
Mini Review
Austin Diabetes Res. 2019; 4(1): 1021.
Role of Amylin in Glucose Homeostasis
Chwalba A1, Dudek A1* and Otto-Buczkowska E2
¹Medical University of Silesia in Katowice, Poland
²Medical Specialist Centre in Gliwice, Poland
*Corresponding author: Aleksandra Dudek, Medical University of Silesia in Katowice, Poland
Received: March 14, 2019; Accepted: April 16, 2019; Published: April 23, 2019
Abstract
The neuroendocrine hormone amylin is co-secreted with insulin by pancreatic islet β cells.
Its secretion is stimulated by food components such as glucose and arginine. The secretion of insulin and amylin is regulated not only by the concentration of glucose in the blood but also increased by the incretin effect. Amylin affects glucagon secretion, inhibits its secretion stimulated by amino acids and reduces endogenous glucose production during the postprandial period. Amylin acts as modulator of glycogen synthesis, glucose consumption and has an influence on insulin resistance induction in skeletal muscle and probably also in the liver. In diabetic patients, ß-cell destruction results not only in the insulin deficiency, but also in C-peptide and amylin reduced secretion.Amylin is clearly involved in glucose homeostasis through the inhibition of gastric emptying and postprandial hepatic glucose production, eventually reducing postprandial glucose fluctuations. Synthetic pramlintide - amylin analogue - has been shown to improve glycemic control in patients with diabetes.
Amylin replacement with pramlintide may be a supplement to insulin therapy in patients with diabetes mellitus.
Keywords: Pramlintide - synthetic amylin analog; Incretin effect; Amyloid; Diabetes
Introduction
In a healthy human, a number of mechanisms maintains glucose homeostasis. For many years, it was thought that normoglycemia is provided by the action of two opposing pancreatic hormones: insulin and glycogen. In later times, the role of the incretin hormone was noted. Insulin and glycogen mechanisms of action are reverse. They affect insulin and glucagon as well as digestive enzymes secretion and regulate digestive tract motility. They act as an important link between the process of food nutrients digestion and absorption and their further metabolic transformation. In addition, kidneys play an important role in maintaining glucose homeostasis. SGLT1 and SGLT2, belonging to SLC5 gene family, are the main part of the intestinal and renal glucose absorption and reabsorption. Neuroendocrine hormone, amylin belongs to the hormones involved in the regulation of glucose homeostasis.
Amylin (islet amyloid polypeptide IAPP)
Amylin, together with insulin and C peptide, is produced by pancreatic β cells. It is also synthesized in the stomach and in the posterior spinal cord ganglia, but to a smaller extent [1]. Pancreatic β cells hormones are secreted as a glucose stimulation response. Nearly a hundred years ago the presence of a substance deemed “amyloid” was found in the pancreas. In 1987 Garth Cooper published the study devoted to the peptide extracted from the amyloid and named it as ‘amylin’ [2]. Amylin is a 37 amino acid peptide hormone obtained by proteolysis of a 89 amino acid precursor molecule – proamylin; proamylin gene is located on chromosome 12 [3]. The American authors have recently reviewed of the literature on the results of long-term amylin studies in rats and humans [4]. They confirmed that the insulin and amylin secretion is regulated not only by the blood glucose concentration, but also by the incretin hormones. In the periprandial period, apart from the pancreatic hormones (such as insulin, glucagon and amylin), intestinal hormones - glucagon-likepeptide 1 (GLP-1) and Gastric Inhibitory Polypeptide (GIP) - plays an additional role in enhancing insulin and suppressing glucagon secretion after a meal. GIP and GLP-1 belong to the glucagon peptide superfamily and thus share amino acid sequence homology. They are secreted by specialized cells in the gastrointestinal tract and have receptors located on pancreatic islet cells as well as in other tissues.
These cells are concentrated primarily in the duodenum and proximal jejunum, although they also can be found throughout the intestine.
The (Figure 1) shows the effect of a meal on the secretion of insulin, amylin and GLP-1
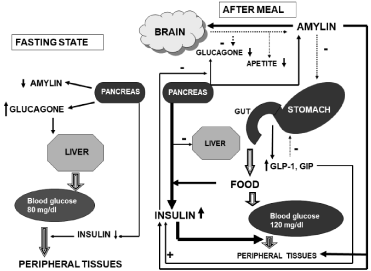
Figure 1: The role of amylin and incretin hormones (GLP-1 and GIP) in maintaining glucose homeostasis.
In the postprandial period, the presence of food in the stomach and intestine leads to an increase in the secretion of insulin, amylin and GLP-1. Insulin causes an
increase in glucose consumption in peripheral tissues, suppression of glucagon secretion and hepatic glucose production. Amylin relieves gastric emptying and
is likely to suppress post-prandial glucagon secretion by means of central action. Animal models have also been found to reduce food intake. GLP-1 slows down
gastric emptying. GIP enhances glucose-dependent secretion of insulin and affects fat metabolism.
In the periprandial period, food is the main source of blood glucose. Physiologically, during the meal, the presence of food in the gastrointestinal tract leads to insulin secretion increase as well as amylin and incretin hormones; subsequently amylin inhibits glucagon secretion and reduces endogenous glucose production in the postprandial period [5]. Insulin and amylin secretion depends on the concentration of serum glucose and is suppressed when the serum glucose decreases. Amylin is a modulator of glycogen synthesis and glucose consumption in skeletal muscle and in the liver; can act as a signal to induce insulin resistance together with insulin. Amylin suppresses hunger and – in this way- can affect glucose homeostasis. In diabetic patients, decreased levels of insulin, amylin and GLP-1, which affects the most insulin and glucagon, secretion, may lead to postprandial hyperglycemia. Deficit of amylin and GLP-1 accelerates gastric emptying and increases blood glucose in parallel with a secondary increase of postprandial glycemia. Increased glucagon secretion leads to intensified hepatic glucose production.
The Swiss authors presented a comprehensive discussion in the physiological effects of amylin dependent on receptor subtypes [6]. According to these authors, diversified amylin influence on glucose homeostasis promotes this hormone as a supportive medicine in the treatment of diabetes and obesity. Amylin role is not only limited to glucose homeostasis regulation. Recently, more attention has been paid to the formation of amyloid deposits in the pancreas. This process may play a role in the pathogenesis of diabetes and its complications.
A comprehensive discussion in the role of amyloid aggregation in the pathogenesis of many diseases has been presented by Chiti et Dobson [7]. Similar observations in the role of Islet Amyloid Polypeptide (IAPP) aggregation in the pathogenesis of diabetes and other diseases were made by other authors [8,9]. It is believed that the formation of fibrillar deposits of misfolded and aggregated peptide is highly toxic to β cells and leads to cell dysfunction, cell loss, pancreas destruction and progress of the disease- a lot of evidence indicates that cytotoxicity of islet amyloid polypeptide aggregates is a major contributor to the loss of β-cell mass in type 2 diabetes. Work is being conducted on the inhibition and disaggregation of amylin aggregates what can be a therapeutic strategy [10,11]. Attempts are made to apply anti-amyloid compounds as therapeutic strategies Islet amyloid polypeptide may induce pancreatic β-cell death. Similar work on the toxic effects of human islet amyloid polypeptide (IAPP) on pancreatic islets was also carried out by other authors [12,13]. The causal factors for amyloid formation are largely unknown and requires further research [14]. A comprehensive discussion of aggregation processes leading to apoptosis and β-cells damage have been presented by Fernández [15]. The aggregation of islet amyloid polypeptide may induce pancreatic β-cell destruction, which is linked to progress of the disease.
The Polish authors have presented a comprehensive discussion of the mechanisms of creating deposits of amylin in the pancreas, but the mechanism and factors favoring the conversion of the native form of amylin to insoluble fibrils remain unexplained [16-18]. The cytotoxic activity of amyloid was also discussed by other authors. A lot of evidence indicates that cytotoxicity of human amylin oligomers to pancreatic islet β-cells impair their function, and reduce mass through disruption of cell membranes and can lead to diabetes. Attempts are made to protect the function of β-cells by reducing body weight or the use of hypoglycemic agents, however, these actions do not allow to stop the progression of cell dysfunction [19,20]. Applying of antiamyloid compounds as therapeutic strategies can be also considered.
The use and role of amylin in the treatment of diabetes
Amylin regulates the secretion of insulin and glucagon. Deficiencies of this anorectic hormone may increase the risk of severe reactive hypoglycemia [21]. Primary pathways for diabetes treatment are stimulation of insulin secretion or insulin substitution, reduction of insulin resistance in peripheral tissues, and modulation of glucose absorption from the gastrointestinal tract. However, in some cases, these methods do not allow for achieving satisfactory metabolic control. In type 1 diabetic patients, the amount of both insulin and amylin is extremely low. The situation is different with type 2 diabetes, because that amount depends on the period of the disease and the degree of destruction of the β cells. In the early stages of type 2 diabetes, the insulin resistance of peripheral tissues is compensated by hyperinsulinemia. As the disease progresses, a progressive dysfunction of β cells, insulin associated reduction and amylin secretion can be observed. Postprandial secretion of amylin is dependent on the phase of secretion. From the onset of the type 2 diabetes, there is an impaired rhythm of insulin secretion in response to a stimulus. During the first phase, there is a marked decrease in the peak of secretion, after which there occurs an increased and prolonged secretion in response to the pronounced insulin resistance of peripheral tissues. These disturbances in insulin secretion rhythms usually coexist with similar changes in amylin secretion, same as in type 1 diabetes with an autoimmune basis [22]. Intensive insulin therapy and maintenance of the normoglycemia in type 1 diabetes is often associated with an increased risk of hypoglycemia and weight gain. Introducing amylin as an adjuvant therapy helps to avoid these complications. The combination of insulin and pramlintide allows for a more physiological diabetes treatment. Some authors believe that such linked treatment improves glycemic control, but increases the risk of hypoglycemia, so therapy must be carefully monitored. Amylin modulates metabolic processes in different tissues, so deficiency can affect the progress of the disease in the autoimmune diabetes. Hence attempts are being made to use amylin analogs in the treatment of type 1 diabetes [23]. The use amylin as an adjuvant therapy for insulin therapy in type 1 diabetes can be also considered [24-26]. After adding pramlintide to insulin therapy, a 30% reduction in insulin dosage compared with what was usual for each patient was obtained to limit the risk of hypoglycemia [27]. Ramkissoon et al. have presented suggestions for a pramlintide and insulin dose optimization model for glycemic control [28]. The authors presented the benefits of using the model of glucose-insulin-pramlintide as a combined therapy for improve glycemic control in type 1 diabetes using an artificial pancreas. Beneficial results of the application subcutaneous pramlintide infusion in patients with type 1 diabetes showed Huffman et al. [29]. Other authors also presented good effects of such therapy in adolescent patients with type 1 diabetes [30-32].
It was also found that in type 1 diabetes juvenile patients, pramlintide slowed gastric emptying and reduced the level of postprandial glucagon and glucose.
Multicenter randomized trials in type 1 diabetes were presented by Herrmann et al., who demonstrated the usefulness of adding pramlintide to the continuous subcutaneous insulin infusion (CSII) therapy [33].
The authors found that the addition of pramlintide to the CSII treatment allowed for better glycemic control in type 1 diabetes patients. Other groups of authors also confirmed beneficial effects of using pramlintide added to insulin therapy in type 1 diabetes. One problem could be a potential for increased incidence of hypoglycemia and nausea [34].
Based on 10 randomized placebo-controlled studies, Qiao et al. confirmed beneficial effects of pramlintide supplement - as an adjuvant therapy - on glycemia and body weight control in patients with type 1 diabetes [35]. The beneficial effects of pramlintide as Weinzimer et al. [36] presented an adjuvant in treatment using closed-loop (CL) insulin therapy in young type 1 diabetics.
The beneficial effect of the therapy with pramlintide as insulinboosters in patients with type 1 diabetes was also observed by other authors [37]. Similar observations were made by Sherr et al. who used such treatment in a group of young patients obtaining compensation for postprandial hyperglycemia [38]. The authors discussed the benefits of pramlintide as an insulin-boosting treatment, comparing the results with those obtained after the use of liraglutide.
The results of studies on the effect of pramlintide on postprandial glucose metabolism in a group of 12 patients with type 1 diabetes were also presented by Hinshaw et al. who found that delayed gastric emptying leads to a reduction in postprandial glycemia [39]. The authors stated that pramlintide analogue modulates postprandial glucose homeostasis via its effects on gastric emptying and glucagon excursions in patients with type 1 diabetes.
Not without significance in attempting therapeutic use of the amylin analogue in diabetic patients is its effect on the lipidogram expressed as an improvement in the LDL / HDL cholesterol index - this suggests the contribution of amylin in reducing the risk of coronary heart disease in diabetic patients [40]. Amylin analogs are also used in the treatment of type 2 diabetes and in obesity [41]. The effectiveness of using pramlintide in lowering postprandial blood glucose in type 2 diabetes was also indicated by other authors [42].
The concentration of amylin in the blood of diabetes 1 patients is low, while in patients with type 2 diabetes, especially associated with obesity, is significantly increased. This refers to the period of disease, when the function of β cells is preserved, and the amylin levels can be many times higher than those observed in healthy people. In type 2 diabetes, the functions of β cells gradually deteriorate as the disease progresses involving insulin therapy.
Amylin supplementation in these patients could allow controlling postprandial hyperglycemia. Amylin participates in the suppression of glucagon secretion and inhibits intestinal glucose absorption. Furthermore, reduces appetite, resulting in promoting weight loss in many patients and, in any case, inhibiting weight gain, which can be caused by insulin therapy.
Obese people usually have hyperamylasemia, hyperglycemia and increased corticosteroid secretion levels. Hyperamylasemia is accompanied by increased resistance to its own action. The use of amylin in therapy may lead to the breakdown of this resistance and, as studies show, the administration of amylin in obese people can lead to normoglycemia and to the reduction of body weight [4,6,16,43,44].
Conclusion
Amylin belongs to the group of pancreatic hormones. Its secretion from B cells proceeds in parallel with the secretion of insulin. In small quantities, it is also produced in other organs. Amylin inhibits food intake, delays gastric emptying, and decreases blood glucose levels, leading to the reduction of body weight. Therefore, amylin as well as insulin play important roles in controlling the level of blood glucose. Amylin is a modulator of glycogen synthesis and glucose consumption in skeletal muscle and has an effect on insulin resistance in muscles as well as in the liver. Fibrillation processes can lead to the formation of pathological deposits called amyloid within the Langerhans islands. Type 1 diabetes has a deficiency in the secretion of amylin resulting from the destruction of B cells. In type 2 diabetes, its level depends on the phase of the disease, in the early periods its level is elevated as well as the level of insulin. In later periods of the disease, as the b cells are being destroyed, this level is reduced.
The multiple effects of amylin indicate its usefulness in the treatment of diabetes.
References
- Hay DL Amylin. Headache. 2017; 57: 89-96.
- Cooper GJ, Willis AC, Clark A, Turner RC, Sim RB, Reid KB. Purification and characterization of a peptide from amyloid-rich pancreases of type 2 diabetic patients. Proc Natl Acad Sci USA. 1987; 84: 8628-8632.
- Rinka TR, Beaumontb K, Kodac J, Youngd A.: Structure and biology of amylin. Trends Pharmacol Sci. 1993; 14: 113.
- Hay DL, Chen S, Lutz TA, Parkes DG, Roth JD. Amylin: Pharmacology, Physiology, and Clinical Potential. Pharmacol Rev. 2015; 67: 564-600.
- Lutz TA. Control of energy homeostasis by amylin. Cell Mol Life Sci. 2012; 69: 1947-1965.
- Boyle CN, Lutz TA, Le Foll C. Amylin - Its role in the homeostatic and hedonic control of eating and recent developments of amylin analogs to treat obesity. Mol Metab. 2018; 8: 203-210.
- Chiti F, Dobson CM. Protein Misfolding, Amyloid Formation, and Human Disease: A Summary of Progress Over the Last Decade. Annu Rev Biochem. 2017; 86: 27-68.
- Akter R, Cao P, Noor H, Ridgway Z, Tu LH, Wang H. et al. Islet Amyloid Polypeptide: Structure, Function, and Pathophysiology. J Diabetes Res. 2016; 2016: 2798269.
- Sun Y, Kakinen A, Xing Y, Pilkington EH, Davis TP, Ke PC, Ding F. Nucleation of β-rich oligomers and β-barrels in the early aggregation of human islet amyloid polypeptide. Biochim Biophys Acta Mol Basis Dis. 2019; 1865: 434- 444.
- Farrukh SUB, Javed I, Ather AQ, Emwas AH, Alazmi M, Gao X, et al. Synthesis and identification of novel pyridazinylpyrazolone based diazo compounds as inhibitors of human islet amyloid polypeptide aggregation. Bioorg Chem. 2019; 84: 339-346.
- Kiriyama Y, Nochi H. Role and Cytotoxicity of Amylin and Protection of Pancreatic Islet β-Cells from Amylin Cytotoxicity. Cells. 2018; 7.
- Montane J, de Pablo S, Castaño C, Rodríguez-Comas J, Cadavez L, Obach M. et al. Amyloid-induced β-cell dysfunction and islet inflammation are ameliorated by 4-phenylbutyrate (PBA) treatment. FASEB J. 2017; 31: 5296- 5306.
- Visa M, Alcarraz-Vizán G, Montane J, Cadavez L, Castaño C, Villanueva- Peñacarrillo M. et al. Islet amyloid polypeptide exerts a novel autocrine action in β-cell signaling and proliferation. FASEB J. 2015; 29: 2970-2979.
- Raleigh D, Zhang X, Hastoy B, Clark A. The β-cell assassin: IAPP cytotoxicity. J Mol Endocrinol. 2017; 59: R121-R140.
- Fernández MS. Human IAPP amyloidogenic properties and pancreatic β-cell death. Cell Calcium. 2014; 56: 416-427.
- Marszałek M. [Amylin under examination. Fibrillogenic polypeptide hormone of the pancreas]. Postepy Hig Med Dosw (Online). 2014; 68: 29-41.
- Marszałek M. [Amylin under examination. Fibrillogenic polypeptide of pancreatic amyloid]. Postepy Hig Med Dosw (Online). 2015; 69: 14-24.
- Marszałek M. [Amylin under examination. Fibrillation - cytotoxic pancreatic polypeptide aggregation]. Postepy Hig Med Dosw (Online). 2015; 69: 309- 319.
- Kanatsuka A, Kou S, Makino H. IAPP/amylin and β-cell failure: implication of the risk factors of type 2 diabetes. Diabetol Int. 2018; 9: 143-157.
- Wijesekara N, Ahrens R, Wu L, Ha K, Liu Y, Wheeler MB, et al. Islet amyloid inhibitors improve glucose homeostasis in a transgenic mouse model of type 2 diabetes. Diabetes Obes Metab. 2015; 17: 1003-1006.
- Mietlicki-Baase EG. Amylin-mediated control of glycemia, energy balance, and cognition. Physiol Behav. 2016; 162: 130-140.
- Otto-Buczkowska E, Jarosz-Chobot P, Machnica Ł. The role of amylin in glucose homeostasis regulation and possible future usage in adolescents with type 1 diabetes Experimental and Clinical Diabetology 2009; 9: 91-94.
- Frandsen CS, Dejgaard TF, Madsbad S, Holst JJ. Non-insulin pharmacological therapies for treating type 1 diabetes. Expert Opin Pharmacother. 2018; 19: 947-960.
- Edelman SV. Optimizing diabetes treatment using an amylin analogue. Diabetes Educ. 2008; 34: 4S-10S
- Harris K, Boland C, Meade L, Battise D. Adjunctive therapy for glucose control in patients with type 1 diabetes. Diabetes Metab Syndr Obes. 2018; 11: 159-173.
- Otto-Buczkowska E, Mazur-Dworzecka U, Dworzecki T. [Role of amylin in glucose homeostasis and its perspective use in diabetes management]. Przegl Lek. 2008; 65: 135-139.
- Riddle MC, Yuen KC, de Bruin TW, Herrmann K, Xu J, Öhman P, Kolterman OG. Fixed ratio dosing of pramlintide with regular insulin before a standard meal in patients with type 1 diabetes. Diabetes Obes Metab. 2015; 17: 904- 907.
- Ramkissoon CM, Aufderheide B, Bequette BW, Palerm CC. A model of glucose-insulin-pramlintide pharmacokinetics and pharmacodynamics in type I diabetes. J Diabetes Sci Technol. 2014; 8: 529-542.
- Huffman DM, McLean GW, Seagrove MA. Continuous subcutaneous pramlintide infusion therapy in patients with type 1 diabetes: observations from a pilot study. Endocr Pract. 2009; 15: 689-695.
- Chase HP, Lutz K, Pencek R, Zhang B, Porter L. Pramlintide lowered glucose excursions and was well-tolerated in adolescents with type 1 diabetes: results from a randomized, single-blind, placebo-controlled, crossover study. J Pediatr. 2009; 155: 369-373.
- Hassan K, Heptulla RA. Reducing postprandial hyperglycemia with adjuvant premeal pramlintide and postmeal insulin in children with type 1 diabetes mellitus. Pediatr Diabetes. 2009; 10: 264-268.
- Kishiyama CM, Burdick PL, Cobry EC, Gage VL, Messer LH, McFann K, et al. A pilot trial of pramlintide home usage in adolescents with type 1 diabetes. Pediatrics. 2009; 124: 1344-1347.
- Herrmann K, Frias JP, Edelman SV, Lutz K, Shan K, Chen S. et al. Pramlintide improved measures of glycemic control and body weight in patients with type 1 diabetes mellitus undergoing continuous subcutaneous insulin infusion therapy. Postgrad Med. 2013; 125: 136-144.
- Herrmann K, Brunell SC, Li Y, Zhou M, Maggs DG. Impact of Disease Duration on the Effects of Pramlintide in Type 1 Diabetes: A Post Hoc Analysis of Three Clinical Trials. Adv Ther. 2016; 33: 848-861.
- Qiao YC, Ling W, Pan YH, Chen YL, Zhou D, Huang YM. et al. Efficacy and safety of pramlintide injection adjunct to insulin therapy in patients with type 1 diabetes mellitus: a systematic review and meta-analysis. Oncotarget. 2017; 8: 66504-66515.
- Weinzimer SA, Sherr JL, Cengiz E, Kim G, Ruiz JL, Carria L. et al. Effect of pramlintide on prandial glycemic excursions during closed-loop control in adolescents and young adults with type 1 diabetes. Diabetes Care. 2012; 35: 1994-1999.
- Galderisi A, Sherr J, VanName M, Carria L, Zgorski M, Tichy E, et al. Pramlintide but Not Liraglutide Suppresses Meal-Stimulated Glucagon Responses in Type 1 Diabetes. J Clin Endocrinol Metab. 2018; 103: 1088- 1094.
- Sherr JL, Patel NS, Michaud CI, Palau-Collazo MM, Van Name MA, Tamborlane WV, et al. Mitigating Meal-Related Glycemic Excursions in an Insulin-Sparing Manner During Closed-Loop Insulin Delivery: The Beneficial Effects of Adjunctive Pramlintide and Liraglutide. Diabetes Care. 2016; 39: 1127-1134.
- Hinshaw L, Schiavon M, Dadlani V, Mallad A, Dalla Man C, Bharucha A, et al. Effect of Pramlintide on Postprandial Glucose Fluxes in Type 1 Diabetes. J Clin Endocrinol Metab. 2016; 101: 1954-1962.
- Marrero DG, Crean J, Zhang B, Kellmeyer T, Gloster M, Herrmann K, et al. Effect of adjunctive pramlintide treatment on treatment satisfaction in patients with type 1 diabetes. Diabetes Care. 2007; 30: 210-216.
- Esquivel MA, Lansang MC. Optimizing diabetes treatment in the presence of obesity. Cleve Clin J Med. 2017; 84: S22-S29.
- Kheirandish M, Mahboobi H, Yazdanparast M, Kamal MA. Challenges Related to Glycemic Control in Type 2 Diabetes Mellitus Patients. Curr Drug Metab. 2017; 18: 157-162.
- Hoogwerf BJ, Doshi KB, Diab D. Pramlintide, the synthetic analogue of amylin: physiology, pathophysiology, and effects on glycemic control, body weight, and selected biomarkers of vascular risk. Vasc Health Risk Manag. 2008; 4: 355-362.
- Younk LM, Mikeladze M, Davis SN. Pramlintide and the treatment of diabetes: a review of the data since its introduction. Expert Opin Pharmacother. 2011; 12: 1439-1451.