
Research Article
Austin Endocrinol Diabetes Case Rep. 2016; 1(1): 1001.
Beneficial Effect of Phloroglucinol against Adverse Metabolic and Electrophysiological Alterations in Streptozotocin Induced Diabetic Rats: a possible Target for the Prevention of Diabetic Peripheral Neuropathy
Bhadri N* and Razdan R
Department of Pharmacology, Al-Ameen College of Pharmacy, India
*Corresponding author: Naini Bhadri, Department of Pharmacology, Al-Ameen College of Pharmacy, Bangalore-560027, Karnataka, India
Received: May 20, 2016; Accepted: June 06, 2016; Published: June 08, 2016
Abstract
Hyperlipidemia is one of the factor responsible for the advancement of DPN and can be targeted to delay the onset. Currently no approved treatment is available for DPN. Therefore in the present study, we investigated the protective effect of phloroglucinol, a natural polyphenol with antioxidant property in preventing the streptozotocin induced lipid alterations due to the progression of Diabetic Peripheral Neuropathy (DPN). Diabetes was induced by a single dose of STZ (streptozotocin) (55mg/kg i.p.) in the rats and were treated with phloroglucinol for 8 weeks. Significant elevation in serum glucose, total cholesterol, triglycerides and LDL were observed while HDL cholesterol and nerve conduction velocity were significantly reduced in diabetic rats. Interestingly, the results further indicated that the lipid abnormalities and altered nerve conduction velocity were ameliorated by phloroglucinol in diabetic rats. A significant improvement in biochemical and electrophysiological deficit was observed in phloroglucinol treated diabetic rats in dose dependent manner. Results indicate the oral administration of phloroglucinol is able to prevent hyperlipidemia caused by diabetes suggest its potential in the treatment in DPN.
Keywords: Diabetic peripheral neuropathy; Lipid alterations; Hyperlipidemia; Nerve conduction velocity
Abbreviations
DPN: Diabetic Peripheral Neuropathy; STZ: Streptozotocin; HDL: High Density Lipoprotein; LDL: Low Density Lipoprotein; TC: Total Cholesterol; TG: Triglycerides
Introduction
Diabetic Peripheral Neuropathy (DPN) is a multifaceted complication appears frequently in more than 50% patient diagnosed with type 1 or type 2 diabetes [1]. The extent of abnormalities markedly increases if the blood glucose is not controlled properly [2]. Prolonged hyperglycemia causes damage of delicate nerve fiber throughout the body by interfering with the ability of the nerves to transmit signals [3]. It also weakens small blood vessels that supply oxygen and nutrients to the different parts of the body [4]. The clinical symptoms of DPN may include numbness, sharp cramps, jabbing or burning pain, muscle weakness etc., [5]. Moreover, several other symptoms including hyperlipidemia is an important contributor to the progression of DPN and are responsible for morbidity. Excessive disturbance of carbohydrates, lipids and protein metabolism leading to an abnormal lipid profile is a major risk factor for the development of DPN [6,7]. Various clinical evidences suggested a significant association between cholesterol, fasting triglycerides and DPN [8,9]. Therefore hyperlipidemia is one of the factors responsible for the advancement of DPN and can be targeted to delay the onset.
Hyperlipidemia is associated with qualitative and quantitative abnormalities in lipoproteins include elevated levels of Total Cholesterol (TC), Triglycerides (TG), Low Density Lipoprotein (LDL) and reduced High Density Lipoprotein (HDL). Multiple etiologies are involved in damage of nerves due to hyperlipidemia associated with diabetes. Previous reports have shown the altered composition of fatty acids in diabetes [10]. Several studies have explored the role of inflammation and oxidative stress in DPN is confirming the reduction in antioxidant potential and increase in lipid peroxidation [11,12]. Systemic oxidative stress results in vascular cellular metabolism, vascular matrix molecule and circulating lipoprotein [13]. These data strengthen the basic hypothesis of the present study that hyperlipidemia is an important contributor in the progression of diabetic neuropathy.
Phloroglucinol (1,3,5- trihydroxybenzene) is a natural polyphenol found in phlorotanin component of brown algae (phaophyceae) and in some plant species [14].Recent studies have shown enormous pharmacological activities of phloroglucinol including antispasmodic, free radical scavenging and anti-inflammatory activity [15]. Polyphenols are known for the prevention of long term diabetic complications, including neuropathy [16,17]. Therefore, in this study the effect of the polyphenolic compound phloroglucinol is evaluated in preventing hyperlipidemia associated with the progression of diabetic neuropathy.
Materials and Methods
Experimental procedures were performed in accordance with the guidelines of the Institutional Animal Ethics Committee of CPCSEA, India. Adult male Wistar rats (250-300 g) were used for the present investigation. Animals were housed in individual cages and were maintained in the animal room at a temperature of 20-24ºC and a humidity 40-70%. Animals had free access to regular rat chow and water. They were acclimatized for a minimum period of one week prior to the beginning of the study.
Drugs and reagents
Phloroglucinol and Streptozotocin (STZ) were purchased from Sigma-Aldrich. Commercial diagnostic kits were obtained from Agappe Diagnostic Pvt. Ltd. All other chemicals and reagents were of analytical grade.
Preparation of drug solution
Aqueous solutions of phloroglucinol were freshly prepared every day administered to rats orally using per oral tube. STZ was dissolved in ice cold citrate buffer (pH 4.5) and used to induce diabetes by intraperitoneal injection.
Induction of diabetes
Diabetes was induced by a single intraperitoneal injection of Streptozotocin (STZ) at a dose of 55 mg/kg body weight freshly dissolved in citrate buffer. Blood samples were collected from the tail vein 72 h after STZ administration. Rats with fasting blood glucose values more than 250 mg/dl were considered diabetic.
Experimental design
After confirmation of diabetes, diabetic rats were divided into four groups (n=6): Diabetic Control (DC), diabetic + phloroglucinol (100mg/kg), diabetic + phloroglucinol (200mg/kg), diabetic + phloroglucinol (250mg/kg). Aged match rats treated with saline were used as Normal Controls (NC). Phloroglucinol was administered by oral gavage daily for eight weeks. The doses of phloroglucinol administered in the present study were decided based on the previously published studies [18]. At the end of the 8th week the animals were anesthetized (ketamine: xylazine 80:5 mg/kg i.p.) and the blood was withdrawn from the retro-orbital plexus using capillary tubes. Blood was collected in clot activator vacutainer for estimation of various biochemical parameters. Tubes were left to clot at 37°C for 10 min, then centrifuged and serum was separated. Noninvasive nerve conduction velocity studies of sciatic nerve were performed to find out the severity of neuropathy.
Estimation of biochemical parameters: Serum estimation for glucose, total cholesterol, triglyceride, HDL cholesterol and LDL cholesterol was estimated using commercial diagnostic kits.
Noninvasive nerve conduction velocity studies: Conduction velocity was measured using power lab data acquisition system [19].
Statistical analysis: All data are expressed as the mean ± Standard Error of Mean (SEM) for six rats in each group of rats. Statistical evaluation of the data was performed by Graph Pad Prism 5 using one way Analysis of Variance (ANOVA), followed by Tukey’s multiple comparison test. Values were considered statistically significant at p<0.05 (confidence level=95%).
Results
Effect of phloroglucinol on serum glucose level
Diabetic animals exhibited significant increased serum glucose after 8 week of STZ administration. Treatment with phloroglucinol (100,200 & 250 mg/kg) for eight weeks showed a significant reduction on the elevated glucose level in the diabetic rats. All three doses of phloroglucinol have shown a significant antihyperglycemic effect (Figure 1).

Figure 1: Effect of phloroglucinol treatment on serum glucose levels in rats.
Values are expressed in mean ± SEM, n=6,*** (p<0.001) when compared with
diabetic control group by using one way ANOVA followed by Tukey’s multiple
comparison test using Graph Pad Prism 5.
Effect of phloroglucinol on serum levels of total cholesterol
Significant increase in the total cholesterol levels were observed in diabetic rats when compared with normal control group. Oral administration of pholorglucinol to diabetic rats inhibited the elevated total cholesterol levels in a dose-dependent manner. The highest significant reduction was observed at the dose 250mg/kg as compared to the diabetic control (Figure 2).

Figure 2: Effect of phloroglucinol treatment on serum levels of total
cholesterol in rats.
Values are expressed in mean ± SEM, n=6,*** (p<0.001) when compared with
diabetic control group by using one way ANOVA followed by Tukey’s multiple
comparison test using Graph Pad Prism 5.
Effect of phloroglucinol on serum levels of triglycerides
Triglycerides levels were markedly increased in the serum of diabetic rats compared with the control group. Treatment with phloroglucinol produced a significant reduction in serum levels of triglycerides in diabetic rats (Figure 3).

Figure 3: Effect of phloroglucinol treatment on serum triglycerides levels in
rats.
Values are expressed in mean ± SEM, n=6,*** (p<0.001) when compared with
diabetic control group by using one way ANOVA followed by Tukey’s multiple
comparison test using Graph Pad Prism 5.
Effect of phloroglucinol on serum level of HDL cholesterol
A significant reduction in serum levels of HDL cholesterol were observed in diabetic rats when compared to normal control group. Oral administration of phloroglucinol produced a dose dependent increase in the serum levels of HDL cholesterol, when compared with diabetic rats. The highest significant elevation in serum HDL level was observed at the dose 250mg/kg as compared to the diabetic control (Figure 4).
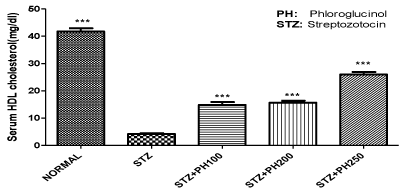
Figure 4: Effect of phloroglucinol treatment on serum HDL cholesterol levels
in rats.
Values are expressed in mean ± SEM, n=6,*** (p<0.001) when compared with
diabetic control group by using one way ANOVA followed by Tukey’s multiple
comparison test using Graph Pad Prism 5.
Effect of phloroglucinol on serum level of LDL cholesterol
Marked elevations in the serum levels of LDL cholesterol were observed in rat treated with STZ when compared to normal control rats. Oral administration of phloroglucinol produced a significant reduction in elevated serum levels of LDL cholesterol when compare with diabetic control group (Figure 5).

Figure 5: Effect of phloroglucinol treatment on serum LDL cholesterol levels
in rats.
Values are expressed in mean ± SEM, n=6, *(p<0.05) *** (p<0.001) when
compared with diabetic control group by using one way ANOVA followed by
Tukey’s multiple comparison test using Graph Pad Prism 5.
Effect of phloroglucinol on non-invasive sciatic nerve conduction velocity
A significant decrease in the sciatic nerve conduction velocity was observed after eight weeks of induction of diabetes. Animal treated with phloroglucinol showed an improvement in the conduction velocity as compared to diabetic control (Figure 6).

Figure 6: Effect of phloroglucinol treatment on non-invasive sciatic nerve
conduction velocity in rats.
Values are expressed in mean ± SEM, n=6,*** (p<0.001) when compared with
diabetic control group by using one way ANOVA followed by Tukey’s multiple
comparison test using Graph Pad Prism 5.
Discussion
Hyperlipidemia is one of the most common complications of diabetes, which causes abnormalities in the lipoprotein metabolism and contributes to the development of Diabetic Peripheral Neuropathy (DPN). Several studies have implicated abnormal lipid profiles are related to the progression of DPN. Although no treatment exists to prevent or cure DPN, lipid lowering drugs may be beneficial for the prevention of DPN. Various dietary plant polyphenols are known to prevent the development and progression of long term diabetic complications.
Thus, we found it worthwhile to investigate the therapeutic potential of phloroglucinol, a naturally occurring polyphenol in preventing the streptozotocin induced lipid alterations due to the progression of DPN.
Experimental DPN develops and progresses within 6 weeks in animals injected with streptozotocin as evidenced by decrease Nerve Conduction Velocity (NCV) [20]. Along with electrophysiological changes there exist many biochemical changes, including alterations in the levels of serum total cholesterol, triglycerides, HDL and LDL cholesterol that accompany DPN [7].
Our obtained results showed that phloroglucinol could reduce the elevated serum glucose level and rats were visibly healthier and more active as compare to STZ treated diabetic rats. The results are in accordance with the previous studies that demonstrated antihyperglycemic properties of phloroglucinol derivative by inhibiting α-glucosidase and α-amylase [21].
Significantly higher serum lipid level was observed in the STZ induced diabetic control group when compared to a normal control group due to a reduction in activity of lipolytic enzyme i.e. lipase and absence of insulin caused by the destruction of beta islet cells by STZ. Lipase is an enzyme that hydrolyzes lipids, the ester bonds in triglycerides to form free fatty acids and glycerol. Insulin inhibits the intracellular lipase in the adipose tissue. The activation of Hormone- Sensitive Lipase (HSL) in the absence of insulin causes elevation in formation of free fatty acid particularly in the plasma [22]. In the liver, β-oxidation of fatty acid causes formation of acetyl CoA and excess of acetyl CoA is converted to triglyceride, cholesterol, and ketone bodies which lead to ketosis [23].
Elevation in the formation of free fatty acid causes increased generation of cytokines or proinflammatory factors. Generation of proinflammatory markers can lead to the progression of diabetic neuropathy [24].
The low concentration of insulin and the presence of peripheral fat depots by glucagons which increases the formation of free fatty acid may lead to abnormally high concentration of serum lipoprotein in the diabetic control group. Some fatty acids converted into triacylglycerol, phospholipids and cholesterol which may be discharged into the blood as lipoproteins [25]. Thus marked hyperlipidemia was observed as STZ induced diabetic rats.
A significant reduction in total cholesterol, triglyceride and LDL levels in diabetic control rats was observed upon oral administration of phloroglucinol for 8 weeks. Phloroglucinol treatment dosedependently decreases the serum level of total cholesterol and LDL cholesterol level. However, serum level of triglycerides decreased independent of dose.
A significant hypolipidemic activity have been shown by the oral administration of phloroglucinol when compared with the diabetic control group might be due to its protective effect against the destruction of pancreatic β-cells and potentiating insulin secretion from surviving β-cells. Lipase gets deactivated in the presence of insulin which leads to immobilization of fatty acid from adipose tissue by glucagons. Thus causes a significant reduction in serum level of free fatty acids. Reduction in serum concentration of fatty acids causes decrease concentration of LDL. In the present study reduction of LDL levels were observed in serum confirms the antihyperlipidemic activity of phloroglucinol.
HDL is considered as a good cholesterol as it carries surplus cholesterol back to the liver for disposal. Recent studies suggest that HDL directly influences glucose metabolism through multiple mechanisms. In various clinical studies decreased HDL levels were reported in diabetic patient. A decrease in HDL cholesterol was observed in the diabetic control group might be due to insulin insufficiency [26,27]. Administration of phloroglucinol for 8 weeks significantly increases the serum HDL in a dose dependent manner. A negative correlation was observed between the levels of HDL cholesterol v/s total cholesterol, triglyceride and LDL. However, it can be inferred from results that phloroglucinol gave its most potent antihyperlipidemic effect at the higher dose (250mg/kg).
Experimental diabetic neuropathy is marked by impaired nerve conduction velocity with the functional changes in the sciatic nerve [28]. The results of the NCV studies show amplitude, conduction velocity of fastest conducting fiber, minimal F-wave latencies, distal latency of compound muscle action and sensory potentials. In our study, we found a reduction in nerve conduction velocity in STZ treated group which confirmed the abnormal functions of nerves due to diabetes. Phloroglucinol treated group showed a significant improvement in NCV. Hence, oral administration of phloroglucinol has shown normalization of nerve speed at which an electrochemical impulse propagates down a neural pathway [29]. The normalization of NCV can be due to reduction in dysglycemia causing diminution of the oxidative stress.
Our results, therefore, suggest that the oral administration of phloroglucinol ameliorated the hyperlipidemia associated progression of diabetic neuropathy.
Conclusion
The present data indicate that antihyperglycemic and antihyperlipidemic property of phloroglucinol may be responsible for protecting against the hyperlipidemia due to the progression of DPN, possibly by suppression of oxidative stress. The findings suggest the therapeutic potential of phloroglucinol where diabetes and hyperlipidemia are involved in the development and progression of DPN.
Acknowledgement
Authors are thankful to Indian Council of Medical Research (ICMR) New Delhi, India for providing senior research fellowship to the corresponding author.
References
- Dixit S, Maiya A. Diabetic peripheral neuropathy and its evaluation in a clinical scenario: a review. J Postgrad Med. 2014; 60: 33-40.
- Callaghan BC, Little AA, Feldman EL, Hughes RA. Enhanced glucose control for preventing and treating diabetic neuropathy. Cochrane Database Syst Rev. 2012; 6.
- Aslam A, Singh J, Rajbhandari S. Pathogenesis of painful diabetic neuropathy. Pain Res Treat. 2014; 412041.
- Zychowska M, Rojewska E, Przewlocka B, Mika J. Mechanisms and pharmacology of diabetic neuropathy - experimental and clinical studies. Pharmacol Rep. 2013; 65: 1601-1610.
- Zhao Y, Ye W, Boye KS, Holcombe JH, Hall JA, Swindle R. Prevalence of other diabetes-associated complications and comorbidities and its impact on health care charges among patients with diabetic neuropathy. J. Diabetes Complications. 2010; 24: 9-19.
- Vincent AM, Hinder LM, Pop-Busui R, Feldman EL. Hyperlipidemia: a new therapeutic target for diabetic neuropathy. J Peripher Nerv Syst. 2009; 14: 257-267.
- Wu S, Cao X, He R, Xiong K. Detrimental impact of hyperlipidemia on the peripheral nervous system: A novel target of medical epidemiological and fundamental research study. Neural Regen. Res. 2012; 7: 392-399.
- Tesfaye S, Chaturvedi N, Eaton SE, Ward JD, Manes C, Ionescu-Tirgoviste C, et al. Vascular risk factors and diabetic neuropathy. N Engl J Med. 2005; 352: 341-350.
- Wiggin TD, Sullivan KA, Pop-Busui R, Amato A, Sima AA, Feldman EL. Elevated triglycerides correlate with progression of diabetic neuropathy. Diabetes. 2009; 58: 1634-1640.
- Poisson JPG, Cunnane SC. Long-chain fatty acid metabolism in fasting and diabetes: relation between altered desaturase activity and fatty acid composition. J. Nutr. Biochem. 1991; 2: 60-70.
- Feldman EL. Oxidative stress and diabetic neuropathy: a new understanding of an old problem. J Clin Invest. 2003; 111: 431-433.
- King GL, Loeken MR. Hyperglycemia-induced oxidative stress in diabetic complications. Histochem Cell Biol. 2004; 122: 333-338.
- Hansel B, Giral P, Nobecourt E, Chantepie S, Bruckert E, Chapman MJ, et al. Metabolic syndrome is associated with elevated oxidative stress and dysfunctional dense high-density lipoprotein particles displaying impaired antioxidative activity. J Clin Endocrinol Metab. 2004; 89: 4963-4971.
- Martinez IJH, Castaneda THG. Preparation and chromatographic analysis of phlorotannins. J Chromatogr Sci. 2013; 51: 825-838.
- Annahazi A, Roka R, Rosztoczy A, Wittmann T. Role of antispasmodics in the treatment of irritable bowel syndrome. World J Gastroenterol. 2014; 20: 6031-6043.
- Bahadoran Z, Mirmiran P, Azizi F. Dietary polyphenols as potential nutraceuticals in management of diabetes: a review. J Diabetes Metab Disord. 2013; 12: 43.
- Sadowska-Bartosz I, Bartosz G. Prevention of protein glycation by natural compounds. Molecules. 2015; 20: 3309-3334.
- Rauniyar BK, Shakya A, Thakur AK, Chatterjee SS, Kumar V. Anti-Stress Activity of Phloroglucinol: A Transient Metabolite of Some Plant Polyphenolics. Pharmacologia. 2015; 6: 21-30.
- Bhadri N, Sanji T, Guggilla MH, Razdan R. Amelioration of behavioural, biochemical, and neurophysiological deficits by combination of monosodium glutamate with resveratrol/alpha-lipoic acid/coenzyme Q10 in rat model of cisplatin-induced peripheral neuropathy. Sci. World J. 2013; 565813.
- Kumar A, Sharma SS. NF-kappa B inhibitory action of resveratrol: a probable mechanism of neuroprotection in experimental diabetic neuropathy. Biochem Biophys Res Commun. 2010; 394: 360-365.
- Nwosu F, Morris J, Lund VA, Stewart D, Ross HA, McDougall GJ. Antiproliferative and potential anti-diabetic effects of phenolic-rich extracts from edible marine algae. Food Chem. 2011; 126: 1006-1012.
- Kraemer FB, Shen WJ. Hormone-sensitive lipase: control of intracellular tri-(di-) acylglycerol and cholesteryl ester hydrolysis. J Lipid Res. 2002; 43: 1585-1594.
- Veech RL. The therapeutic implications of ketone bodies: The effects of ketone bodies in pathological conditions: Ketosis, ketogenic diet, redox states, insulin resistance, and mitochondrial metabolism. Prostaglandins Leukot Essent Fat Acids. 2004; 70: 309-319.
- Sandireddy R, Yerra VG, Areti A, Komirishetty P, Kumar A. Neuroinflammation and oxidative stress in diabetic neuropathy: futuristic strategies based on these targets. Int J Endocrinol. 2014; 10.
- Pari L, Latha M. Antidiabetic effect of Scoparia dulcis: effect on lipid peroxidation in streptozotocin diabetes. Gen Physiol Biophys. 2005; 24: 13- 26.
- Hollenbeck CB, Chen YDI, Greenfield MS, Lardinois CK, Reaven GM. Reduced plasma high density lipoprotein-cholesterol concentrations need not increase when hyperglycemia is controlled with insulin in noninsulindependent diabetes mellitus. J Clin Endocrinol Metab. 1986; 62: 605-608.
- Chen YD, Jeng CY, Reaven GM. HDL metabolism in diabetes. Diabetes Metab Rev. 1987; 3: 653-668.
- Coppey LJ, Davidson EP, Dunlap JA, Lund DD, Yorek MA. Slowing of Motor Nerve Conduction Velocity in Streptozotocin-induced Diabetic Rats is preceded by Impaired Vasodilation in Arterioles that Overlie the Sciatic Nerve. International Journal of Experimental Diabetes Research. 2000; 1: 131-143.
- Zangiabadi N, Ahrari MN, Nakhaee N. The effect of omega-3 fatty acids on Nerve Conduction Velocity (NCV) and F-wave latency in patients with diabetic polyneuropathy. Am J PharmacolToxicol. 2007; 2: 1-3.