
Review Article
Austin Endocrinol Diabetes Case Rep. 2017; 2(1): 1009.
Impacts of Metal and Metal Oxide Nanoparticle on Embryos
YK Lahir*
Department of Biophysics, University of Mumbai, India
*Corresponding author: Yogendrakumar Lahir, Department of Biophysics, University of Mumbai, Vidyanagari, Santa Cruz (E), Mumbai-400 098, MS, India
Received: June 15, 2017; Accepted: July 03, 2017; Published: July 10, 2017
Abstract
In the recent past numerous studies related to nanomaterials and biological experimental models are being carried out. These are basically converging to enhance the understanding the mode, mechanism and resultant impacts on biota of the ecosystem. Extensive applications of nanotechnology are responsible for the excessive and unproportional use of nanomaterials/nanoparticles in present day life. As a result the existence of nanomaterials as discarded waste matter in ecosystem is quite obvious. This discarded bulk of nanomaterials become the prime factor that interferes in the life of biosystem. This compels to take review of such studied that are related to the detrimental effects on biosystem and redesign the research. Embryonic phase of life cycle of any metazoan biological entity exhibiting sexual reproduction is an essential, sensitive and vulnerable phase. It is very responsive to the fluctuations in the ecosystem. Embryonic phase of biosystem may be accomplished either within the body of the maternal parent or outside in open in the aquatic and/or soil aspects of ecosystem. It becomes pertinent to review the impact of nanomaterials on the biotic and abiotic components of the ecosystem specifically when nanomaterials are involved in wide varieties of products of day to day life. Nanomaterials are of diverse nature and because of their specific physicochemical properties are being exploited in biomedical, pharmaceutical, chemical, electronics, and many industrial fields. These nanomaterials are of various types like metals and metallic oxides, carbon based nanomaterials, quantum dots, polymeric nanomaterials, nanocomposites, nano-alloys and most of these are specifically functionalized for the targeted purposes. Of all these nanomaterials metals and metal oxide nanoparticles appear to be most exploited materials as a consequence are released in huge amount in the ecosystem relatively more in comparison to the rest. The metal and metal oxide nanoparticles get dispersed in air, water and soil relatively much easily and remain there either in pristine or combined forms. These features make them more detrimentally effective. In this review an effort is made to understand the intricacies involved specifically during the embryonic stage. Thus an effort is made to review the pertinent literature on the impacts of metals and metal oxide nanoparticles specifically on embryos of vertebrates.
Keywords: Nanomaterials; Metal-nanoparticles; Metal oxide nanoparticles; Placenta; Reproductive phase
Over View
Embryo
The term ‘Embryo’ has its origin from Greek and Latin, (grow or get filled up); this world denotes growth at the maximum rate in most of the biosystems exhibiting sexual reproduction. Duration of embryonic phase varies with respect to the species. Embryo is the product of post fertilization process i.e., zygote is a unicellular diploid stage under goes cell proliferation resulting in blastula, gastrula and three germinal layer stage, cell differentiation, cell migration, demarcation of presumptive zones, morphometric movements, organogenesis. In these cases the embryos are relatively more protected than those developing outside their respective mother. Among mammals female individuals are provided with varied forms of placenta in different groups of mammals. The placenta is a morphological and physiological bridge between the embryo and the uterus of maternal parent which meets all requirements of developing embryo. This feto-maternal organ gets rooted within blastocyst and is delivered with the fetus at birth. The placenta ensures the implantation, interface to supply and eliminate nutrient and the metabolites, to regulate maternal recognition of pregnancy, maintains immune environment suitable for the fetus and establishes paracrine and endocrine homeostasis [1]. Among most of the invertebrates and lower vertebrates embryos are left in their natural environment (mostly outside their maternal parent) for their embryonic growth.
Mostly embryos are considered as suitable model for developmental biology and biomedical research specifically embryos of zebra fish, medake fish (Oryzias latipes), Pelophylax perezi, Xenopus, avian embryos (chick embryo) [2-4]. In case of reptilian and avian embryos the embryonic development is accomplished outside the maternal parent and morphologically the developmental stages are relatively distinct and easily manipulative for experimentation in comparison to other animals. In these experimental models the entry port for the nanomaterials is albumin, air sac; these components of the egg are likely to affect the exact concentration that reaches the tissue under study, thereby may affect the implications of the nanoparticles. The eggs and embryos of fishes and amphibians are covered with a jelly like materials made of proteoglycans, glycoproteins; this mucoidal layer is the probable protective and preventive in nature.
Egg, zygote and early developing stages are enclosed within vitelline membrane; this membrane mostly consists of protein fibers and protein receptors that are needed for the binding of sperm, these protein receptors are species – specific in nature. As a result of signal transduction Ca+2 increase and ‘cortical reaction’ is induced, these cortical granules get added on to the Vitelline Membrane (VM) involving exocytosis and converting VM in to fertilization membrane [5]. The potential of contractibility of actomyosin can induce effective forces that plays major role in permitting the cells to sense and response to varied mechanical stimuli [6]. These developing stages are relatively smaller in size, have lower metabolic activities, potentially lower energy reserves probably due to higher growth rate and distribution limitation. Lister et al, have suggested that most of the embryos and larval stages are provided with ‘Mycosporine-Like- Amino Acids’ (MAAs), these are the gifts from their maternal parent. The levels of such amino acids may vary with respect to the type of food consumed by the maternal parents [7]. Vitelline membrane is the membranous structure which is protective and regulates the embryonic pattern. There are four major Vitelline Membrane Proteins (VMPs) having cystine. The critical cystine involves in isomerizing intermolecular disulfide bond enzymatically and facilitates assembly of egg shell [8].
Entry-ports for any toxicants are the vulnerable sites in and on the embryo that facilitate the entry; reproductive openings, pre and post fertilizational stages, larval stages in case of aquatic animals and transplacental route in case of mammals [9]. There are likely to be some deviations in the toxicological investigations when conducted in vivo and in vitro this is because within organism there is a ‘molecular crowding’ which is not there in ‘test tube’, but overall the pattern is likely to represent the derogative impacts of the toxicant under study. There are number of types of nanomaterials and their detrimental influences on biota are vast. In this presentation derogative effects due to metal and metal oxide nanoparticles pertaining to vertebrates have been elucidated.
Metal and metal oxide nanoparticles
Nanomaterials are identified and characterized because of their nanosize and specific physicochemical properties. These are fascinating materials – products of multidisciplinary nanotechnology. These have been suitably used in variety of disciplines ranging from basic and fundamental science to most of the applied branches of industries; as such these wonder particles have gained prime attention of researchers, scientists and industrialists. Nanoparticles have specific physicochemical properties that include chemical composition, size, shape, surface modification or properties, agglomerate or single nanoparticle state [10]. Nanoparticles exhibit properties that favor surface coatings, surface charge, and ‘Zeta potential’ change in shape and because of such abilities nanoparticles are functionalized to carry out targeted functions [11]. All these properties enable them to pass though almost all types of biological barriers in an organism. Sometimes these factors are likely to results in bioaccumulation and induce some biochemical, molecular and developmental defects in organism. Nanoparticles and the allied products are well known for their higher degree of interactions with biomolecules. The synthesis and engineering of nanomaterials are precisely controlled in relation to the physicochemical properties, surface modifications and other features specifically in accordance to the target specifications [12]. Parameters of nanomaterials like size, shape and core composition, purity of the metals and their precursor, surface properties, pH, ionic strength, nature of the biomolecules present in the system also affect their interactions with biomolecules [13,14]. As a result of these interactions the biomolecules are likely to undergo changes involving shift of energy within specific thermodynamics, kinetics and physicochemical limitations [15,16]. Generally greater numbers of nanoparticles are used in colloidal form at least in biomedical field and related industries. The size of nanoparticles renders them to be more towards spherical shape showing curvatures rather a particle with flat nature, this feature reflects on their assumed colloidal behavior. The colloidal behavior of nanoparticles is related to their curvature, small radii, and higher percentage of atom at their surface. These features affect their electronic structure, surface charge, behavior and reactivity; these features play a decisive role towards their dispersal or colloidal behavior. As such these nanoparticles in most probability follow “Derjguin-Landan-Overwey-Overbeek” and “Extended-Derjguin-Landan-Overwey-Overbeek” models in their behavior. This behavior of nanoparticles is related with the repulsive and attractive forces like van der Waals, electrostatic forces and double layer etc. All these forces affect the distribution of net potential energy between the nanoparticles in spite of the state of nanoparticles whether in dispersed or agglomerate state [17].
The interaction between nanomaterials and macromolecules of biosystem are likely to be either beneficial, derogative in nature or may exhibit delayed reaction, further this behavior is dose dependant [18]. Cho et al, observed that that surface charge and uptake of nanoparticles exhibit some type of correlation; negatively charged nanoparticles were found to be less adsorbed on the cell membrane surface enhance the degree of internalization of such nanoparticles was low [19]. Dose dependant histological impacts exhibit disorganization and atrophy in the hepatic tissue in case of chick embryo when treated with zinc oxide nanoparticles [20]. Biocompatibility of nanomaterials is one of the prime parameters affecting the beneficial and derogative impacts on any aspect of biosystem. This parameter plays significant role in the safety of the biosystem from the nanomaterials and appropriate application in biomedical and related fields. Any material that is intended to interface within biological system and to evaluate, treat, augment, replacement of tissue/organs and participate any functional aspect is considered to be biomaterial. Biocompatibility of such materials can be understood by following its pathways: in biosystem the biomaterial may exhibit molecular adsorption, experience mechanical, biophysical and biochemical impetus. These aspects influence structural and functional unit of biosystem and as a result it may be defensive, be a target or may interfere; when defensive there may be no effect, it is a good outcome, when a target-it can exhibit adverse effect eliciting adverse outcome, if as interferer then the response may be no interaction then it is neutral, a good outcome, if interaction occurs then response may be either poor or adverse [21]. There are many properties which contribute to the biocompatibility like physicochemical properties, surface characters, hydrophilicity and/or hydrophobicity that can enhance the suitability of biomaterial in the biomedical and related fields [22].
Some aspects related to the interactions between metal, metal oxide nanoparticles and embryo
Concentration of nanoparticles, i.e., (nanoparticles /cm3) affects the animal tissues depending on the type of tissue and type of metal nanomaterials [10]. Lee et al found that when the embryos of zebra fish were exposed to higher concentration of silver nanoparticles it resulted in higher number of deformed embryos and higher mortality [23], similar observations were recorded in case of embryos of Oryzias latipes and Pimphales promelas when subjected to higher concentration of silver nanoparticles [24,25]. Dose dependent toxic effects were observed in case of embryos of zebra fish and chick when exposed to ZnO-nanoparticles [20,27]. Browning et al did not observe dose dependent impact on the embryos of zebra fish when exposed to higher concentration of silver nanoparticles instead accumulation of silver nanoparticles in the body of embryos was noticed [27].
Other parameter that may affect toxic effect is agglomerated state of nanoparticles because there is a change in the surface area and chemical properties; it is of common observation that most of the nanoparticles undergo agglomeration when come in contact with water, ZnO nanoparticles is good example [10]. Agglomerated ZnO nanoparticles have ability to cause relatively more toxic effects and higher degree of mortality [26]. It is of common observation that agglomerated nanoparticles get deagglomeration and after sonication these can be used; Laban et al studied the toxicity of agglomerated ZnO nanoparticles and did not observe any toxic effects with respect to the size [25]. There are some of the nanoparticles that release ions when subjected to physiological fluid; this feature plays an effective role in the respective toxicity. When embryo of zebra fish were exposed to silver nanoparticles having 10 nm size and coated with citrate 10/50nm coated with polyvinylpyrrolidone showed changed swimming behavior, delayed hatching, inflation of bladder, derogative morphological impacts and silver ions, both were investigated and mortality; Ag ions induced hyperactivity with respect to changes in light but coated nanoparticles were not very effective in this aspect [28]. Li et al observed the toxic effects of 50μm sized Ag nanoparticles and silver ions on the developing stage –in blastula of mouse that is referred as blastocyst resulted in decline in number of cells, elevated apoptosis, declined the rate of implantation, loss of weight of embryonic tissue and higher degree of resorption of Ag nanoparticles in the post implanted embryos [29]. Toxic impacts of copper nanoparticle and nickel salt on embryos of zebra fish; copper nanoparticles with 0.1mg/l concentration reduced hatching, morphologically deformed larvae and caused death of larvae at gastrula stage while lower concentration 0.01mg/l and 0.05mg/l and Cu+2 at 0.006 and 0.03mg/l appeared to be not very effective, thus indicating higher toxicity at 0.1 mg/l than Cu+2 at 0.06mg/l [30]. ZnO nanoparticles in water form aggregates, small aggregates 142.4nm and big aggregates 517.7nm, with concentration 50 and 100mg/L both appeared to be toxic as the embryos under study were killed; the concentration of 1-25mg/L retarded the growth (body length) of the larvae and rendered defective tail formation at 96 h of exposure [31]. Hydrophobicity and hydrophilicity are related to the distribution and surface feature of nanoparticles and helpful in developing predictive models of nanoparticle; Principal Component Analysis (PCA) is an effective mode for controlling many surface molecular features. It is important to identify specific features of the materials and its specific chemistry that is responsible for the biological interactions; this consequently will help in safe designing of nanoparticles [32].
Interactions between metal, metal oxide nanoparticles and vertebrates
Embryo is biologically and metabolically active and important phase in the life of a metazoan, may it be invertebrate or vertebrates. In the given ecosystem nanomaterials are available to biota and abiota because these are likely to be released either as waste or unused or discarded intentionally or accidently. Whatever the case may be most of the biological systems get affected by these materials. Embryo is metabolically active and relatively more vulnerable to the negative or derogative impacts of the nanomaterials present in ecosystem. In the current scenario the embryos are the prime target to study the effects of nanomaterials on the developmental phase of a biosystem. Nanoparticles are ubiquitous in environment and their dose and the exposure time for the organisms is changing day by day possibly causing change in the threshold toxic dose. This unnoticed slow exposure to the organisms is likely to be either fetal or injurious and/ or may result in malfunctioning or malformation in embryo and offspring.
Whenever nanomaterials either interact externally and/or internalized there is a ‘conception period’; during this period there occur molecular and cellular restructuration in biosystem; during this span of time it is very difficult to observe the response of biosystem. Nanoparticles have the ability to cross over most of the biological barriers; this feature ensures their influence on the cellular viability of the organism (Flow Chart).
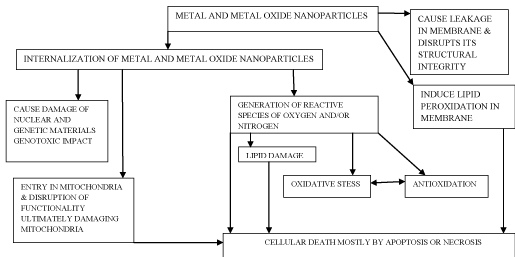
Figure 1: Broad outline involved during n interaction between metal and metal oxide nanoparticles.
Review of literature reflects that mostly nanoparticles of gold, silver, titanium oxide and zinc oxide have been studied mostly in relation to fecundated eggs (ova) and the developing embryos of many animals [33].
Fish Embryo
The zebra fish and its embryo both are preferred as experimental model for toxicity investigations because of some of some specific features like transparent embryos, high degree of homology to human genome, economically feasible and most striking point is ‘mediumthroughput screening for non evasive real time assessment’ of toxicity, high reproducibility, fast and distinct development, distinct organogenesis, can be handed comfortably and because of having high fecundity produces large numbers of larvae [34,35]. Silver nanoparticles capped with starch and BSA was tested for toxicity on the embryos of zebra fish. The experimental embryos exhibited higher mortality, hatching disorder, pericardial edema and irregular cardiac functions in response to the graded concentrations of these Ag nanoparticles. Further, there were changes in the embryos like change in the axes of the body, twisted notochord, slow blood flow; the silver ions stabilizers used along with Ag nanoparticles could not cause any change in the developing process. Ag nanoparticles were found to be distributed in organs like brain, heart, yolk and blood when tested using TEM and electron-dispersive X- ray analysis. Apoptosis was commonly seen in the tissues of experimental embryos [36].
Toxicity of colloidal silver and silver nanoparticles having 3, 10, 50 and 100nm size was investigated on embryos of zebra fish, colloidal Ag ions resulted in 100% mortality while colloidal Agnanoparticles caused below 3% mortality. Colloidal silver and colloidal-Ag-nanoparticles caused different degrees of toxicity profile; colloidal silver was much more toxic while colloidal-Agnanoparticles with size mentioned above were found to be less toxic. Colloidal Ag-nanoparticles exhibited the toxicity profile as embryonic malfunctioning; this impact is mostly due to the release of Ag ions during the destabilizing of nanoparticles [34]. Ag nanoparticles (30 -70nm) get internalized the embryo of zebra fish via simple diffusion and Brownian motion through chorionic pores and reach chorionic space, these remain within the inner mass of the embryo throughout the developmental process. Normal developmental process gets affected when concentration increases from 0.0 to 0.2nm and number of deformed/malformed and dead embryos increased. There was 100% mortality at 0.2nm concentration. Embryonic deformities were observed in heart and yolk sac edema, and abnormal head and eyes exhibited micro opthalmia. Tail also showed abnormalities such as disorganized tissue at the base of tail, abnormal and disorganized structure of fin fold. Single Ag nanoparticles were found to be embedded in most of the body tissues [37]. The charge on the surface of Ag nanoparticles also a potentials parameter to induce toxic impact; Ag nanoparticles having positive charge (Ag-CALNNK NPs+ζ) were found to be more biocompatible in comparison to (Ag- CALNNK NPs-ζ), negatively charged nanoparticles exhibited greater degree of toxicity in the embryo of zebra fish [38]. Silver nanoparticles having 97±13nm size caused abnormal embryonic effects in embryos of zebra fish with 2 hours of exposure duration and concentration within 0 -24pm; it was noticed that earlier stages of development when exposed to Ag nanoparticles resulted in head abnormality and also these were without eyes, thus exhibited toxic effects with respect to developmental stages [39]. van Aerle et al compared the toxic effects of silver nanoparticle (10nm), silver bulk (0.6–1.6μm) and AgNO3 (0.25μg/L) in case of embryos of zebra fish. The mortality rate was 4.6±2.5, and the gene expression showed fluctuations with Ag nanoparticles, Silver bulk and Ag ions with reference to the bioavailability; mortality was highest with Ag ions, moderate with Ag nanoparticle and least with silver bulk with exposure time 48 and 96 hours.
Gene expression may be affected by the photoperiod available in laboratory to the developing embryos. Gene expression possibly cannot be explained based on Ag ions only but this may be the function associated with particle matter [40]. When the embryos of zebra fish were exposed to nanoparticles of Zinc oxide, Titanium oxide and Cerium oxide with dose ranging from 3.5mg/l to 9.1mg/l were observed to be toxic as a result of 24 hour exposure. Physicochemical properties of these engineered metal oxides nanoparticles appeared to be the major cause of toxicity; out of these three metallic nanoparticles ZnO was observed to release 40% to 89% Zn+2 and these ions were the cause of toxicity rendering ZnO to be most toxic of the three nanoparticles [41]. Uptake of 4nm sized Ag nanoparticles was much faster in comparison to the 10nm sized Ag nanoparticles. Ag nanoparticles were found more distributed in head as compared to the trunk region but free Ag ions were not observed. The neurological development was observed to be adversely affected due to acute exposure of Ag nanoparticles. The experimental embryos showed reduced heart, hypoblastic hind brain, reduced eyes, and cardiac defects. Gene expression profile related to neural development were affected ; ATP binding cassette transporter sub family member gene and metal-sensitive metallothioneins gene were found to be drifted from their normal profile expression. It can be inferred from these observations that Ag nanoparticles may used as a potential neurotoxic agent [42]. Ag nanoparticles (20nm) and Cd1−1 of CdS (~4nm) exhibited LC50 at 0.529mg/l in embryos of zebra fish; CdS nanoparticle caused elevated embryonic malformation as compared to Ag nanoparticles. SiO2 could induce toxic impact in early developing stages probably due to the presence of chorion membrane. The fluorescent study demonstrated that SiO2 nanoparticles were located on gill lamellae; their elimination was probably via intestine because these nanoparticles were noticed along the inner lining of the intestine [43]. Bohme et al investigated the uptake and distribution of the metal composed nanoparticles and their cations using eggs, chorion and vitelline spaces and embryos of zebra fish. Metals ions were internalized in ova and chorion space in the order as Au>Ag>Zn>Cu but not the nanoparticles. ZnO nanoparticles were more in the tissues of embryo rather than in ova [44].
Amphibian Embryo
Amphibian eggs and embryos have also attracted the attention of researchers at least in the field of environmental toxicology and teratological investigation. Ag nanoparticles (2.75mg/L) changed the expression of transcription related to T3 and path ways that mediate stress response but ZnO nanoparticles could not affect this interaction; low concentration (0.6 to 550μg/L) affected signaling path way related to T3 but no induction of stress in the Rana catesbeiana tadpoles [45]. Comparative cytotoxic effects related in human kidney cell 293 (HEK293) and embryo of Xenopus as result of exposure of carboxylated nanodimonds (50μg ml -1) were studied and this exposure caused potential embryonic toxicity and teratogenicity; derogative impacts on gastrula, neural developmental stages, morphological dysfunctions and mortality were noticed [46]. Exposure of TiO2 (56nm) and Fe2O3 (44nm) could induce toxic impacts on the embryos of Xenopus laevis. When the embryos of Xenopus were exposed to ZnO, TiO2, Fe2O3 and CuO nanoparticles (20-100nm) with concentration 1000mg L -1 no mortality was observed; developing deformities in the organs like gastrointestinal, spinal cord with mild dose (3.16mg L -1) of CuO and ZnO nanoparticles were observed [47]. EC50 dose for ZnO was around 10.3mg L -1 and embryos were totally deformed. The minimum dose of 10.0mg L -1 of CuO and ZnO prevented the growth of the tad pole indicating the selective chances of specific toxicity and/or malfunctioning in embryo of Xenopes due to selected metal oxide nanoparticles [47]. Embryos of Xenopus laevis, did not exhibit mortality when exposed to TiO2 and ZnO nanoparticles (5ppm -320ppm) for 96 hours, even enzymes biomarkers like acetylcholinestrase, carboxylesterase, glutathione S- transferase, glutathione reductase, lactase dehydrogenase, and aspartate aminotransferase activities were not affected; these enzymes activities were carried out in dead as well as in living specimens during the experiments [48]. A novel Zn-doped CuO nanocomposites may not cause derogative effects on the embryo of frog and whatever the mild toxic impacts are observed were of very low intensity in comparison to those caused due to ZnO nanoparticles. The rate of uptake of these novel nanocomposites is concentration dependent; Cu ions and Zn ions from dissolved nanoparticles can do more harm than CuO and ZnO nanoparticles. Zn-doped CuO nanocomposites appears to have similar mechanism i.e., these involve oxidative response [49]. Tadpoles of Lithobates sylvaticus (frog) were exposed for 55 days to Au nanoparticles (18.1±3.5nm) having concentration (0.05pm, 0.5pm and 5pm) no derogative impact on metamorphosis was observed [50].
Reptilian Embryo
There appears to be very less investigatory work related to the impacts of nanomaterials on reptilian ova and embryos, there is a need of such investigating. Reptiles like crocodiles, alligators and some species of turtles inhabit water bodies like marshes, wet lands, rivers, lakes etc, although their embryonic development takes place outside the maternal parent but the eggs are laid near water bodies which are likely to be affected by the nanomaterials that are released either intentionally or unintentionally in and around such water bodied. These animals can act as a marker of the levels of toxicants in these ecological habitats. Thus, there appears to be a greater need to conduct such toxicity related studies to have the better understanding of the mechanism involved from teratogenic and phylogenic point of view. It is evident that most of such reptiles are under ‘protection act’ but some restricted investigations in this direction may give some insight to understand the derogative impacts of nanomaterials in these habitats and some protective measures may be devised.
Avian Embryo
Avian embryonic development is accomplished outside the maternal parent, within eggs. The morphologically the developmental stages are relatively distinct in comparison to other animals. This developmental process can be easily manipulated in laboratory conditions amicably without disturbing the incubation and developmental cycles by providing appropriate conditions of temperature, humidity etc. Thus these are easy and convenient models for toxicological investigations. Embryos of broilers and layers of 10, 13, 16 and 19 days old were used as experimental models for texting Ag and Au nanoparticles toxicity. It was noticed that rate of oxygen consumption was affected in layers embryo, Ag and Au nanoparticles could not induce a change in metabolic rate in broiler embryo but in layer embryos the metabolic rate was increased. Overall growth and embryonic development in broiler and layer embryo were not affected due to both nanoparticles [51]. Ag nanoparticles influence metabolic rate in case of layer embryos and number of microbes in digestive tract and even immunoglobulins in broiler embryos but these nanoparticles could not enhance or affect fat uptake, energy retention or performance of growth [51]. Platinum nanoparticles having 2 to 19μm diameter when administered in albumin of the eggs of Gallus domesticus in concentration ranging from 1μg/ml to 2.0μg/ml could not affect the growth of the embryo but in brain apoptotic cells were noticed, the authors are of the opinion that Pt nanoparticles may be a good option to be a carrier for the cytostatic/ cancer drugs at least to treat brain cancer [52]. Copper nanoparticles affected the metabolic rate in Lohmann hybrid chicken; there was decline in oxygen consumption, reduced lipid oxidation and loss of weight of internal organs like heart, liver, intestine etc. There was no change in the concentration of IgM and IgG (immunoglobulins) and expression of mRNA, NF-Kb and TNF-α remained unaffected indicating that copper nanoparticles used in this study could not affect immunoglobulins and gene expression [53].
Histopathological impacts of nanoparticles plays important role in understanding and evaluating the structural effects on avian embryonic tissues. Pardeshi et al reported the derogative implications of hepatic tissue of chick embryo; ZnO nanoparticles (40-60 nm in size) with single dose of 50μg/g and 150μg/g egg weights were administered in chick embryos on the 5th day of embryonic cycle and observations were made after 48 h and 96 h. After 48 h the 50μg/g dose did not alter the compactness of hepatic tissue but there were more intercellular spaces and the hepatic chords were distorted, the blood sinuses were slightly deviated from those found in controls. After 48 h the dose 150μg/g blood cells were distributed in hepatic sinuses and blood capillaries completely distorted., fat storage cells were more in numbers. Hepatic cells were less organized but there was loss of cytoplasm and vacuole formation and with turgid nuclei. After 96 h of exposure the hepatic lobes showed distorted hepatic chords, and disturbed arrangement of hepatocytes with more intercellular space. The cells of endothelial lining of blood sinuses and capillaries showed signs of disappearance allowing blood cell to dispersed in nearby space, more numbers of fat storage cells. There was total loss of orientation of hepatic tissues, cytoplasm of hepatocytes, diminished, indistinct or scattered nuclei [20]. Metals and metal oxide nanomaterials can influence proteomes quantitatively [54]. ZnO nanoparticles (5μg/ml) got internalized in the cells of hen’s ovarian granulosa and inhibited the growth of the cells. The gene expression was also affected and so are the proteins due to ZnO nanoparticles. This path way of influencing gene expression appears to be different than that followed by Cu+2. It may be assumed that ZnO nanoparticles have the potential to influence the female reproductive physiology [54]. Copper plays an effective role in growth and development of blood vessels and muscles but Cu nanoparticles exhibit similar ability with different mode that followed by Cu ion. When chick embryo (chorioallantoic membrane) at 3,6,9,12,18, and 20 days of incubation were treated with Cu nanoparticles, the embryonic development was not affected and weight of the body and organs like heart, liver and spleen were not changed. The Cu nanoparticles appeared to be proangiogenic in nature; this is authenticated by the observations on m-RNA concentration and mRNA gene expression favoring angiogenesis and proliferative process [55]. There seems to be some lacunae in the information about the toxicity of Au, Ag and goldsilver alloy; gold nanoparticles are relatively more biocompatible but are toxic to spermatozoa depending on the dose/concentration. This toxic contact can be prevented or interrupted by the protein corona but if the size of Au nanoparticles is less than 2 nm they impart toxic effects. Ag nanoparticles are relatively more toxic than Au nanoparticles. Although there is very faint distinction between threshold toxic values, mostly toxicity of Ag nanoparticle is related to the Ag ions released in medium. These nanoparticles get internalized oocyte and the cumulus cell surrounding oocyte. Au nanoparticles cause lower degree of embryotoxic impact than Ag nanoparticles. Oocyte maturation is affected by Ag nanoparticles of size 6nm 6-20nm by 46 hours exposure [56].
Mammalian Embryo
Female mammals are quite different than their counterpart in lower vertebrates. The female individuals are provided with varied form of placenta in different groups of mammals. The placenta has been attractive target for research since the early industrial revolution to understand the effects of various chemicals or toxins on the fetus whenever maternal parent is exposed to them either intentionally or unintentionally [9]. In human female placenta is a round flat organ and connects fetus and the maternal uterine wall abridging maternal parent and fetus. Structurally it is composed of two major components namely ‘fetal placenta – chorion frondosum’ and this develops from blastocyst (fetal origin) the second part is called ‘maternal placenta – ‘deciduous basalis or placenta uterine’, it develops from maternal uterine wall [57]. Placenta – materno-fetal tissue is only means of exchange of materials between mother and fetus; this tissue is made of maternal endothelium, mesenchyme and epithelium trophoblast, mesenchyme and fetal vascular endothelium [58]. The susceptibility of the administrated nanoparticles is likely to change because of the developmental changes due to gestational process involving structural and physiological fluctuations. These changes affect the degree of placental permeability in relation to gestational development [59]. Permeability and viability of placental tissue is very selective and specific in nature and this is physiologically feasible in maintaining molecular homeostasis between maternal and fetal body fluids. Although placental tissue is physiologically active but to greater extent hormones like laptin and human chorionic gonadotropin play a major role in the physiological maintenance of placenta. The metabolic activity of the placenta can be investigated by finding the correlation between the total changes in the total contents in the maternal and fetus in a given time. This can be carried out by perfusion technique and calculating consumption of glucose or production of lactate [60].
The assessment of potential danger to the developing fetus from the inhaled nanoparticles or intravenous administration of very fine and small amount of nanomaterials circulating (systemic availability) in the maternal parent is of significance because these nanoparticles move across the one of the most efficient biological barrier. Commonly very less percentage of nanoparticles of the systemic circulation enters placenta and the great challenge is to measure this percentage of nanomaterials in placenta [58]. Krystek et al have proposed a technique to detect vary minute amount and this technique is called ‘Inductive Coupled Plasma Mass Spectroscopy (ICP-MS)’, its reported efficiency is around 50ng/g of tissue [61]. Very small changes in kinetic profile can be investigated involving different particles; there is a possibility that may not be related to the difference between primary particles size and/or hydrophobicity, the inhaled particles or administered particles [62]. Metallothioneins play regulatory role during the physiological and detoxification processes at materno-fetal-interface and during fetal organogenesis; they also protect the cells of trophoblast against the disturbances caused in redox homeostasis due to pregnancy and retention of ‘neuro-immuno-endocrine homeostasis, movements and storage of essential metals that are required for the fetal organogenesis and protect it against microbial infection [63].
Although placenta is considered to be one of the effective biological barriers for many biomolecules but intravenous administration of silica (70nm) and TiO2 (35nm) moved across this biological fetomaternal barrier and reach fetal liver and brain and placenta itself; this indicates that placenta is the via route for the nanosized particles and resulted in size reduction of fetus and uteri [64]. When TiO2 (25-70nm) with surface area 20-25m2/g were administered subcutaneously in pregnant mice brought changes in genital organs and detrimental impact noticed included declined sperm production, activation of enzyme system (caspase-3) that promotes apoptosis in the cells of olfactory lobes [65]. To investigate the impact of metal nanoparticles at cellular level ‘Mouse embryo stem cells’ and ‘embryoid bodies’ were exposed to Ag nanoparticles (10nm; 5μg/ml, 50μg/ml) for 24, 48 and 72h, the overall impact included increased cell death, ROS, loss of mitochondrial membrane potential, elevated alkaline phosphatase activity, apoptosis that followed by necrosis depending on the dose used. There was greater degree of change in the gene expression, growth factor both these parameters inhibited cellular functions like cell cycle phase and the degree of self renewal ability [66].
Small nanoparticles have better ability to move across placenta in comparison to large nanoparticles; SiO2 nanoparticles with size range 70, 300 and 3000nm were administered intravenously in pregnant mice with 0.8 mg/mice. All these nanoparticles were found in liver of pregnant mice but only 70nm sized SiO2 nanoparticles were found in the placental trophoblast, fetal liver and brain. In case of gold nanoparticles with size 1.4nm and 18nm administered in pregnant rats only 1.4nm sized Au nanoparticles were noticed in fetal indicating that this sized nanoparticles can move across the placenta [57]. Sprague-Dawley rats were exposed for the duration of 5-19 gestational days to ZnO nanoparticles having 20nm size and positive charged (0mg/kg/day, 100mg/kg/day, 200mg/kg/day and 400mg/kg/ day), the numbers of corpora leutea and implantation did not change much with the increase of dose; body weight of the fetuses showed irregular fluctuations but numbers of fetal deaths increased with the rise of concentrations. Further numbers of fetuses with deformed visceral morphology like disorganized and disoriented thymus, ureter and kidneys were noticed. Fetal skeleton malformation like incomplete ossification of skull, dumbbell ossification or incomplete ossification of thoracic centrums and variations in size and number of ribs were observed. There was minimum effects on intrauterine fetal growth and there were no observable adverse toxic effects on maternal and fetal growth with 00mg/kg/day ZnO (20nm) with positive charge [67]. Phospholipids micelle enclosing CdSe/CdS/ZnS quantum dots were administered intravenously in mice (dose 0.81mgCd/kg for two weeks prior their mating). It was seen that the quantum dots were distributed in most of the major organs of the experimental mice but none of the complications related to pregnancy were noticed and also no change in the pregnancy out-come were observed. There appears to be an indication that nanosized metal in modified form as quantum dots enclosed in micelle does not inflict detrimental impact of embryo in case of mice. There is dire need to confirm this type of behavior of other metal and metal oxide nanoparticles on embryo before exploiting them for any biomedical and molecular applications.
Conclusion
This review is an effort is made to elucidate the derogative role of metal and metal oxide nanoparticles during embryonic development and their consequences. Pre and post embryonic stages exhibit higher degree of metabolic activities, potentially lower energy reserves probably due to higher growth rate and distribution limitations. Generally toxic/detrimental effects are mostly dose and time dependant. Whenever nanomaterials either interact externally and/or internalized there is a ‘conception period’; during this period molecular and cellular restructuration in biosystem take place and these changes are difficult to be noticed. The derogative impacts depend on the nature of the nanomaterials and the species. These nanomaterials involve modes of inter actions like release of metal ions, production of ROS, change in the potential of the cell membrane or membrane of cell organelles, form an additive or ligand complexes. The metabolic aspects of the detrimental effects may involve interference with enzyme action, gene expression, phases of cell cycle and cell functioning that may induce dysfunctioning of repair of cell, inflammation/necrosis, apoptosis, DNA damage, cell signaling process, etc. The data on toxicological impact presented from time to time will be help full in evaluation of the environmental status in relation to toxicological aspects and assist in remedial steps. The metal and metal oxide based formulations can be designed to attain a specific oriented target. Metal and metal oxide nanomaterials alone or in combination with other nanomaterials quantum dots, dendrimers and other nano forms depending on their ability to move across biological membrane can be utilized in treatment of a specific tissue. Metal nanoparticle like Pt nanoparticles may be considered as an option to be used as a carrier for the cytostatic/cancer drugs at least to treat brain cancer. One of the most futuristic approach can be that application of nanotoxicity in the destruction of cancerous cells, this will involve the precise target oriented designing of those selected nanomaterials that can be exploited in dealing with clinical and some of the physiopathological conditions.
References
- Mondal S. Physiological Genomics of Placental Growth and Development. In: Reproductive Genomics in Domestic Animals. Zhihua Jiang, Troy L. Ott (Eds). Blackwell Publication, USA. 2010; 251-268.
- Rashidi H, Sottile V. The chick embryo: hatching a model for contemporary biomedical research. Bioessays. 2009; 31: 459-465.
- Salvaterra T, Alves MG, Domingues I, Pereira P, Rasteiro MG, Carvalho RA, et al. Metabolic effects of a short-term exposure to nanoparticles of titanium silicate in tadpoles of Pelophylax perezi (Seoane), Aquat Toxicol. 2013; 128-129: 190-192.
- Umanzor-Alvarez J, Wade EC, Gifford A, Nontapot K, Cruz-Reese A, Gotoh T. Near-infrared laser delivery of nanoparticles to developing embryos: a study of efficacy and viability. Biotechnol J. 2011; 6: 519-524.
- Becker WM, Kleinsmith LJ, Hardin J. The world of cell, 5th Ed, Pearson Education Inc, Singapore, ISBN. 2004; 81: 297-0415.
- Wozniak MA, Chen CS. Mechanotransduction in development: a growing role of Contractility, Molecular Cell Biology, Nature Rev. 2009; 10:34-44.
- Lister KN, Lamare MD, Burritt DJ. Sea ice protects the embryos of Antarctic sea Urchin Strechinus neumayeri, from oxidation damage due to naturally enhanced level of UB-B radiation. The J of Exp Biol. 2010; 213: 1967-1975.
- Wu T, Manogaran AL, Beauchamp JM, Waring GL. Drosophila vitelline membrane Assembly: a critical role for evolutionary conserved cystine in the VM domain of SV23. Dev Biol. 2010; 347: 360-368.
- Lahir YK. Principles and applications of toxicology. See Kay Publications, Banglore, India. ISBN: 978-81-924169. 2013; 5-3.
- Cela P, Vesela S, Matalova E, Buchtova M. Embryonic toxicity of nanoparticles, Cell Tissues Organs. 2014; 199: 1-23.
- Rogers NJ, Franklin NM, Apte SC, Batley. The importance of physical and chemical Characterization in nanoparticles toxicity studies. Integr Environ Assess Manag. 2007; 3: 303-304.
- Lahir YK. Some aspects of interactions between nanomaterials and the cytoskeleton of eukaryotic cells. Adv Clin Toxicol. 2016; 1: 000111.
- Baer D, Munusamy P, Karakoti A, Thevuthasan S, Kuchibatta S, Seal S. Ceria nanoparticles: Environmental impact on particle structure and chemistry. In Bulletin of the American Physical Society, APS, March Meeting, Boston, MA, USA. 2012; 57.
- Teske SS, Detweir CS. The biomechanism of metals and metal oxide nanoparticles interaction with cells. Int J Environ Res Public Health. 2015; 12: 1112- 1134.
- Auffan M, Rose J, Wiesner MR, Bottero JY. Chemical stability of metallic nanoparticles, A Parameter controlling their potential cellular toxicity in vitro. Environ Pollu. 2008; 157: 1127-1133.
- Hotze EM, Phanrat celebrate T, Lowry GV. Nanoparticles aggregation: Challenges to understanding transport and reactivity in the environment. J Environ Qual. 2010; 39: 1909-1924.
- Zhang L, Gu FX, Chan JM, Wang AZ, Langer RS, Farokhzad OC. Nanoparticles in medicine: Therapeutic applications of development. Clin Pharmacol Therapeutic. 2008; 83: 761- 769.
- Shirsekar PP, Kanhe N, Mathe VL, Lahir YK, Dongre PM. Interaction of zinc oxide with Human red blood cells. Bionano Frontier. 2016; 9: 99-104.
- Cho EC, Xie JW, Wurm PA, Xia YN. The effects of size, shape and surface functional group of gold nanostructures on their adsorption and internalization by cell. Nano letters. 2009; 9: 1080.
- Pardeshi P, Nawale AB, Mathe VL, Lahir YK, Dongre PM. Effects of zinc, Nanoparticles on the hepatic tissue of chicken embryo: A histopathological approach. Bionano Frontier. 2015; 7: 176-180.
- Williams DF. There is no such a thing as a biocompatible material, Biomaterials. 2014; 35: 10009-10014.
- Keel D, Hocker H. Polymers for biomedical applications: improvement of the interface compatibility, Adv Polymer Sci. 1999; 149: 1-57.
- Lee KJ, Nallathamby PD, Browning LM, Desai T, Cherukuri, PK, Xu XH. Single nanoparticle spectroscopy for real-time in vivo quantitative analysis of transport and toxicity of single nanoparticles in single embryos. Analyst. 2012; 137: 2973-2986.
- Wu Y, Zhou Q, Li H, Liu W, Wang T, Jiang G. Effects of silver nanoparticles on the development and histopathology biomarkers of Japanese medaka (Oryzias latipes) using the partial-life test. Aquat Toxicol. 2010; 100: 160-167.
- Laban G, Nies LF, Turco RF, Bickham JW, Sepulveda MS. The effects of silver nanoparticles on Fat head minnow (Pimephales promelas) embryos. Ecotoxicology. 2010; 19: 185-195.
- Zhu X, Zhu L, Duan Z, Qi R, Li Y, Lang Y. Comparative toxicity of several metal oxide nanoparticles aqueous suspensions to zebra fish, Danio rerio, early developmental stage. J Environ Sci Health. 2008; 43: 278-284.
- Browning LM, Lee KJ, Huang T, Browning LM, Lee KJ, Lowman JE, et al. Random walk of single Gold nanoparticles in zebra fish embryos leading to stochastic toxic effects on embryonic developments, Nanoscale. 2009; 1: 138-152.
- Powers CM, Slotkin TA, Seidler FJ, Badireddy AR, Padilla S. Silver nanoparticles alter zebra fish development and larval behavior: distinct roles for particle size, coating and composition. Neurotoxicol Teratol. 2011; 33: 708-714.
- Li PW, Kuo TH, Chang JH, Yeh JM, Chan WH. Induction of cytotoxicity and apoptosis in mouse blastocysts by silver nanoparticles. Toxicol Lett. 2010; 197: 82-87.
- Bai W, Tian W, Zhang Z, He X, Ma Y, Liu N. Effects of copper nanoparticles on the development of zebra fish embryos. J Nano Sci Nanotechnol. 2010; 10: 8670-8676.
- Bai W, Zhang Z, Tian W, He X, Ma Y, Zhao Y. Toxicity of zinc oxide nanoparticles to zebra fish embryo: a physicochemical study of toxicity mechanism. J Nanopart Res. 2010; 12: 1645-1654.
- Zhou Z, Son J, Harper B, Zhou Z, Harper S. Influence of surface chemical properties on toxicity of engineered ZnO nanoparticles. Beilstein J Nanotechnol. 2015; 6: 1568-1579.
- Teusan A, Baranov N, Dmour R, Vizitiu A, Jelihovchi I. The effects of metal nanoparticles on embryos of different animal species. Int J Med Dent. 2015; 5:179-182.
- Bar-Ilan O, Albrecht RM, Fako VE, Furgeson DY. Toxicity assessments of multisized gold and silver nanoparticles in zebrafish embryos. Small. 2009; 5: 1897-1910.
- Chakraborty C, Sharma AR, Sharma G, Lee SS. Zebra fish: A complete animals model to Enumerate the nanoparticle toxicity. J Nanobiotechnology. 2016; 14: 65.
- Asharani PV, Lian WU, Gong Z, Valiyaveettii S. Toxicity of silver nanoparticles in zebra fish. Nanotechnology. 2008; 19: 255102.
- Lee KJ, Nallathamby PD, Browning LM, Desai T, Cherukuri PK, Xu XH. In vivo quantitative Study of size-dependent transport and toxicity of single Ag nanoparticle using zebra fish embryos. Chem Res Toxicol. 2012; 25: 1029-1046.
- Lee KJ, Browning LM, Nallathamby PD, Xu XN. Study of charge-dependent transport and toxicity of peptide-functionalized silver nanoparticles using zebra fish embryos and single nanoparticle plasmonic spectroscopy. Chem Res Toxicol. 2013; 26: 904-917.
- Browning LM, Lee KJ, Browning LM, Lee KJ, Xu XN. Silver nanoparticles incite size and dose dependent developmental phenotypes and nanotoxicity in zebra fish embryo. Chem Res Toxicol. 2013; 26:10.
- van Aerle R, Lange A, Moorhouse A, Paszkiewicz K, Ball K, Johnston BD, et al. Molecular mechanism of toxicity of Ag nanoparticles in zebra fish embryo. Environ Sci Technol. 2013; 47: 8005-8014.
- Wehmas LC, Andrers C, Chess T, Punnoose A, Pereira CB, Greenwood JA, et al. Comparative metal oxide nanoparticles toxicity using embryos of zebra fish. Toxicol Rep. 2015; 2: 702-715.
- Xin 1, Rotchell JM, Cheng JY, Zhang Q. Silver nanoparticles affects neural development of Embryos of Zebra fish. J Appli Toxicol. 2015; 35: 1481-1492.
- Lacave JM, Retuerto A, Unai VP, Gilliland D, Oron M, Cajaraville MP, et al. Effects of Metal bearing nanoparticles (Ag, Au, CdS, ZnO, SiO2) on developing zebra fish embryo. Nanotechnol. 2016; 27: 325102.
- Bohme S, Baccaro M, Schmidt M, Potthoff A, Stark HJ, Reemtsma T, et al. Metal uptake and distribution in zebra fish embryo Danio rerio difference between Nanoparticles and metalions. Environmental Sci Nano. 2017.
- Hinther A, Vawda S, Skiroow RC, Veldhoen N, Collins P, Cullen JT, et al. Nanometals induce stress and alter thyroid hormone action in amphibian at or below North America water quality guidelines. Environ Sci Technol. 2010; 44: 8314-8321.
- Marcon L, Riquet F, Vicogne D, Szunents S, Bodast JF, Boukherroub R. Cellular and in vivo toxicity of functionalized nanodimonds in Xenopus embryo. J Material Chem. 2010; 20; 8064-8069.
- Nations S, Wages M, Canas JE, Maul J, Theodorakis C, Cobb GP. Acute effects of Fe3O4, TiO2, ZnO and CuO on Xenopus Laevis, Chemosphere. 2011; 83: 1053-1061.
- Birhanli A, Fatma BE, Sayilkan F, Gungorodo A. Effects of TiO2 nanoparticles on the development of Xenopus embryo. Turkish J of Biology. 2014; 38; 283-288.
- Mantacca P, Moschini E, Bonfanti P, Facio U, Perelshtein I, Lipovsky A, et al. Toxicity evaluation of New Zn-doped CuO nanocomposites with high effective antibacterial properties. Toxicol Sci. 2015; 146: 16-30.
- Fong PP, Lucas B, Thompson B, Gerardo LF, Car F, Sitton AJ. Long term exposure to Ag Nanoparticles accelerate larval metamorphosis without affecting mass in wood frog Lithobates sylvaticus at environmentally relevant concentrations, Environmental Toxicol. 2016; 35: 2304-2310.
- Pineda L, Sawisz E, Hotowy A, Elnif J, Sawosz F, Ali A, Chwalibog A. Effects of nanoparticles of gold and silver on metabolic rates and development of broiler and layer embryos. Comparative Biochemistry and physiology part-a: molecular and integrative physiology. 2012; 161: 315-319.
- Prasek M, Sawasz E, Jaworski S, Gradzik M, Ostaszewaska T, Kamszewaski M, et al. Influence of nanoparticles of platinum on chick embryo development and brain morphology. Nanoscale Res Lett. 2013; 8; 251.
- Pineda L, Sawosz E, Vadalasetty KP, Chwalibog A. Effects of copper nanoparticles on metabolic rate and development of chick embryo. Anim Feed Sci Technol. 2013; 186: 125-129.
- Zhao Y, Li L, Zhang PF, Shen W, Liu J, Yang FF, et al. Differential regulation of gene and protein expression by ZnO nanoparticles in hen’s ovarian granulosa cell: specific role of nanoparticles. PLoS-ONE. 2015; 10: 0140499.
- Sosnowska NM, Sawosz E, Vadalasetty KP, Lukasievicz M, Niemiec J, Wierzbicki M, et al. Nanoparticles of copper stimulate angiogenesis at systemic and molecular levels. Int J Mol Sci. 2015; 16: 4838-4849.
- Taylor U, Tiedemann D, Rehboek C, Kuess WA, Barcikowski S, Rath D. Influence of gold, silver and Au-Ag alloy nanoparticles on germ cell functions and embryo development. Beilstein J Nanotechnol. 2015; 6: 651-664.
- Sun J, Zhang Q, Wang Z, Yan B. Effects of nanotoxicity on female reproductivity and fetal development in animals models. Int J Mol Sci. 2013; 14: 9314-9337.
- Hougaard S, Champagnolo L, Pascale CP, Tarade A, Delphine RR, Valentino S, et al. A perspective on the development toxicity of inhaled nanoparticles, Reproductive Toxicol. 2016; 56: 118-140.
- Rubinchik-Stem M, Eyal S. Drug interactions at human placenta, What is the evidence? Front Pharmacol. 2012; 3: 126.
- Wick P, Malek A, Manser P, Meili D, Macder-Althaus X, Diener L, et al. Barrier capacity of human placenta for nanosized materials. Environ Health Persp. 2010; 118: 432-436.
- Krystek P, Tentschert J, Nia Y, Trouiller B, Noel L, Goetz M E, et al. Method development and inter laboratory comparison about the determination of titanium from TiO2 in tissues by the inductivity coupled plasma mass spectroscopy. Anal Bioanal Chem. 2014; 406: 3853-3861.
- Geraets L, Oomen AG, Krystek P, Jacobson NR, Wallian H, Laurentic M, et al. Tissue distribution and elimination after oral and intravenous administration of different TiO2 nanoparticles in rats, Particle and Fiber Toxicol. 2014; 11.30.
- Jakovac H, Grebic D, Mrakovcic-suitic I, Rukavina D, Biserka RS. Expression of metallothionines in placenta and fetal tissue in undisturbed and PGM-Zn treated synergic pregnancy. Am J BioSci. special issue-Oxidants-antioxidants. The biological balance. 2015; 3: 1-7.
- Yashimata K, Yoshioka Y, Higashisaka K, Mimura K, Morishita Y, Nozaki M, et al. Silica and TiO2 nanoparticles cause pregnancy complications in mice, Nature Nanotechnol. 2011; 6: 321-328.
- Tekedo K, Suzuki K, Ishihara A, Miyoko K, Fujimoto R, Tabata M, et al. Nanoparticles transferred from pregnant mice to their offspring can damage the genital and cranial nerve system. J Health Sci. 2009; 55: 95-102.
- Krishnamurthy PR. Toxicity of Ag nanoparticles in mouse embryonic stem cells and chemical based reprogramming of somatic cells to sphere cells in case of mouse. Ph D dissertation. University of Dayton, Ohio. UMI NO-36721550. 2011.
- Hong JH, ParK HC, MK, Kim MS, Shin HC, Lim JH, et al. Effects of ZnO nanoparticles on dams and embryo fetal development in rat. Int J Nanomedicine. 2014; 9: 145-157.