
Rapid Communication
Austin J Dermatolog. 2021; 8(1): 1093.
Serum Interleukin 17 and Interleukin 33 in Vitiligo Egyptian Patients
Ibrahim ElGhareeb M*, Elmokadem S, Fathi M, Khalifa N and Gado M
Department of Dermatology, Zagazig University, Egypt
*Corresponding author: Mohamed Ibrahim ElGhareeb, Department of Dermatology, Zagazig University, Egypt
Received: December 15, 2020; Accepted: January 07, 2021; Published: January 14, 2021
Abstract
Background: Vitiligo is an acquired depigmented disorder characterized by milky-white macules in the skin. Interleukin-17 has important role in autoimmune diseases development like vitiligo. Interleukin-33 is released after tissue damage or cell death. It may reflect recent disease activity.
Aim: To measure serum levels of IL17 and IL-33 in vitiligo patients and to assess their relationship with disease activity.
Methods: Levels of IL17 and IL-33 in serum samples taken from 21 healthy normal subjects and 21 vitiligo patients were measured by ELISA. All the patients were subjected to careful history taking , general examination and local examination to assess extent ,activity of vitiligo and VASI score.
Results: IL17 and IL-33 serum levels were highly statistically significant increase in patients with vitiligo than the normal control subjects(p≤0.001). Their levels were related to disease activity and the extent of the disease. Interleukin 17 serum levels were positively correlated with IL-33 serum levels.
Conclusion: Increased serum levels of IL-17 and IL-33 in vitiligo patients and their levels were related to disease activity and the extent of the disease indicating that IL-17 and IL-33 may play a role in the pathogenesis of vitiligo.
Introduction
Vitiligo pathogenesis was explained by many theories like autoimmune, neurogenic, genetic, autocytotoxic, microenvironmental hypotheses [1]. The role of autoimmunity in vitiligo is supported by the presence of autoantibodies against melanocytes, and presence of perilesional T-lymphocytes infiltrates [2].
Cytotoxic CD8+ T lymphocytes are the responsible cells of melanocytes destruction. Many cytokines secreted within the skin help those T cells to be directed to targeted melanocytes. Interferons increases the expression of CXCL10, which allows tissue invasion by cytotoxicT-cells. It has been reported increased expression of other cytokines as IL-17 and IL-33 in patients with vitiligo [3].
IL-17 binds to its receptor on keratinocytes, which leads to increase in the production of IL-1β, IL-8, IL-6, and TNF-a that may lead to melanocyte destruction. IL-17 effects on keratinocytes also include the upregulation of human β -defensin 2 and CCL20 gene expression [4]. CCL20 together with VEGFs increase the migration of cytotoxic T cells as well as neutrophils to the targeted melanocytes. Neutrophils migration leads to increase in ROS production. ROS may destroy melanocytes directly or indirectly through increased production of IL-1,IL-6, and TNF-a from the surrounding keratinocytes that eventually lead to melanocytes destruction [5,6].
IL-33 increases Th1 cytokines, as IFN- γ. Cytotoxic T cells expresses IL-33 receptor which is called ST2L on their cell surface [7].
It was found that IL-33 levels were increased in both blood and tissue samples in vitiligo patients. The expression of ST2 receptors in tissue samples were also increased [8].
IL-33 affects the function of keratinocytes that surround the targeted melanocytes by inhibiting the release of SCF and bFGF (which are paracrine melanogenic factors) , and at the same time increasing TNF- a and IL-6 release(which are paracrine melanocyte destroying factors). Peripheral nerve endings in the vitiliginous skin microenvironment release neuropeptides, as corticotropin- releasing hormone and neurotensin that synergize with IL-33 [9,10].
Methods
Twenty one vitiligo patients (14 females and 7 males) were enrolled in the study with age ranging from 8 to 65 years. Twenty one age-matched healthy subjects (13 females and 8 males) were used as controls. The duration of vitiligo ranged from 1 to 42 years.
None of the patients had immune mediated comorbidities such as insulin-dependent diabetes, atopic dermatitis or psoriasis. Patients had to refrain from systemic therapy for vitiligo for at least 4 weeks before the study. All the patients were subjected to careful history taking, general examination and local examination to assess extent, activity of vitiligo and VASI score. Nine patients (42.9%) had segmental vitiligo while 12 patients (57.1%) had non-segmental vitiligo. Eight patients (38.1 %) were stable while 13 patients (61.9%) had active vitiligo. The VASI ranged from 2.5%- 14.25% ,with mean of 6.71±3.67. Family history of vitiligo was present in 8 vitligo patients.
Upon informed consent, a blood sample(6ml), performed for routine analysis, was taken to determine the circulating serum levels of IL-17 and IL-33. Serum levels of IL-17 and IL-33 were measured in the patients and controls using sandwich Enzyme-Linked Immunosorbent Assay (ELISA) kit according to the manufacturer’s recommendations. Data were expressed as ng/L.
The collected data were analyzed by computer using Statistical Package of Social Services version 24 (SPSS).
Results
IL - 17 and IL-33 serum levels were highly statistically significant increased in vitiligo patients as compared with controls (p≤0.001) (Table 1).
Variable
Patient group (N=21)
Control group (N=21)
Mann Whitney U test
P-value
Serum level of IL-17 (ng/L)
Mean ± SD
48.48 ± 8.09
8.85 ± 3.19
0.001*
Median (Range)
45.6(36.2-65.6)
8.80(4.5-13.58)
Mean ± SD
28.75 ± 15.88
8.2 ± 3.73
0.001*
Median (Range)
24.5(7.56-67.11)
7.01(4.04-18.85)
Table 1: Comparison of serum level of IL-17 and serum level of IL-33 between the two groups.
There was a positive significant correlation between serum IL- 17 and serum IL-33 (r =.568 and P-value < 0.05). There was also a high statistically positive correlation between serum IL-17 levels and VASI score (r = 0.992 and P-value < 0.05). There was also a positive significant correlation between VASI score and IL-33 (r = 0.585 and P-value < 0.05). The higher VASI score, the higher IL-17 and IL-33 levels. There was a positive significant correlation between duration of vitiligo and VASI score (r =-.609 and P-value < 0.05). There was a positive correlation between IL-17 and duration of vitiligo (r =.632 and P-value < 0.05), while there was no correlation between IL-33 and duration of vitiligo (Figures 1-3).
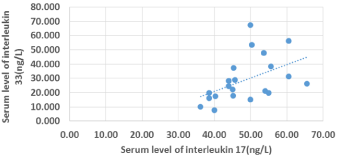
Figure 1: Scatter diagram for correlation between IL-17 and IL-33.
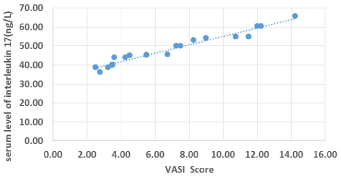
Figure 2: Scatter diagram for correlation between VASI score and IL-17.
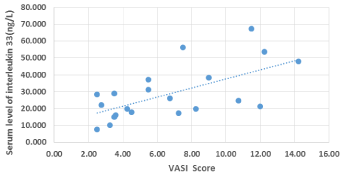
Figure 3: Scatter diagram for correlation between VASI score and IL-33.
The mean of serum levels of IL-17 among the patient group was higher in active vitiligo (51.86±7.18 ng/L) than stable vitiligo (42.98±6.54 ng/L).The mean of serum levels of IL-33 among the patient group was statistically higher in active vitiligo (37.01±14.67 ng/L) than stable form (15.33±4.39 ng/L).
There was a statistically significant relation of serum levels of IL- 17 with extent of disease (p<0.05) where it was higher with generalized non-segmental vitiligo than in segmental vitiligo. Serum levels of IL- 33 among segmental vitiligo was statistically lower than generalized non-segmental vitiligo (16.53±5.44 vs. 37.9±14.9 ng/L) respectively with a highly statistically significant difference(p<0.001) (Figure 4).
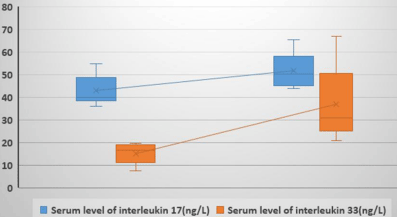
Figure 4: Box plot for comparison of serum level of IL-17 and serum level of
IL-33 in relation to activity state among the patient group.
As regard ROC curve for cutoff point, IL-33 >21.5 ng/L had a sensitivity of 92%, and a specificity of 100% with a high statistical significance in predicting activity state of vitiligo. It was also found that IL-17 >44.5 (ng/L) had a sensitivity of 84.6%, and a specificity of 62.5% with a statistical significance in predicting activity state of vitiligo (Figure 5).
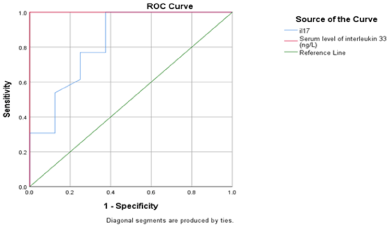
Figure 5: ROC curve of serum level of IL-17 and serum level of IL-33 in
relation to activity of disease in patient group.
Discussion
This study aimed to measure the serum levels of IL-17 and IL-33 in patients with vitiligo to explore their role in vitiligo pathogenesis. It aimed to determine whether there is correlation between IL-17 and IL-33 levels and extent and activity of vitiligo.
The mean of serum level of IL-17 among the patient group was statistically higher than mean of serum level of IL-17 in the healthy control group. There was positive correlation between mean of serum levels of IL-17 and VASI score. There was a statistically significant difference between serum levels of IL-17 in patients with generalized non-segmental vitiligo and those with segmental vitiligo with higher levels in generalized non-segmental type. It showed also significantly high levels in patients with active than those with stable vitiligo.
The mean of serum level of IL-33 in patient group was statistically higher than its level in the healthy control group. There was a positive correlation between VASI score and serum IL-33 levels, the higher VASI score, the higher IL-33 levels. Serum level of IL-33 was statistically higher among generalized non-segmental vitiligo than segmental vitiligo and in active vitiligo than stable vitiligo.
Regarding the relation between serum levels of both IL-17 and IL-33, we found positive correlation between them, which assist the possibility of involvement of both cytokines in pathogenesis of vitiligo and their interaction in extent and course of the disease.
When IL-33 level was more than 21.5 (ng/L), it had sensitivity of 92%, and specificity of 100% in predicting activity state of vitiligo. While IL-17 level more than 44.5 (ng/L) had sensitivity of 84.6%, and specificity of 62.5% in predicting activity state of vitiligo. This mean that IL-33 could be more valuable in following the activity of vitiligo.
In accordance with the results of the present study, Sushama et al., (2018) , Bhardwaj et al., (2017) and Bassiouny and Shaker (2011) found that serum IL-17 levels were significantly higher in patients with vitiligo than in healthy controls and higher in patients with generalized than localized vitligo and in active than stable vitiligo . They found a positive correlation of serum IL-17 level with duration, extent and activity of vitiligo [11-13].
Moreover, Elela et al., (2013) and Zhou et al., (2015) found that IL-17 serum levels were significantly higher in patients than in controls with positive correlation between serum levels of IL-17 and VASI score, extent and activity of vitiligo, but not with duration of disease [14,15].
However, Aly et al., (2017) and Habeb et al., (2013) reported significantly higher serum levels of IL-17 among vitiligo patients versus controls, they found no significant difference between active and stable or generalized and localized vitiligo patients and they explained that by the presence of other factors affecting the serum level of IL-17 and the disease activity [16,17].
On the other hand, Osman et al., (2015) found that there was no significant difference in IL-17 serum levels between vitiligo patients and control group. Jandus et al., (2008) investigated the role of Th- 17 cells in the pathogenesis of vitiligo. They didn’t find any increase in the number of these cells in peripheral blood of vitiligo patients [18,19].
Regarding IL-33 serum levels, our results were in agreement with Vaccaro et al ., (2016) and Li et al ., (2015) who reported that IL-33 serum levels were statistically significantly higher in patients affected by vitiligo as compared with controls. They found that serum IL-33 levels were higher in patients with generalized than localized vitiligo and in active vitiligo than stable type [20,8].
However, Ebrahim et al ., (2018) reported that serum IL-33 levels were higher in patients with vitiligo than in controls, they showed that there was negative correlation between serum level of IL-33 and duration of vitiligo but no correlation with extent of vitiligo. They explained that their patients reported long durations of vitiligo with a stable course [21].
The difference between our results and these studies may be contributed to multifactorial etiology and complicated pathogenesis of vitiligo and/or different criteria of patient selection and different populations.
Conclusion
From this study, It can be concluded that vitiligo is associated with increased serum levels of IL-17 and IL-33. Their levels are related to disease activity and the extent of the disease indicating that IL-17 and IL-33 may play a role in the pathogenesis of vitiligo. Interleukin 17 serum levels were positively correlated with IL-33 serum levels. Sensitivity and specificity of IL-33 serum levels were higher than sensitivity and specificity of IL-17 serum levels in relation to activity of vitiligo. This may be valuable in predicting activity of vitiligo.
References
- Picardo M and Taieb A. Pathophysiology overview. In: Vitiligo. Edited by Picardo M and Taieb A. Vitiligo.: Springer, Berlin/Heidelberg. 2010: 149–154.
- Razmi M and Prasad D. Recent advances in pathogenesis and medical management of vitiligo, In: Pigmentary Skin Disorders, Editted by Prasad K. Springer. 2018: 123-138.
- Wang X, Wang Q, Wu J. Increased Expression of CXCR3 and its Ligands in Vitiligo Patients and CXCL10 as a Potential Clinical Marker for Vitiligo. Br J Dermatol. 2016; 37: 1-27.
- Dyring-Andersen B, Honor´e T, Madelung A. Interleukin (IL)-17A and IL-22- producing neutro phils in psoriatic skin. Br J Dermatol. 2017; 177: 321-322.
- Singh R, Lee K, Vujkovic-Cvijin I. The role of IL-17 in vitiligo: A review. Autoimmun Rev. 2016; 15: 397-404.
- Suzuki E, Mellins E, Gershwin M. The IL-23/IL-17 axis in psoriatic arthritis. Autoimmun Rev. 2014; 13: 496-502.
- Silver M, Margulis A, Wood N. IL-33 synergizeswith IgE-dependent and IgEindependent agents to promote mast cell and basophil activation. Inflamm Res. 2010; 59: 207–218.
- Li P, Ma H, Han D. Interleukin-33 affects cytokine production by keratinocytes in Vitiligo. Clin Exp Dermatol. 2015; 40: 163-170.
- Laddha N, Dwivedi M, Begum R. Increased Tumor Necrosis Factor (TNF)- and its promoter polymorphisms correlate with disease progression and higher susceptibility towards vitiligo. PLoS ONE. 2012; 7: e52298.
- Drube S, Heink S, Walter S. The receptor tyrosine kinase c-Kit controls IL-33 receptor signaling in mast cells. Blood. 2010; 115: 3899-3906.
- Sushma S, Dixit N, Gautam R. Cytokine profile (IL-2, IL-6, IL-17, IL-22, and TNF- a) in vitiligo—New insight into pathogenesis of disease. J Cosmet Dermatol. 2018: 1-5.
- Bhardwaj S, Seema R, Niharika S. Increased systemic and epidermal levels of IL-17A and IL-1 β promotes progression of non-segmental vitiligo. Elsevier Cytokine. 2017; 91: 153–161.
- Bassiouny D, Shaker O. Role of interleukin-17 in the pathogenesis of vitiligo. Clinical and Experimental Dermatology. 2011; 36: 292–297.
- Elela M, Hegazy R, Fawzy M. Interleukin 17, Interleukin 22 and FoxP3 expression in tissue and serum of non-segmental vitiligo: A case- controlled study on eighty-four patients. Eur J Dermatol. 2013; 23: 350-355.
- Zhou L, Yu-Ling S, Kai L. Increased circulating Th17 cells and elevated serum levels of TGF-beta and IL-21 are correlated with human non-segmental vitiligo development Pigment Cell Melanoma Res. 2015; 28: 324–329.
- Aly D, Faisal M, Khadiga S. Is There a Relation between Vitamin D and Interleukin-17 in Vitiligo? A Cross-Sectional Study. Dermatology. 2017; 233: 413-418.
- Habeb A, Al Hefnawy M, Elsayed S. Expression of interleukin-17 mRNA in vitiligo patients. Egypt J Dermatol Venerol. 2013; 33: 67–60.
- Osman A, Mukhtar M, Bakheit K. Plasma levels of interleukin-17, interleukin-23, and transforming growth factor-β in Sudanese patients with vitiligo: A case-control study. Indian J Dermatol. 2015; 60: 635.
- Jandus C, Bioley G, Rivals J. Increased numbers of circulating polyfunctional Th17 memory cells in patients with seronegative spondylarthritides. Arthritis Rheum. 2008; 58: 2307-2317.
- Vaccaro M, Francesca C, Carmen M. IL-33 circulating serum levels are increased in patients with non-segmental generalized vitiligo. Arch Dermatol Res. 2016; 308: 527–530.
- Ebrahim A, Ahmed M, Enas S. Evaluation of serum interleukin-33 in nonsegmental vitiligo. Benha Medical Journal. 2018; 35: 54–59.