pract Concept. 2014; 4: 77-82.
- Soyer HP, Argenziano G, Zalaudek I, Corona R, Sera F, Talamini R, et al. Three-point checklist of dermoscopy. A new screening method for early detection of melanoma. Dermatology. [Validation Studies]. 2004; 208: 27-31.
- Zalaudek I, Argenziano G, Soyer HP, Corona R, Sera F, Blum A, et al. Threepoint checklist of dermoscopy: an open internet study. British Journal of Dermatology. 2006; 154: 431-437.