
Special Article - Psoriasis
Austin J Dermatolog. 2019; 6(1): 1088.
Coronary Microvascular Dysfunction as an Early Culprit in the Pathophysiology of Myocardial Involvement in Psoriasis
De Michieli L, Dal Lin C and Tona F*
Department of Cardiac, Thoracic, Vascular Sciences and Public Health, University of Padova, Italy
*Corresponding author: Francesco Tona, Department of Cardiac, Thoracic, Vascular Sciences and Public Health University of Padova, Padova, Italy
Received: April 18, 2019; Accepted: May 13, 2019; Published: May 20, 2019
Abstract
Psoriasis is a chronic, immune-mediated disorder that mainly affects the skin and has a complex genetic and autoimmune physiopathology, with an estimated global prevalence of 2-3% and a highly disabling disease burden. It is indeed characterized by several comorbidities, such as arthritis, metabolic syndrome or components of the syndrome, Cardiovascular (CV) disorders, and several other diseases. Life expectancy of patients with psoriasis is substantially reduced, with cardiovascular diseases contributing the most. Cardiovascular involvement is partially correlated with an increased prevalence of traditional CV risk factors such as diabetes, hypertension, metabolic dyslipidemia, tobacco use and obesity; on the other hand, cardiac involvement is substantially correlated with chronic inflammation that results in endothelial dysfunction and coronary microvascular dysfunction. The latest is responsible for diastolic and systolic myocardial dysfunction and could cause microvascular angina. The aim of this review is to briefly describe psoriasis’s physiopathology and to analyze causes and modalities of myocardial involvement through the available scientific literature.
Keywords: Psoriasis; Coronary microvascular dysfunction; Cardiovascular disorders
Introduction
Psoriasis is a chronic, immune-mediated disorder that mainly affects the skin and joints and has a complex genetic physiopathology, with an estimated global prevalence of 2-3% [1-3]. Women and men are affected equally. Psoriasis can manifest at any age, but onset usually occurs between 18 and 39 years of age or between 50 and 69 years of age [4].
Five main types of psoriasis have been described: plaque psoriasis, guttate or eruptive psoriasis, inverse psoriasis, pustular psoriasis, either palmoplantar pustulosis or generalized pustular psoriasis and erythrodermic psoriasis [1].
Besides skin and joints manifestations, disease burden is further increased by several comorbidities, which include metabolic syndrome or components of the syndrome, Cardiovascular (CV) disorders, and several other diseases such as anxiety and depression, non-alcoholic fatty liver disease, Crohn’s disease, and lymphoma [3]. Life expectancy of patients with psoriasis is substantially reduced, with cardiovascular diseases contributing the most [5].
On one hand, CV disorders in psoriasis patients seem to be determined by traditional CV risks factors but, on the other hand, the chronic inflammatory state caused by the disease itself plays an important role on CV involvement in these patients.
In this setting, patients with psoriasis have increased prevalence of traditional CV risk factors such as diabetes, hypertension, metabolic dyslipidemia, tobacco use and obesity [6]. A Norway study in 2018 reported a positive association between psoriasis and objective measures of Body Mass Index (BMI), waist circumference and highsensitivity C-reactive protein, but no clear association with blood pressure and blood lipids. People with moderate/severe psoriasis had an odds ratio for being overweight of 1.94, whereas the odds ratio for metabolic syndrome was 1.91. Psoriasis was also positively associated with self-reported diabetes, myocardial infarction and angina pectoris. These associations were strongest for people with moderate/ severe psoriasis [7]. Moreover, several studies showed early vascular abnormalities in psoriasis, represented by impaired Flow-Mediated vasodilation (FMD) of brachial artery and increased Intimal Medial Thickness (IMT) of common carotid artery [8].
However, the association between severe psoriasis and increased CV risk has been reported to be independent of traditional risk factors in the majority of the studies performed [9,10].
Patients with severe psoriasis have approximately a sevenfold increased risk of myocardial infarction compared with matched controls for age, sex, BMI and CV risk factors. According to a population-based, prospective, cohort study from the United Kingdom, the risk of cardiovascular mortality is increased by 57% [9]. Furthermore, a correlation between the severity of psoriasis and the odds of having a myocardial infarction has been proved so that a 30-year-old patient with severe psoriasis has an approximate twofold increase in the risk of experiencing a first myocardial infarction [9]. Large-scale, population-based epidemiological studies have indeed demonstrated that psoriasis is associated with an increased risk of cardiovascular events beyond traditional risk factors and BMI [11].
Pathophysiology of psoriasis
Involvement of the immune system in psoriasis is now clearly accepted and proved; in particular, dysregulated interactions of innate and adaptive components of the immune system with resident cutaneous cell types has been described [12]. Psoriasis is therefore thought to origin from abnormal interaction between genetics, the immune system, and environmental exposures [6]. There is evidence for a genetic predisposition involving genes such as PSORS1, IL- 23R, IL-12R and other, with a central role of inflammatory Dendritic Cells (DC) and T-lymphocytes, in particular Th1 and Th17 cells [13]. Accordingly, cytokines such as TNFa or IL-23 and more recently IL- 17 produced by DC and Th17 cells appears to be a highly effective modality for the treatment of psoriasis as well as psoriatic arthritis [14]. However, new evidences show that the cutaneous nervous system via releasing neuropeptides appears to play a role in the development of psoriatic lesions and, moreover, the microbiome of the skin is known to interact with the native and adaptive immune system and thereby contributes to psoriatic inflammation [13].
Myocardial involvement in psoriasis
As mentioned before, chronic psoriasis is associated with other conditions that are caused, in part, by chronic inflammation [15], such us CV disorders, Crohn’s disease, and lymphoma. Helper T-cells type 1 (Th-1) chronic inflammation, typical of psoriasis, is also an important factor in the pathophysiology of other conditions such as insulin resistance, atherosclerosis, and plaque rupture leading to thrombotic events.15 Correlation between chronic inflammatory condition and cardiovascular disease has been proved also for other diseases such as lupus erythematosus and rheumatoid arthritis [16].
It’s well known that immune cells dominate early atherosclerotic lesions, that their effector molecules accelerate progression of the lesions and that activation of inflammation can elicit acute coronary syndromes [17]. Chronic exposure to pro-inflammatory cytokines also leads to expression of adhesion molecules on vascular endothelial cells, inducing leukocyte recruitment, smooth muscle cell growth factor release, enzymes degrading the connective tissue matrix secretion, LDL oxidation and lipid deposition on arterial wall, that cause the development of atherosclerotic lesions [15]. Inflammatory exacerbation may eventually determine the destabilization and rupture of atherosclerotic plaques, by increasing the expression of matrix metalloproteinases degrading the collagen of the fibrous cap [18].
Atherosclerosis underlies the majority of the coronary arteryrelated events, primarily because of development of epicardial arteries plaques which can cause typically Type I and Type II myocardial infarction [19]. Despite this, individuals with inflammatory immunemediated diseases may have just mild atherosclerosis as estimated by angiography [10]. Therefore, alterations in coronary microvascular function is considered to contribute to the increased cardiovascular morbidity and mortality observed in psoriasis.
The term Coronary Microvascular Dysfunction (CMD) has been introduced to describe abnormalities in the regulation of myocardial blood flow which are not explained by disease of the epicardial coronary arteries [20]. Endothelial dysfunction is one of the main actor in CMD, being due to multifactorial causes, showing a possible etiological correlation with smoking, obesity, hypercholesterolemia, and systemic inflammation [21] (Figure 1). On the basis of the clinical settings in which it occurs, CMD can be classified into four types: dysfunction occurring in the absence of Coronary Artery Diasease (CAD) and myocardial diseases, dysfunction in the presence of myocardial diseases, dysfunction in the presence of obstructive epicardial CAD, and iatrogenic dysfunction.
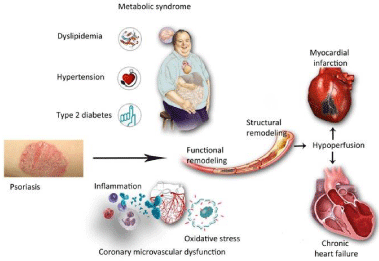
Figure 1: Psoriasis, both cutaneous and arthritic, is a low-grade chronic,
systemic inflammatory disease associated with increased circulating proinflammatory
cytokines and oxidative stress, and increased prevalence
of cardiovascular risk factors such as obesity, diabetes, hypertension and
metabolic syndrome. These conditions can induce a coronary microvascular
remodeling, firstly functional and subsequently also structural. This
microvascular dysfunction/damage may leads to an ischemic myocardial
insult and/or to an organ dysfunction secondary to hypoperfusion up to heart
failure.
Coronary microvascular dysfunction may be sustained by several pathogenetic mechanisms, like structural, functional and extravascular mechanisms. The functional mechanisms include endothelial dysfunction (inflammatory state, smoking, hyperlipidemia, diabetes), dysfunction of smooth muscle cell (hypertrophic cardiomyopathy, arterial hypertension) and autonomic dysfunction (Figure 1) [20].
Early microvascular impairment in psoriasis patients might be hypothesized as the consequence of prolonged and sustained systemic inflammation and might explain the increased CV risk conferred by the disease. Many inflammation biomarkers are detected in the blood of patients with psoriasis and are associated with disease activity [22]. Systemic inflammation induces insulin resistance and reduction in the release of vasodilating factors such as nitric oxide by endothelial cells. The resulting vascular stiffness is known as endothelial dysfunction [1], which is the main responsible of coronary microvascular disfunction (Figure 1). In the study by Osto et al. [10], 56 young patients with severe psoriasis without clinical evidence of cardiovascular disease, and 56 controls matched for age and gender were studied with coronary flow velocity in the left anterior descending coronary artery detected by transthoracic echocardiography at rest and during adenosine infusion. Coronary Flow Reserve (CFR) was the ratio of hyperemic Diastolic Flow Velocity (DFV) to resting DFV. Coronary Flow Reserve (CFR) is dependent on the combined effects of epicardial coronary flow and coronary microvascular function. Therefore, impaired CFR may reflect the presence of coronary microvascular dysfunction in the absence of obstructive coronary artery narrowing. CFR was abnormal in 22% of psoriasis patients vs 0% of controls; moreover, the severity of psoriatic disease (analyzed with Psoriasis Area Severity index, PASI score) was found to be an independent determinant of CFR reduction under 2.5. They concluded that CFR in young patients with severe psoriasis was reduced suggesting a coronary microvascular dysfunction, independently related to the severity and extension of psoriasis.
Similarly, in the study by Recio-Mayoral et al. [23], using positron emission tomography, resting and hyperaemic (adenosine, 140 mg/ kg/min) Myocardial Blood Flow (MBF) was measured in 25 patients with Systemic Lupus Erythematosus (SLE) or Rheumatoid Arthritis (RA); they found a reduced CFR in the absence of significant coronary disease is suggestive of coronary microvascular disfunction. They speculate that this was the consequence of prolonged systemic inflammation, which may precede and contribute to premature coronary artery disease in these patients [23]. Therefore, microvascular dysfunction may be considered as an early culprit of the myocardial involvement in psoriasis. Microvascular dysfunction can be responsible for different type of cardiac involvement, such as diastolic dysfunction and subsequent Heart Failure (HF) with preserved Ejection Fraction (HFpEF), subclinical and clinical systolic dysfunction, microvascular angina.
As mentioned earlier, systemic proinflammatory state induces coronary microvascular endothelial cells to produce Reactive Oxygen Species (ROS), which limits Nitric Oxide (NO) bioavailability for surrounding cardiomyocytes; that decreases Protein Kinase G (PKG) activity in cardiomyocytes inducing concentric Left Ventricle (LV) remodeling and stiffens the cardiomyocyte because of hypophosphorylation of the protein titin. Stiff cardiomyocytes and increased collagen deposition by myofibroblasts cause diastolic LV dysfunction [24]. Left ventricular relaxation could be impaired as well; it is indeed dependent on both cross-bridge detachment and sarcoplasmic reticular calcium reuptake, but NO signaling is involved as well through cyclic Guanosine Monophosphate (cGMP) that normally reduces myofilamentary calcium sensitivity and thereby facilitates cross-bridge detachment. As cross-bridge detachment is an energy-consuming process, slow LV relaxation can also result from a myocardial energy deficit [25]. Slow LV relaxation may reduce LV stroke volume, especially at high heart rates [26]. Stiff cardiomyocytes, increased collagen deposition by myofibroblasts and impaired left ventricle relaxation can cause diastolic LV dysfunction, main pathophysiological feature of HFpEF.
A recent Italian Study by Gorga et al. enrolled 52 patients with the diagnosis of chronic plaque psoriasis, compared with a control group not affected by any relevant systemic diseases and inflammatory disorders. They performed conventional echocardiographic and Tissue Doppler (TDI) imaging. Left ventricular diastolic dysfunction was found in 36.5% patients in the psoriasis group versus 0% in control group, and significant reduction of the E/A ratio was found also for the right ventricle. Systolic function can be impaired as well; among others, Shang et al. in 2011 [27] performed a study in which 94 patients with psoriatic Arthritis (PsA) but without clinical evidence of CV diseases and 63 healthy subjects were enrolled. All underwent conventional echocardiography and tissue Doppler imaging. They reported that early LV remodeling was found in psoriasis patients, such as a thickened posterior wall, increased relative wall thickness, and higher prevalence of concentric remodeling, compared to controls. Furthermore, subclinical impairment of LV function was also detected thanks to tissue Doppler imaging with 47.2% patients having evidence of subclinical LV dysfunction as defined by mean S ‹ 4.4 cm/s, lateral E’ ‹ 11.5 cm/s, and/or lateral E/E’ › 10. Among these patients, 19.4% had only diastolic dysfunction, 8.3% had only systolic dysfunction, while 19.4% had both systolic and diastolic dysfunction. It must be underlined that PsA patients with subclinical LV dysfunction were found to be older, with a higher age at diagnosis of PsA and of psoriasis and a longer disease duration. They were found also to have a higher prevalence of hypertension and hyperlipidemia, higher levels of serum creatinine, and more antihypertensive treatment than those with normal LV function. Multivariable regression showed that age at diagnosis of PsA > 40 years and hypertension were independent predictors of subclinical LV dysfunction [27]. In a study of 2014 [28], 50 patients with psoriasis and 50 age- and sexmatched control subjects were included and LV dyssynchrony was investigated by color-coded tissue Doppler imaging. In the psoriasis group, peak A velocity, deceleration time, isovolumetric relaxation time, and E/E'values were higher in the psoriasis group; LV systolic dyssynchrony parameters [including standard deviation of Ts of the 12 LV segments (time from QRS onset to peak systolic velocity), maximal difference in Ts between any two of the 12 LV segments, standard deviation of Ts of the six basal LV segments, and maximal difference in Ts between any two of the six basal LV segments] were found to be higher in the psoriasis group. The patients with ventricular dyssynchrony were higher in the psoriasis group than the control group. They concluded that patients with psoriasis with normal ejection fraction and narrow QRS, LV systolic dyssynchrony is an early manifestation of heart involvement and may coexist with diastolic dysfunction.
Coronary microvascular dysfunction can also result in microvascular angina; chest pain without obstructive epicardial CAD is a common entity, occurring in up to 30% of patients undergoing invasive coronary angiography for chest pain. To our knowledge, as far as the association between psoriasis (and inflammatory states) and microvascular dysfunction has been proved, it is reasonable to think that psoriasis patients may as well suffer from microvascular angina. To date there are no studies defining prevalence and correlation of these pathologies. However, a 2016 Study by Zutt et al. [21] assessed various comorbidities in 50 psoriasis patients without clinical symptoms of cardiac disease. Myocardial scintigraphy was employed to detect cardiac risk and exercise-induced ischemia; more than half of the patients had pathological findings on myocardial scintigraphy (56%); 43% of these individuals showed evidence of coronary singlevessel disease, 14% evidence of prior myocardial infarction, 7% abnormal ventricular wall movement. The most frequent finding on myocardial scintigraphy was that of small-vessel disease (50%). Prior to the study, no patient had reported clinical symptoms of cardiac disease. In the study, however, there was no significant correlation between the severity of psoriasis or any comorbidities and pathological findings on myocardial scintigraphy. They concluded that myocardial scintigraphy could be considered as a very sensitive, noninvasive method for the early detection of cardiac comorbidities in psoriasis patients (its true diagnostic value will require larger studies with control subjects and control methods such as coronary angiography).
Unlike epicardial coronary arteries, the coronary microcirculation cannot be directly imaged by coronary angiography [20]; as mention earlier, CFR can be used as a surrogate of microvascular function. CFR using invasive testing and myocardial perfusion reserve using Positron Emission Tomography (PET) or Cardiac Magnetic Resonance (CMR) are the current gold standard for clinically assessing microvascular function [29]. The cut-off to define CMD is still unclear; however, several contemporary prognostic studies among patients with and without coronary artery disease have found CFR to have prognostic impact at thresholds of 1.5 to 2.6. [29]. Our group found that in psoriasis patients without hemodynamically significant epicardial stenosis as detected by coronary multi-slice computed tomography, CFR assessed by transthoracic echocardiogram may be a reliable prognostic marker for cardiovascular events-free survival providing a rationale for assessing CFR to identify psoriasis patients at higher risk for developing cardiovascular complications (data under review). In order to better describe a complete general overview of psoriasis patients, it must be underlined that they can also suffer, among others, from impaired aortic elastance [30], hypertension with non-dipping nocturnal blood pressure [31] and increased frequency of pulmonary hypertension [32].
The treatment and control of psoriasis and its related systemic inflammation using powerful drugs blocking major drivers of skin and vascular inflammation (namely TNF-alfa and IL-17), may eventually impart benefits toward overall CV risk. Recently, CANTOS study [33] showed that IL-1 inhibition by Canakinumab given once every three months among patients with a prior myocardial infarction and a persistent increase in C-reactive protein reduced the hazard ratio for cardiovascular events of 15% compared with placebo.
The treatment of HFpEF and diastolic dysfunction is still controversial; no treatment has yet been shown, convincingly, to reduce morbidity or mortality in these patients. However, since once HFpEF is established patients can be highly symptomatic, an important aim of therapy may be to alleviate symptoms and improve well-being. Diuretics will usually improve congestion thereby improving symptoms and signs of HF; evidence about beta-blockers and MRAs improving symptoms is lacking; inconsistent evidence for an improvement in symptoms in those treated with ARBs and Ace- Inhibitors is present [34]. Apart from treating the underlying disease, treatment of small-vessel disease patients primarily focuses on symptom relief using calcium antagonists, beta blockers, or nitrates. Analgesics and tricyclic antidepressants may also have a therapeutic role [35].
Conclusion
Psoriasis is a highly prevalent disease with important comorbidities including those involving the cardiovascular system; despite traditional risk factors, which have a higher prevalence in psoriasis patients compared to non-affected population, cardiovascular involvement seems to be strictly correlated with the chronic inflammatory state of the primary disease. Microvascular dysfunction, mainly cause by endothelial dysfunction, is an early and important culprit of the development of myocardial diastolic dysfunction, systolic dysfunction and microvascular angina. Aggressive psoriasis treatment seems to be a high valuable weapon also against the cardiovascular disease progression.
References
- Boehncke WH, Schön MP. Psoriasis. The Lancet. 2015; 386: 983–994.
- Lebwohl M. Psoriasis. The Lancet. 2003; 361: 1197–1204.
- Christophers E. Psoriasis - epidemiology and clinical spectrum. Clinical and Experimental Dermatology. 2001; 26: 314–320.
- Parisi R, Symmons DPM, Griffiths CEM, Ashcroft DM. Global Epidemiology of Psoriasis: A Systematic Review of Incidence and Prevalence. Journal of Investigative Dermatology. 2013; 133: 377–385.
- Abuabara K, Azfar R, Shin D, Neimann A, Troxel A, Gelfand J. Cause-specific mortality in patients with severe psoriasis: a population-based cohort study in the U.K.: Cause-specific mortality in patients with severe psoriasis. British Journal of Dermatology. 2010; 163: 586–592.
- Mehta NN, Azfar RS, Shin DB, Neimann AL, Troxel AB, Gelfand JM. Patients with severe psoriasis are at increased risk of cardiovascular mortality: cohort study using the General Practice Research Database. European Heart Journal. 2010; 31: 1000–1006.
- Snekvik I, Nilsen TIL, Romundstad PR, Saunes M. Psoriasis and cardiovascular disease risk factors: the HUNT Study, Norway. Journal of the European Academy of Dermatology and Venereology. 2018; 32: 776–782.
- Mosca S, Gargiulo P, Balato N, Di Costanzo L, Parente A, Paolillo S, et al. Ischemic cardiovascular involvement in psoriasis: A systematic review. International Journal of Cardiology. 2015; 178: 191–199.
- Gelfand JM, Neimann AL, Shin DB, Wang X, Margolis DJ, Troxel AB. Risk of Myocardial Infarction in Patients With Psoriasis. JAMA. 2006; 296: 1735.
- Osto E, Piaserico S, Maddalozzo A, Forchetti G, Montisci R, Famoso G, et al. Impaired coronary flow reserve in young patients affected by severe psoriasis. Atherosclerosis. 2012; 221: 113–117.
- Ahlehoff O, Gislason GH, Charlot M, Jørgensen CH, Lindhardsen J, Olesen JB, et al. Psoriasis is associated with clinically significant cardiovascular risk: a Danish nationwide cohort study: Psoriasis and cardiovascular risk. Journal of Internal Medicine. 2011; 270: 147–157.
- Nickoloff BJ, Qin JZ, Nestle FO. Immunopathogenesis of Psoriasis. Clinical Reviews in Allergy & Immunology. 2007; 33: 45–56.
- Luger TA, Loser K. Novel insights into the pathogenesis of psoriasis. Clinical Immunology. 2018; 186: 43–45.
- PUIG L. The role of biologics in the treatment of moderate-to-severe plaque psoriasis. Giornale Italiano di Dermatologia e Venereologia. 2016; 152: 28- 35.
- Hansson GK. Inflammation, Atherosclerosis, and Coronary Artery Disease. New England Journal of Medicine. 2005; 352: 1685–1695.
- Faccini A, Kaski JC, Camici PG. Coronary microvascular dysfunction in chronic inflammatory rheumatoid diseases. European Heart Journal. 2016; 37: 1799–1806.
- Geovanini GR, Libby P. Atherosclerosis and inflammation: overview and updates. Clinical Science. 2018; 132: 1243–1252.
- Libby P. Inflammation in atherosclerosis. Nature. 2002; 420: 868–874.
- Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, et al. Fourth Universal Definition of Myocardial Infarction (2018). Global Heart. 2018; 13: 305–338.
- Camici PG. Coronary Microvascular Dysfunction. N Engl J Med. 2007: 11.
- Zutt M, Rudolph H, Kaune KM, Wosniok W, Gärtner U, Linke R. Myocardial scintigraphy - a method for detecting cardiac comorbidity in psoriasis patients?: Myocardial scintigraphy in psoriasis patients? JDDG: Journal der Deutschen Dermatologischen Gesellschaft. 2016; 14: 1007–1014.
- Armstrong AW, Voyles SV, Armstrong EJ, Fuller EN, Rutledge JC. A tale of two plaques: convergent mechanisms of T-cell-mediated inflammation in psoriasis and atherosclerosis: T-cell Immunology in psoriasis and atherosclerosis. Experimental Dermatology. 2011; 20: 544–549.
- Recio-Mayoral A, Mason JC, Kaski JC, Rubens MB, Harari OA, Camici PG. Chronic inflammation and coronary microvascular dysfunction in patients without risk factors for coronary artery disease. European Heart Journal. 2009; 30: 1837–1843.
- Paulus WJ, Tschöpe C. A Novel Paradigm for Heart Failure With Preserved Ejection Fraction. comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. Journal of the American College of Cardiology. 2013; 62: 263–271.
- Borlaug BA, Paulus WJ. Heart failure with preserved ejection fraction: pathophysiology, diagnosis, and treatment. European Heart Journal. 2011; 32: 670–679.
- Wachter R, Schmidt-Schweda S, Westermann D, Post H, Edelmann F, Kasner M, et al. Blunted frequency-dependent upregulation of cardiac output is related to impaired relaxation in diastolic heart failure. European Heart Journal. 2009; 30: 3027–3036.
- Shang Q, Tam LS, Yip GWK, Sanderson JE, Zhang Q, Li EKM, et al. High Prevalence of Subclinical Left Ventricular Dysfunction in Patients with Psoriatic Arthritis. The Journal of Rheumatology. 2011; 38: 1363–1370.
- Bulbul Sen B, Rifaioglu EN, Ekiz O, Buyukkaya E, Kurt M, Karakas MF, et al. Assessment of left ventricular dyssynchrony in patients with psoriasis. International Journal of Dermatology. 2014; 53: 1221–1227.
- Löffler AI, Bourque JM. Coronary Microvascular Dysfunction, Microvascular Angina, and Management. Current Cardiology Reports. 2016; 18.
- Ardic I, Kaya MG, Yarlioglues M, Karadag Z, Dogan A, Yildiz H, et al. Impaired aortic elastic properties in normotensive patients with psoriasis. Blood Pressure. 2010; 19: 351–358.
- Bacaksiz A, Akif Vatankulu M, Sonmez O, Erdogan E, Tasal A, Turfan M, et al. Non-dipping nocturnal blood pressure in psoriasis vulgaris. Wiener klinische Wochenschrift. 2012; 124: 822–829.
- Gunes Y, Tuncer M, Calka O, Guntekin U, Akdeniz N, Simsek H, et al. Increased frequency of pulmonary hypertension in psoriasis patients. Archives of Dermatological Research. 2008; 300: 435–440.
- Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. New England Journal of Medicine. 2017; 377: 1119–1131.
- Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. European Heart Journal. 2016; 37: 2129–2200.
- Kaski JC. Pathophysiology and Management of Patients With Chest Pain and Normal Coronary Arteriograms (Cardiac Syndrome X). Circulation. 2004; 109: 568–572.