
Research Article
Austin J Dermatolog. 2019; 6(1): 1087.
Narrow-Band Imaging: A Useful Tool to Early Recognize Oral Lichen Planus Malignant Transformation. A Pilot Study
Cozzani E¹, Russo R¹, Mazzola F², Garofolo S², Camerino M¹, Burlando M¹, Peretti G² and Parodi A¹*
¹Di.S.Sal. Section of Dermatology, University of Genoa, Italy
²Department of Otorhinolaryngology Head and Neck Surgery, University of Genoa, Italy
*Corresponding author: Parodi A, DI.S.SAL Section of Dermatology, University of Genoa, Largo Rosanna Benzi, Genoa, Italy
Received: January 17, 2019; Accepted: February 21, 2019; Published: February 28, 2019
Abstract
Background: Oral Lichen Planus (OLP) lesions have an overall malignant transformation rate of 1.37%. The diagnosis of malignancy is made by means of a histopathological examination, executed on the basis of a clinical suspicion. Narrow Band Imaging (NBI), a promising endoscopic technique, uses a filtered light with specific wavelengths to highlight microvascular abnormalities associated with subclinical neoplastic changes of the upper aerodigestive tract epithelium.
Objective: The study aims to analyze the value of NBI in selecting which patients need to undergo a biopsy before the emergence of clinical changes, allowing early detection of oral malignancies arising from OLP.
Methods: A prospective study was conducted enrolling thirty-two consecutive patients with a histological diagnosis of OLP, no previous diagnosis of oral cancer, no other oral inflammatory diseases. Patients with suspicious NBI lesions underwent biopsies; other patients were included in follow-up.
Results: Two patients were judged positive at NBI-evaluation; they were both histologically diagnosed of neoplastic lesions. None of the other patients developed clinical features of malignancies during follow-up.
Limitations: Patients with negative NBI-evaluation did not undergo biopsies.
Conclusion: NBI evaluation could both increase the accuracy of detecting subclinical neoplastic transformation in OLP lesions and help clinicians perform biopsies only in selected cases.
Keywords: Oral lichen planus; Narrow band imaging; Oral squamous cell carcinoma; Malignant transformation; Oral cancer; Oral diseases
Abbreviations
OLP: Oral Lichen Planus; HCV: Hepatitis C Virus; DIF: Direct Immunofluorescence; SCC: Squamous Cell Carcinoma; NBI: Narrow Band Imaging; HDTV: High-Definition Television; WL: White Light; CIS: Carcinoma In Situ.
Introduction
Oral Lichen Planus (OLP) is one of the most common chronic inflammatory skin diseases occurring in the oral cavity. Its worldwide prevalence has been estimated between 0.5% and 2% [1,2]. The female/male ratio is 2:1 [1]. The onset-age is between the forth and seventh decade, with very few cases reported in pediatric population [1,3,4].
The prevalent theory regarding the etiopathogenesis relies on a T-lymphocyte-mediated immuno-pathological reaction, probably induced by a series of exogenous triggers as the cause of an alteration of the endogenous and surface antigens of the oromucosal keratinocytes, which ultimately enter in apoptosis [1,5]. Such triggers include: the so-called Koebner phenomenon, which precisely delineates skin lesion appearance at the site of an injury; [6,7] psycho-organic stress (anxiety disorders, depression); [8] mechanisms linked to autoimmunity phenomena; [9] viral (Hepatitis C Virus, HCV), [1,10] and bacterial infections (Fusobacteria and Campylobacter species) [11]. A genome-wide association study has recently recognized single-nucleotide polymorphisms that might be used to identify HCV-positive patients at risk for OLP [12].
Six clinical subtypes of OLP can be seen individually or in combination: reticular, papular, plaque, erosive/ulcerative, atrophic and bullous [1,13].
The clinical features of the lesions, particularly when they occur bilaterally and with Wickham’s classic lattices, are strongly indicative of OLP, allowing a diagnosis based on the clinical appearance alone [13]. However, such a characteristic appearance is found in a low percentage of cases; therefore a histological examination is recommended (gold standard) [1,13].
The typical histopathological features of lichen planus are: saw-tooth rete ridges; hyperparakeratosis and hyperortokeratosis; thickening of the cells of the granular layer; liquefaction of basal layer cells and apoptosis of basal keratinocytes; homogenous infiltrate band of lymphocytes and hystiocites along the epithelium-connective tissue interface in the superficial dermis; cytologically normal maturation of the epithelium; hyaline colloidal bodies (Civatte bodies). Civatte bodies are thought to represent apoptotic keratinocytes and other necrotic epithelial components, which are transported to the connective tissue by phagocytosis [1,14].
Especially if lesions are ulcerated with secondary inflammation, Direct Immunofluorescence (DIF) testing has proven to be a valuable method for diagnosing bullous, erosive and ulcerative diseases of the oral mucosa [15,16]. Peculiar DIF findings in patients with OLP are fibrinogen deposition along the basal membrane area and colloidal bodies containing IgA, IgG and IgM [1,17].
OLP undergoes periods of remission and exacerbation, thus a scheduled follow-up is strongly recommended [10]. Treatment is normally reserved only for symptomatic patients. A topical therapy is generally administered (medium-high potency corticosteroids as first choices, cyclosporine, pimecrolimus and tacrolimus if the lesions are unresponsive, antimycotics to treat or to prevent oral candidiasis due to prolonged corticosteroids use); besides, systemic folic acid and variants of vitamin B may have therapeutic effects on OLP patients, since they often have deficiencies [18-27].
Regarding OLP, a critic topic is about its possibility to undergo a malignant transformation. A recent review identified an overall malignant transformation rate of 1.37%, with an annual transformation rate of 0.2% [2].
An important clinical characteristic of carcinomas arising in OLP lesions is their tendency to multifocality, according to the concept of field cancerization, possibile in oral cavity neoplasms [28].
In their histopathological aspect, most of the malignant neoplasms developed in OLP lesions are well-differentiated Squamous Cells Carcinomas (SCCs); together with atypical cells, aberrant microvascular patterns are considered as early histologic signs of malignant transformation [1,29].
Malignant transformation has a higher incidence in immunosuppressed patients, smokers, alcohol users, and HCVpositive patients [2,30]. Tongue lesions and erosive OLP lesions are more likely to progress towards malignant transformation [2,31- 33]. Supported by the current biological knowledge, recent studies reported the possible role of Candida spp., which may over-infect OLP lesions both at initial diagnosis and during immunosuppressive therapy, in carcinogenesis [2,34,35]. Chronic inflammation of oral cavity (Koebner phenomenon, poor oral hygiene) may determine molecular alterations favoring OLP malignant transformation [36].
Nowadays, there is still a lack of clear guidelines for clinicians: the diagnosis of malignancy is made by means of a histopathological examination, executed on the basis of a clinical suspicion.
Narrow Band Imaging (NBI) is a new promising endoscopic technique serving the concept of “biologic endoscopy” [37]. It consists of the use of a blue filtered light with specific (narrow) wavelengths that highlight hemoglobin so as to enhance, inside and around a target lesion, submucosal microvascular abnormalities associated with subclinical preneoplastic and neoplastic changes of the upper aerodigestive tract epithelium [37-39]. Regarding head and neck tumors, its diagnostic value has already been applied to various tasks, such as defining the superficial extension of malignancies or detecting persistent/recurrent disease after (chemo-) radiotherapy and surgery, synchronous and metachronous tumors, and unknown primary squamous cell carcinoma [40].
To the best of our knowledge, no study has been published with the specific aim of investigating the impact of NBI examination in the identification of subclinical signs of malignant transformation of OLP lesions.
Our study aims to analyze the value of NBI in selecting which patients need to undergo a biopsy before the emergence of clinical changes, allowing early detection of oral malignancies arising from OLP.
Materials and Methods
A prospective study was conducted at the Department of Dermatology and at the Department of Otorhinolaryngology–Head and Neck Surgery of San Martino Polyclinic Hospital, University of Genoa, Italy. Thirty-two consecutive patients affected by OLP were enrolled between May 2015 and December 2016, and follow-up visits were conducted until May 2018. Patients were 14 men and 18 women; their ages ranged from 49 to 81 years (median=67 years). Inclusion criteria were (1) diagnosis of OLP confirmed by histological examination (2) no previous diagnosis of oral cancer (3) no other oral inflammatory diseases.
All patients underwent at the time T0 a dermatological and otorhinolaryngological examinations.
The study was approved by the San Martino Human Ethics Review Committee. All patients enrolled in this protocol received information material and signed specific informed consent.
T0 Dermatological examination
A clinical examination of oral mucosa was performed, even evaluating the subtype of OLP (reticular, papular, plaque, erosive/ ulcerative, atrophic and bullous) and the presence or absence of active lesions. Patients’ skin and genital mucosa were also evaluated to look for lichen planus lesions. An assessment of oral cancer risk factors (cigarette smoking, alcohol abuse, HCV infection) and treatments used (corticosteroids, immunosuppressant’s, antimycotics, other products) was made.
T0 Otorhinolaryngological examination
The oral cavity was evaluated at first by conventional oral examination; then the entire oral cavity, with particular attention to the macroscopic lesion and surrounding mucosa, was investigated using a rigid 0° endoscope (Olympus Medical System Corporation, Tokyo, Japan) with High-Definition Television (HDTV) White Light (WL) and then NBI.
Aberrant changes in microvascular pattern visualized during HDTV-NBI evaluation are considered an early sign of malignant transformation: according to recent Inoue classification, modified by Takano, (Figure 1) that describes four typical vascular patterns due to capillary loops in the intraepithelial papillae (intrapapillary capillary loops, IPCLs) of the oral cavity, we considered type II, III and IV as an indicator of neoplastic progression, so they were judged as “positive” [41].
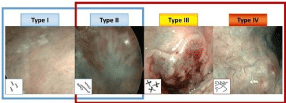
Figure 1: Takano’s classification for oral cavity lesions. In type I, point and small Intrapapillary Capillary Loops (IPCLs) appear widespread on the mucosa.
Elongated IPCLs with dilated caliber belong to type II. Extremely tangled IPCLs with larger caliber so as to create a dotted pattern are typical feature of type III. Type
IV is characterized by irregular vessels, extremely variable caliber and no loops. Vascular patterns of type II, III, IV were considered as an indicator of neoplastic
progression, so they were judged as “positive” [41].
Follow-up
After the first visit all patients eliminated smoke and any proinflammatory factors, as poor oral hygiene and dysfunctional dentures; all patients received anti-inflammatory topical therapy (clobetasol propionate 0.05% mixed with 4% hydroxyethyl cellulose gel).
Dermatology and otorhinolaringology consultations were carried out, combining conventional oral examination, with rigid 0° endoscope HDTV-WL and NBI evaluation.
The entire cohort of patients was divided into two Groups. The first Group (Group A) included patients with no suspicious lesions at NBI endoscopy at the first examination. In such cases a second NBI examination were rescheduled at three months (T1), then every six months (T2). The second Group (Group B) encompassed patients with positive lesions in whom a re-evaluation one month later was planned.
Patients with positive endoscopic findings (Group B) were stratified in two subgroups. Group B1: patients judged negative at the NBI re-evaluation after one month that followed the follow-up policy at three months (T1) and then every six months as Group A; Group B2: patients with endoscopic findings still positive in whom an excisional biopsy was performed. Group B2 patients were again divided into two subgroups. Group B2a: patients whose histology was negative for SCC, who were re-evaluated after three months; Group
B2b: patients whose histology was positive for SCC, that underwent a wider re-resection and were endoscopically re-evaluated after three months (Figure 2).
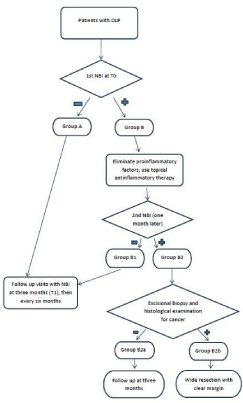
Figure 2: Patient management flow-chart.
Results and Discussion
Oral lichen planus is a chronic inflammatory disease, treated by topical therapy only when symptoms are present; [18,20,25,26,42,43] that was confirmed by our results on disease duration and treatments used. Epidemiological data, types and phase of OLP, risk factors, previous drug therapies and duration of disease of groups A, B, B1, and B2, are summarized in Table 1. Totals sum up to 31 patients, since we excluded a patient because the protocol was not followed in his case. The only patient excluded from the study had a positive lesion at the first NBI evaluation but he presented with a worrying clinical appearance together with strong risk factors. He was a 67-year-old man with a clinically active erosive OLP, despite the therapy with corticosteroids. Therefore, the patient did not join group B and straightly underwent a histological examination. Although the positivity at the NBI evaluation and the worrisome clinical appearance and history, the histological examination was negative for cancer. The patient was consequently included in follow-up: currently no evidence of malignancy was ever observed in that patient.
Group A (N=25)
Group B (N=6)
Group B1 (N=4)
Group B2b (N=2)
Age, median (range), y
66(49-81)
71 (50-81)
71(60-81)
65(50-80)
Sex, No. (%)
Male
8 (32)
3 (50)
2(50)
1(50)
Female
17 (68)
3 (50)
2(50)
1(50)
Type of OLP, No. (%)
Reticular
9 (36)
1 (16.5)
0
1
Papular
1 (4)
0 (0)
0
0
Plaque
5 (20)
1 (16.5)
1
0
Atrophic
1 (4)
0 (0)
3
0
Erosive
9 (36)
4 (67)
0
1
Mixed
0(0)
0 (0)
0
0
OLP Phase, No (%)
Remitting
9(36)
2 (33)
2
0
Active
16 (64)
4 (67)
2
2
Risk Factors, No (%)
Smoke
7(28)
1 (16.5)
0
1
Alchool
2 (8)
1 (16.5)
0
1
HCV
3(12)
0 (0)
0
0
Previous Therapies, No (%)
Steroid
3(12)
2 (33)
2
0
None
8 (32)
1 (16.5)
1
0
Antimycotics
1 (4)
0 (0)
0
0
Immunosuppressant
0(0)
1 (16.5)
0
1
Other
6 (24)
0 (0)
0
0
Association
7(28)
2 (33)
1
1
Duration of disease , median (range), months
48(2-408)
120(6-360)
99(6-192)
270(180-360)
Table 1: Epidemiological, types and phase of OLP, risk factors, and previous drug therapies of groups A, B, B1 and B2b. None of the patients has been placed ingroup B2a.
In our series (Table 1), erosive (48%) and reticular (32%) types were the most represented; 21 patients (68%) presented with active lesions. The duration of disease ranged from 2 months to 34 years. At least two drugs were administered in 29% of patients. Five patients had cutaneous or genital involvement.
All patients from Group A underwent further NBI examinations at every follow-up visit. No positive lesions were found in any of them (Figure 3). Nowadays, there is no evidence of malignancy in any of them.
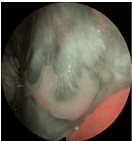
Figure 3: Oral lichen planus (Group A). Peri-lesional small dark spots
and augmented vascularization without recognizable vessels indicate an
inflammatory but benign lesion of the cheek mucosa.
In Group B, one month after T0 four patients were judged negative at second-look examination in NBI (Group B1, Figure 4), while two patients still had positive lesions (Group B2) so they both underwent an excisional biopsy. In one of them Carcinoma in-situ (Cis) was diagnosed, completely excised with free margins (Figure 5). Nowadays there is no evidence of relapse. The other patient was diagnosed of invasive squamous cell carcinoma and was treated by a transoral excision of cheek mucosa extended to retromolar area without any need for reconstruction: final histological report revealed a SCC staged pT1 with free margins. During the follow-up, three months after surgical treatment, a suspicious novel lesion appeared on a preexisting reticular OLP lesion involving the gingival mucosa, beside inferior incisor teeth. The novel lesion was judged positive at NBI examination; therefore, an excisional biopsy from gingival mucosa was performed. The histological examination showed an insitu squamous cell carcinoma. The patient presented no evidence of disease at the last follow-up visit: a single erosion in the soft palate was detected, non-suspicious to NBI evaluation. Therefore, both patients were placed in Group B2b. None of the patients has been classified in Group B2a.
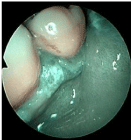
Figure 4: Oral lichen planus (Group B1). Small dark intra-papillary capillary
loops surround a keratotic gingival lesion with intra-lesional thicker spots.
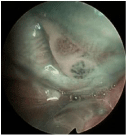
Figure 5: Carcinoma in situ of the retro-molar area/cheek mucosa (Group
B2b). Intra-lesional thick dark intra-papillary capillary loops with variable
caliber, suspicious for malignant mucosal transformation of the lingual border
are clearly recognizable.
In summary, both patients with lesions judged positive at the second NBI-evaluation were histologically diagnosed of neoplastic lesions one month after the first visit.
In our experience, evaluations by HDTV-NBI turned out to be useful diagnostic tools to precociously identify pre-neoplastic and neoplastic changes in patients affected by OLP.
In fact, in our series, two cases raised suspect of oral cancer after we repeated HDTV-NBI evaluations twice (group B2b). Those two cases (6,5%) have been histologically confirmed as squamous cell carcinomas: such a percentage is higher than that described in the literature, 2 probably because of the small size of our sample of patients.
None of the patients with NBI-suspicious lesions underwent biopsy for non-neoplastic lesion (group B2a). Maybe because of the small sample of patients, we experienced that histological examination has been performed only for neoplastic lesions.
In our experience, NBI examination guided us to choose the correct patients that needed a histological evaluation of the lesion. Notably, performing NBI evaluation, we were able to find a case of in-situ squamous cell carcinoma in a patient with a clinically classical OLP, with a reticular pattern and without any suspicious feature calling for biopsy.
One month after T0, four patients (group B1) were judged negative at second-look examination in NBI: we believe that such a result is due to the removal of any inflammatory factor for the oral mucosa in the period between the first and the second evaluation, obtained even by topical therapy. No evidence of malignancy was detected in any of those patients during subsequent follow-up visits.
The patient excluded from the study, who had one positive NBI evaluation at T0 and was immediately subjected to a biopsy, was negative for cancer at histological examination. We suppose that a high degree of inflammation, due to erosive kind of OLP, may have altered the microvascular component of the lesion, therefore simulating the NBI patterns of a neoplastic lesion - as already highlighted in literature [38-44]. Likely, this patient would have taken part in the B1 group, showing no suspicious feature one month after T0, once any pro-inflammatory factor was eliminated.
During the follow-up, each patient from group A (negative NBI examination at T0) was evaluated by clinical assessment and HDTV-NBI examination, without biopsies: currently, no patient ever presented macroscopic malignant transformations.
Undoubtedly, a limit of our study is not having performed biopsies on patients with negative NBI lesions. This prevents us from saying that NBI examinations avoid biopsies, which are definitely useless, since we cannot prove that those patients were free from histological findings of cancer. Anyway, since patients were all included in a twoyear follow-up program and none of them showed clinical changes for two years, we are confident that no cases of cancer have been unidentified performing our protocol.
The results of our study need to be confirmed by larger, randomized studies; if so, it will be clear the need to insert this diagnostic and follow-up tool in the daily management of patients affected by OLP. Of course, it is necessary to schedule a protocol of NBI use, since a single examination could bring about false positive results, due to concomitant inflammatory factors-the case of the patient excluded from the study demonstrates that. Patients with NBI-positive lesions need to be re-evaluated one month later with the same technique after eliminating every possible confounding factor, such as inflammatory insults: we experienced that NBI evaluations did not bring any false positive result when performed so.
Conclusion
In the light of our results, we conclude that the routine association of clinical evaluation and HDTV-NBI examination could both increase the accuracy of detecting neoplastic transformation in OLP lesions and help clinicians perform biopsies only in selected cases. NBI evaluation proved to be a cutting-edge choice in the follow-up of patients affected by OLP, allowing the identification of malignancies in cases with clinical features absolutely not indicative of neoplastic transformation, and avoiding biopsy, an invasive technique, in a large number of patients, thus reserving it for really at risk individuals. Patients with stable or non-suspicious OLP lesions would extremely benefit from this conservative approach.
References
- Alrashdan MS, Cirillo N, McCullough M. Oral lichen planus: a literature review and update. Arch Dermatol Res. 2016; 308: 539-551.
- Giuliani M, Troiano G, Cordaro M, Corsalini M, Gioco G, Lo Muzio L, et al. Rate of malignant transformation of oral lichen planus: a systematic review. Oral Dis. 2018; 1-17.
- Farhi D, Dupin N. Pathophysiology, etiologic factors, and clinical management of oral lichen planus, part I: facts and controversies. Clin Dermatol. 2010; 28: 100-108.
- Kanwar AJ, De D. Lichen planus in childhood: report of 100 cases. Clin Exp Dermatol. 2010; 35: 257-262.
- Kurago ZB. Etiology and pathogenesis of oral lichen planus: an overview. Oral Surg Oral Med Oral Pathol Oral Radiol. 2016; 122: 72-80.
- Muller S. The lichenoid tissue reactions of the oral mucosa: Oral lichen planus and other lichenoid lesions. In: Richardson MS, ed. Current Concepts in Head and Neck Pathology. Philadelphia: Saunders. Surg Pathol Clin. 2011: 1005-1026.
- Patterson J. The lichenoid reaction pattern (“interface dermatitis”). In: Weedon’s Skin Pathology. 4th London: Churchill Livingston Elsevier; 2016: 39.
- Rojo-Moreno JL, Bagan JV, Rojo-Moreno J, Donat JS, Milian MA, Jimenez Y, et al. Psychologic factors and oral lichen planus. A psychometric evaluation of 100 cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998; 86: 687-691.
- Sugerman PB, Satterwhite K, Bigby M. Autocytotoxic T-cell clones in lichen planus. Br J Dermatol. 2000; 142: 449-456.
- Cassol-Spanemberg J, Rodriguez-de Rivera-Campillo ME, Otero-Rey EM, Estrugo-Devesa A, Jané-Salas E, López-López J. Oral lichen planus and its relationship with sistemic disease. A review of evidence. J Clin Exp Dent. 2018; 10: e938-944.
- Wang K, Miao T, Lu W, He J, Cui B, Li J, et al. Analysis of oral microbial community and Th17-associated cytokines in saliva of patients with oral lichen planus. Microbiol Immunol. 2015; 59: 105-113.
- Nagao Y, Nishida N, Toyo-oka L, Kawaguchi A, Amoroso A, Carrozzo M, et al. Genome-wide association study identifies risk variants for lichen planus in patients with hepatitis C infection. Clin Gastroenterol Hepatol. 2017; 15: 937e4-944e5.
- Gorouhi F, Davari P, Fazel N. Cutaneous and mucosal lichen planus: A comprehensive review of clinical subtypes, riskfactors, diagnosis, and prognosis. Scientific World J. 2014; 2014: 742-826.
- De Rossi SS, Ciarrocca K. Oral lichen planus and lichenoid mucositis. Dent Clin North Am. 2014; 58: 299-313.
- Gandolfo S, Carbone M, Carrozzo M, Gallo V. Oral lichen planus and Hepatitis C Virus (HCV) infection: is there a relationship? A report of 10 cases. J Oral Pathol Med. 1994; 23: 119-122.
- Laskaris G, Sklavounou A, Angelopoulos A. Direct immunofluorescence in oral lichen planus. Oral Surg. 1982; 53; 5: 483-487.
- Moliaoglu N. Oral lichen planus: a review. Brit J Oral & Maxillo Surgery. 2000; 38: 370-377.
- Al-Hashimi I, Schifter M, Lockhart PB, Wray D, Brennan M, Migliorati CA, et al. Oral lichen planus and oral lichenoid esions: diagnostic and therapeutic considerations. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007; 103: S25: e1-e12.
- Carrozzo M, Gandolfo S. The management of oral lichen planus. Oral Dis. 1999; 5: 196-205.
- Cribier B, Frances C, Chosidow O. Treatment of lichen planus. An evidence-based medicine analysis of efficacy. Arch Dermatol. 1998; 134: 1521-1530.
- Eisen D, Ellis CN, Duell EA, Griffiths CE, Voorhees JJ. Effect of topical cyclosporine rinse on oral lichen planus. A doubleblind analysis. N Engl J Med. 1990; 23: 290-294.
- Voute AB, Schulten EA, Langendijk PN, Nieboer C, van der Walle I. Cyclosporin A in an adhesive base for treatment of recalcitrant oral lichen planus. An open trial. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1994; 78: 437-441.
- Conrotto D, Carbone M, Carrozzo M, Arduino P, Broccoletti R, Pentenero M, et al. Ciclosporin vs. clobetasol in the topical management of atrophic and erosive oral lichen planus: a double- blind, randomized controlled trial. Br J Dermatol. 2006; 154: 139-145.
- Sieg P, Von Domarus H, von Zitzewitz V, Iven H, Farber L. Topical cyclosporin in oral lichen planus: a controlled,randomized, prospective trial. Br J Dermatol. 1995; 132: 790-794.
- Carbone M, Conrotto D, Carrozzo M, Broccoletti R, Gandolfo S, Scully C. Topical corticosteroids in association with miconazole and chlorhexidine in the long-term management of atrophic-erosive oral lichen planus: a placebo-controlled and comparative study between clobetasol and fluocinonide. Oral Dis. 1999; 5: 44-49.
- Lodi G, Tarozzi M, Sardella A, Demarosi F, Canegallo L, Di Benedetto D, et al. Miconazole as adjuvant therapy for oral lichen planus: a double-blind randomized controlled trial. Br J Dermatol. 2007; 156: 1336-1341.
- Nosratzehi T. Oral Lichen Planus: an Overview of Potential Risk Factors, Biomarkers and Treatments. Asian Pac J Cancer Prev. 2018; 19: 1161-1167.
- Hande AH, Mohite DP, Chaudhary MS, Patel M, Agarwal P, Bohra S. Evidence based demonstration of the concept of ‘field cancerization’ by p53 expression in mirror image biopsies of patients with oral squamous cell carcinoma - an immunohistochemical study. Rom J Morphol Embryol. 2015; 56: 1027-1033.
- Lo Muzio L, Mignogna MD, Favia G, Procaccini M. The possible association between oral lichen planus and oral squamous cell carcinoma: a clinical evaluation on 14 cases and a review of the literature. Oral Oncol. 1998; 34: 239-246.
- Aghbari SMH, Abushouk AI, Attia A, Elmaraezy A, Menshawy A, Ahmed MS, et al. Malignant transformation of oral lichen planus and oral lichenoid lesions: a meta-analysis of 20095 patient data. Oral Oncol. 2017; 68: 92-102.
- Mignogna MD, Lo Muzio L, Lo Russo L, Fedele S, Ruoppo E, Bucci E. Clinical guidelines in early detection of oral squamous cell carcinoma arising in oral lichen planus: a 5-year experience. Oral Oncol. 2001; 37: 262-267.
- Kaplan BR. Oral lichen planus and squamous carcinoma: case report and update of the literature. Rhode Island Dental Journal. 1991; 24: 5-9.
- Bombeccari GP, Guzzi G, Tettamanti M, Giannì AB, Baj A, Pallotti F, et al. Oral lichen planus and malignant transformation: a longitudinal cohort study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011; 112: 328-334.
- Bombeccari GP, Giannì AD, Spadari F. Oral Candida colonization and oral lichen planus. Oral Dis. 2017; 23: 1009-1010.
- Marable D, Bowers L, Stout T, Stewart CM, Berg KM, Sankar V, et al. Oral candidiasis following steroid therapy for oral lichen planus. Oral Dis. 2016; 22: 140-147.
- Mignogna MD, Fedele S, Lo Russo L, Lo Muzio L, Bucci E. Immune activation and chronic inflammation as the cause of malignancy in oral lichen planus: is there any evidence? Oral Oncol. 2004; 40: 120-130.
- Piazza C, D Bon F, Peretti G, Nicolai P. 'Biologic endoscopy: optimization of upper aerodigestive tract cancer evaluation. Curr Opin Otolaryngol Head Neck Surg. 2011; 19: 67-76.
- Piazza C, Cocco D, Del Bon F, Mangili S, Nicolai P, Majorana A, et al. Narrow band imaging and high definition television in evaluation of oral and oropharyngeal squamous cell cancer: a prospective study. Oral Oncol. 2010; 46: 307-310.
- Piazza C, Dessouky O, Peretti G, Cocco D, De Benedetto L, Nicolai P. Narrow-band imaging: a new tool for evaluation of head and neck squamous cell carcinomas. Review of the literature. Acta Otorhinolaryngol Ital. 2008; 28: 49-54.
- Filauro M, Paderno A, Perotti P, Marchi F, Garofolo S, Peretti G, et al. Role of Narrow-Band Imaging in Detection of Head and Neck Unknown Primary Squamous Cell Carcinoma. Laryngoscope. 2018; 128: 2060-2066.
- Takano JH, Yakushiji T, Kamiyama I, Nomura T, Katakura A, Takano N, et al. Detecting early oral cancer: narrowband imaging system observation of the oral mucosa microvasculature. Int J Oral Maxillofac Surg. 2010; 39: 208-213.
- Carbone M, Arduino PG, Carrozzo M, Gandolfo S, Argiolas MR, Bertolusso G, et al. Course of oral lichen planus: a retrospective study of 808 northern Italian patients. Oral Dis. 2009; 15: 235-243.
- Holmstrup P, Schiøtz AW, Westergaard J. Effect of dental plaque control on gingival lichen planus. Oral Surg Oral Med Oral Pathol. 1990; 69: 585-590.
- Piazza C, Cocco D, De Benedetto L, Del Bon F, Nicolai P, Peretti G. Role of narrow band imaging and high-definition television in the surveillance of head and neck squamous cell cancer after chemo and/or radiotherapy. Eur Arch Otorhinolaryngol. 2010; 267: 1423-1428.