
Research Article
Austin Diabetes Res. 2024; 9(1): 1030.
The Molecular Immunoregulatory Effect of Human Periodontal Ligament Stem Cells on Dendritic Cells of New-onset Type 1 Diabetes Mellitus Patients: In Vitro-Study
Al Marahelh M¹; AlHabashneh R²; Abuarqoub D³; Jafar H¹; Awidi A¹; Al Jabary L²*
1Cell Therapy Center, The University of Jordan, Amman, Jordan
2Department of Preventive, Faculty of Dentistry, Jordan University of Science and Technology, Irbid, Jordan
3Department of Biomedical Sciences and Pharmacology, The University of Petra, Amman, Jordan
*Corresponding author: Al Jabary L Department of Preventive, Faculty of Dentistry, Jordan University of Science and Technology, Irbid, Po box: 11183, Jordan Tel: 00962795066085 Email: leenjabary@hotmail.com
Received: March 18, 2024 Accepted: April 26, 2024 Published: May 03, 2024
Abstract
The immunosuppressive effects of human periodontal ligament stem cells (PDLSCs) on cells of the immune system have been reported on since PDLSCs were discovered as a viable source of the rapeutic stem cells. Their therapeutic application in the context of type 1 diabetes mellitus is inadequately reported on and merits further evaluation. The objective of this in-vitro research aims to evaluate the immunomodulatory capacity of human Periodontal Ligament Stem Cells (PDLSCs) in dendritic cell-mediated T-cell immune responses by assessing their ability to influence phenotype, differentiation, maturation and gene profile of monocyte derived DCs isolated from Type One Diabetes Mellitus (T1DM) patients.
This was done by deriving mature dendritic cells mDCs from monocytes of type one diabetic, characterized by flow cytometry at different stages (monocyte, immature DCs, and mature DCs) from four different donors. mDCs where then co-cultured with PDLSCs for two days and changes in level of maturation and costimulatory molecules measured by flow cytometry. qPCR was subsequently done to analyse the gene transcription profile of co-cultured mDCs in comparison to mDCs in relation to their upregulation or downregulation of the following cytokines, IL-6, IL-10, TGF-b, IL-1b, TNF-a.
Results showed that PDLSCs exerted an immunosuppressive effect on fully mature dendritic cells by significantly reducing expression of all maturation markers. A significant upregulation of the immunoregulatory cytokine IL6 by 8 folds was noted along with a significant downregulation of immunostimulatory cytokine TNF-a by 2 folds. This supports the immunosuppressive role of PDLSCs and their immunomodulatory capacities in T1DM context.
Keywords: Diabetes; Stem Cells; Periodontal Ligament Stem Cells; Type 1 Diabetes; Cell Therapy; Immunotherapy; Cytokines; Immunosuppression
Introduction
Type 1 Diabetes Mellitus (T1DM) is a chronic disease of an autoimmune pathogenesis in which cellular immunity plays a pivotal role in the selective destruction of insulin-producing pancreatic beta (β) cells, thus leading to a metabolic dysfunction. According to a recent systematic review [1-3] Type 1 Diabetes is prevalent in 9.5% of populations worldwide and is at an increase [1].
Preclinical and early clinical studies have implicated CD8+ effector T cells as the mediators of β cell apoptosis, with the final common pathway involving a multitude of cells including autoreactive CD4 and CD8 cells, B cells, coupled with malfunctioning regulatory T-cells (Tregs) subsets [4,5]. Features of pancreatic β cells that alter the cells lability to apoptosis are also thought to be involved. The release of β cell antigens, majorly being glutamic acid decarboxylase 65, GAD-65, which the Antigen Presenting Cells (APCs), mainly being Dendritic Cells (DCs) uptake and present to CD4 and CD8 T cells is believed to trigger the destructive autoimmune response in diabetes [4-6].
Furthermore, cytokines released by dendritic cells and other cells of the immune system play a crucial role in orchestrating complex multicellular interactions between pancreatic β cells and immune cells in the development of Type 1 Diabetes (T1D) and are thus potential immunotherapeutic targets for this disorder [7].
Owing to the autoimmune nature of T1DM, stem cell therapies have been considered as an effective treatment modality due to their ability to immunosuppress many cells of the immune system including Dendritic Cells (DCs), the main antigen presenting cells in T1DM. Mesenchymal Stem Cells (MSCs) have emerged in recent years as a safe and promising treatment strategy for autoimmune diseases, including T1DM [8,9]. Mesenchymal Stem Cells (MSCs) have immunomodulatory features, secrete cytokines and immune receptors that regulate the microenvironment in the host tissue which coupled with their multilineage potential makes them an effective tool in the treatment of chronic diseases [10].
MSCs exert trophic properties and have been shown to supress or modulate the activity of immune cells including antigen-presenting cells, Natural Killer (NK) cells, B cells and T cells [10]. Their unique surface marker expression hinders alloreactivity and protects them from NK cell lysis which permit MSCs to be a feasible stem-cell source for cell transplantation experiments [11].
Not only were these cells able to escape T-cell recognition and supress T cell responses [12] but have also been found to increase the conversion from Th2 T helper cells 2 to Th1 T helper cells 1 through modulation of interleukin IL4 and interferon IFN-γ levels in effector T Cells [12,8].
Dental-tissue-derived MSC-like populations offer an affordable and convenient source of MSCs and are a widely researched potential source of stem cells for clinical regenerative medicine. MSCs that are isolated from the dental pulp and periodontal ligament appear to be the most promising [13]. Periodontal ligament stem cells possess MSCs properties [14] that have in vitro showed fibroblast-like morphology, a cologeneic nature, and the ability to differentiate into adipocytes, osteoblasts and chondrocytes in a suitable induction media [15]. Surface marker expression of these cells was also similar to that of the mesenchymal stem cell. Their low immunogenicity and immunosuppressive influence on cells of the immune system has been reported on in multiple studies [16-18] making PDLSCs a viable source for therapeutic and immunoregulatory stem cells.
A previous pioneer in vitro study conducted by our university group reported on PDLSCs ability to immunomodulate mature dendritic cells mDCs from T1DM patients by reducing all maturation markers.[19] The detection of high levels of anti-inflammatory cytokines in the co-culture supernatant media was also noted but it was not clear whether this is secreted by the PDLSCs or the mDCs as the study did not look at the molecular changes taking place upon co-culture in the dendritic cell.
Further studies are needed hence needed to evaluate the mechanism of action of PDLSCs and the viability of their application in the context of T1D. The current study aims to further evaluate the influence PDLSCs have on dendritic cell-mediated T-cell immune responses by studying their effect on maturation and differentiation of monocyte derived DCs isolated from Type One Diabetes Mellitus (T1DM) patients and their influence, if any, on gene profile of DCs for a selection of immunoregulatory and immunostimulatory cytokines.
Methodology
Isolation and Generation of mDCs
The plastic adherence method suggested by Obermaier [20] was followed with some modifications to isolate CD14+ monocytes from Peripheral Blood Mononuclear Cells (PBMNCs) of full heparinized blood from four donors. Four donors, including three males and one female, diagnosed with early disease onset and a positive INF-y response to GAD65 were included in this study after gaining informed consent. The protocol was approved by Institutional Review Board (IRB) committee at Jordan University of Science and Technology (IRB NO 45783).
Following isolation of CD14+ monocytes, they were resuspended in a medium of RPMI 1640 complete medium (Euroclone. S.P.A, ITALY) supplemented with 1% L-glutamine, 1% streptomycin and 10% Platelet Lysate (PL). Following incubation and seeding, cells were characterized via flow cytometry to confirm viability of CD14+ monocytes and phenotype was confirmed by observing the cells under inverted microscopy.
To induce differentiation into Immature DCs (iDCs), monocytes were cultured in a 6- well plates and stimulated with 1000IU/ML GM-CSF and 500IU/ML IL-4 (R&D Systems, USA) for six days. At day six, the isolated iDCs were cultured in another 6- well plate with 10% Actiplate, GM-CSF (1000IU/mL), IL-4 (500IU/mL), IL-1β (500IU/mL) and TNF-α (500IU/mL) to stimulate their differentiation into mature DCs (mDCs) which were subsequently pulsed with recombinant human GAD-6 (ABCAM, UK) at a concentration of 10μg/1 × 106 cells/ml for 48hours.
Characterization of both iDCs and mDCs was performed by flow cytometry to analyse expression of CD14 (PE-CY7), CD80 (APC-H7), CD40 (BV51), CD83 (FITC), CD86 (BV421), CD1a (PE), CD209 (APC) and HLA-DR (Percp-CY5.5) using conjugated monoclonal antibodies (BD, USA). Samples were analysed using BD FACS Canto II flow cytometer using BD FACS Diva 8 software. Phenotype of these cells was confirmed with inverted phase-contrast microscopy (Axiovert, Zeiss,Germany).
Isolation of PDLSCs
Using the enzymatic method [21] PDLSCs were isolated from impacted third molar teeth within 24 hours of extraction from healthy donors. The surface marker expression of these cells was also analysed to ascertain their MSC like surface expression. BD FACS Canto II flow cytometer was used, BD stem flow TM hMSC Analysis Kit (BD, USA) was used to analyse the surface marker expression of the isolated cells. Three samples were sub-cultured and used at passages 3-5 for co-culturing with mDCs.
Co-Culture of mDCs with PDLSCs
GAD-65 pulsed mDCs were added to three PDLSCs samples at a ratio of 1:1 and the co-culture was left to incubate for 48 hours. The level of maturation of conditioned mDCs was subsequently measured by flow cytometry to check for surface markers CD14, CD80, CD40, CD 83, CD86, CD1a, CD209 and HLA-DR. Phenotype of the cocultured cells was also studied using inverted phase-contrast microscope.
Q-PCR for Quantification of Gene Expression
Since this study also aimed to investigate the effect of co-culturing DCs with PDLSCs on their gene expression for a selection of immunoregulatory and immunostimulatory cytokines that are vital to the pathogenesis of T1D, Q-PCR was performed to determine the expression of the target genes at the mRNA level. GAD-65 pulsed mDC and conditioned DCs were lysed by Trizol-hybrid method for RNA extraction using miniRNeasy kit (Qiagen, USA). The extracted RNA was quantified by a Nanodrop (Thermofisher, USA). To synthesize cDNA, 0.5 μg total RNA was reverse transcribed by using the PrimeScript RT Master Mix (Cat No. RR036A, Takara, China) using T100™ Thermal cycler PCR instrument (BioRad, USA). Primers were designed using Primer-BLAST (RRID:SCR_003095) and obtained from IDT (USA) (Table 1). The selected cytokines included, IL-10, IL-6, TGF-β, IL-1β, and TNF-αlisted each with their primer sequencing in table 1.
Each sample was performed in triplicate, and a mean value was calculated. Data were analysed according to 2−DDCT method using CFX Maestro™ Software - Bio-Rad.
Data Analysis
IBM SPSS Statistics software V.22 (IBM Corp, IBM SPSS Statistics for Windows, Version 22.0 Armink, NY: IBM Corp). One-Way ANOVA was used to test differences in percentages of surface markers studies between iDCs, mDCs and conditioned DCs within test group. qPCR data were analysed according to 2−DDCT method using CFX Maestro™ Software - Bio-Rad. In all analyses P values <0.05 were considered significant.
Results
Characterization of PDLSCs
Morphology and surface marker expression of PDLSC at passage 3 was evaluated. When viewed under the inverted microscope, PDLSCs showed typical MSCS morphology with the typical fibroblast like appearance and were adherent to the tissue culture plate (Figure 1). Their surface marker expression was typical of MSCs with 100% positive for CD90 (p=0.00), 97% for CD73, 90% CD105, and 100% for CD44 (p=0.00). Meanwhile negative cocktail expression was 3% and included the antibodies CD45, CD34, CD11b, CD19 and HLA-DR (Figure 2).
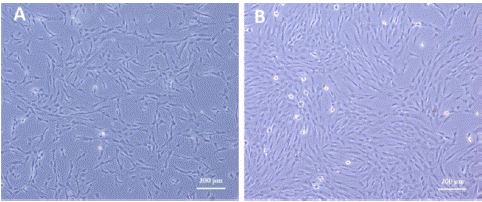
Figure 1: PDLSCs at 4X under inverted light microscope showing typical fibroblast like appearance (A) primary culture of PDLSCs, (B) PDLSCs in confluence at passage 3.
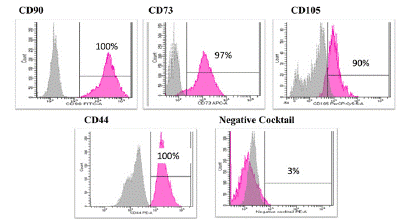
Figure 2: Representative flow cytometry histograms showing high expression of CD90, CD105, CD73 and CD44 and lack the expression of negative MSCs cocktail (CD34, CD11b, CD19, CD45 and HLA-DR). Gray peak corresponds with isotype control and the violet peak corresponds with the antibodies. Data were analyzed by using FACS canto II.
Morphology and Phenotype of iDCs, mDCs and Co-Cultured DCs
Monocyte derived iDCs, exhibited a rounded appearance with short dendrites and were larger in size than their monocyte precursor. Upon maturation of iDCs into mDCs, these cells showed pronounced and numerous longer dendrites (Figure 3). Co-cultured mDCs showed a morphology similar to that of iDCs with shorter dendrites (Figure 3). The change of mDCs morphology strongly correlated with a previous study [21].
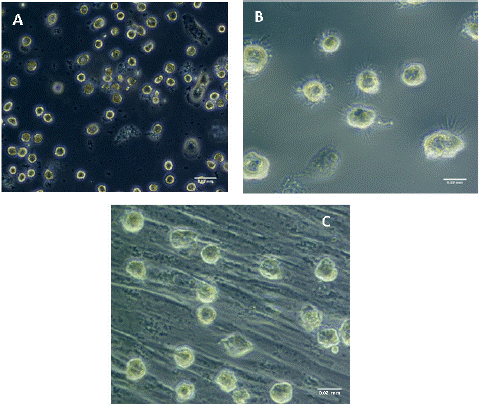
Figure 3: Morphological features of monocyte derived iDCs, mDCs, and co-cultured mDCs under inverted phase-contrast microscope (a) iDCs at 20X Magnification exhibiting round appearance and short dendrites. Scale bar 0.03mm. (b) mDCs pulsed with GAD-65 at 40X Scale bar 0.02 (c) mDCs after co-culture with PDLSCs at X40, showing a morphology that is similar to that of the iDCs with short dendrites. Scale bar 0.02.
Effect of Co-Culturing with PDLSCs on GAD65 Pulsed mDCs Profile
Phenotypic analysis of co-cultured mDCs showed skewing of mDCs towards an immature state with a decrease in expression of all costimulatory and maturation surface marker including CD14, CD80, CD83, CD86, CD40, CD1a, CD209 and HLA-DR. Flow cytometry was performed to determine the level of maturity and expression profile at the level of iDCs, mDCs, and co-cultured DCs.
CD14 expression at all cell levels was negligible which is expected following monocyte differentiation as CD14 is characteristic of monocyte detection. Expression of CD80, CD83, CD40, CD1A, and HLA-DR was significantly increased upon maturation (p<0.05). After co-culturing for 48hours, expression of all surface markers was significantly decreased (p<0.05) as expected. This is presented in Figure 4 below.
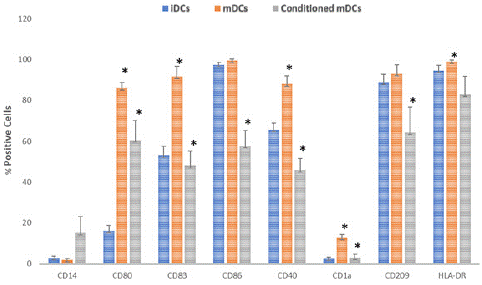
Figure 4: Maturation surface marker expression CD14, CD80, CD83, CD86, CD40, CD1a, CD209, HLA-DR, as expressed by iDCs, mDCs, and conditioned DCs. Data shown as mean ± SD, significant difference among different cell type shown as an *(p < 0.05).
Gene Expression Profile of Cocultured mDCs for Immunoregulatory and Immunostimulatory Cytokines
TNFα, IL-6, IL-1β, TGF-β and IL10
As shown in Figure 5 gene expression profile was evaluated in conditioned mDCs. Results showed significant upregulation in the expression of immunoregulatory genes (IL-6, IL-10 and IL-1β). The IL-6 gene was upregulated by up to 8 folds. An increase of 4 folds was also noted in the IL-10 gene but results were not significant. Despite a 15 folds increase in IL-1β gene, results were also not significant.
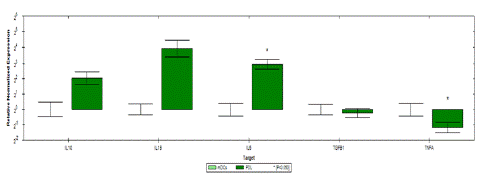
Figure 5: Relative normalized expression of immunomodulatory and immunostimulatory genes of mDCs after co-culture with PDLSCs from four donors as compared to uncultured mDCs (A) upregulated genes. (B) downregulated gene. Results were normalized to 18S rRNA Each sample was performed in triplicate, and a mean value was calculated. Data were analysed according to 2−DDCT method using CFX Maestro™ Software - Bio-Rad. *p ≤ 0.01 and fold change ≥ 1.5.
A significant downregulation of up to 2 folds was noted in the immunostimulatory TNFα gene.
No change between mDCs and conditioned DCs was noted in TGF-β gene expression. Fold regulation results shown in table 2 confirmed the upregulation and downregulation of mDCs target genes.
Discussion
Type 1 diabetes is a chronic condition characterized by specific adaptive immunity against β-cell antigens that ensues when an imbalance occurs between regulatory and inflammatory T-cells. The autoimmune nature of the diabetes type 1 warrants the need to pursue an immune based treatment that could halt disease progression. The challenge lies in finding a clinically efficient treatment with no adverse short- or long-term reactions and one that can be applied to the target organ and localized to the immune pathway in question.
Mesenchymal stem cells have been widely studied for their immunosuppressant qualities in autoimmune diseases including type 1 diabetes, but there in vivo application is limited owing to the sensitivity of these cells to immune-mediated environment and cell senescence due to overexpansion of cells. Hence a debate exists on how to enhance the immunomodulatory effects of MSCs.
Recently, PDLSCs have emerged as an appealing alternative source of stem cells due to their high differential potential and a morphological appearance similar to that of MSCs. The effects of PDLSCs in the context of T1D have however not yet been widely explored, making this research a pioneer study in evaluating the immunoregulatory properties of PDLSCs on dendritic cells of diabetic patients. The resemblance in morphology between PDLSCs and MSCs has been confirmed in this study by flow cytometry analysis of the costimulatory molecules and maturation markers which in compliance with the criteria suggested by the International Society for Cellular Therapy showed positive expression of CD105, CD44, CD90, CD73 (>90%) and negative expression of DC45, CD34, CD11b, CD19 and HLA-DR (<3%). [22] This agrees with the current literature on characteristics of PDLSCs being similar to bone marrow stromal cells [8].
The skewing of the mature dendritic cells towards an immature phenotype that was observed in this study after co-culturing with PDLSCs, provides evidence of the immunomodulatory effect of PDLSCs and their capability to produce tolDCs. Conditioned DCs adopted an immature morphological appearance, with the loss of long dendritic process and skewing towards an immature phenotype with increase CD14 expression and reduction in all other maturation markers. Tolerogenic DCs express low amounts of co-stimulatory molecules, increased produced of anti-inflammatory cytokines, and are capable of driving T cells to differentiate into Tregs [23].
This follows with other studies that show BM-MSCs conditioned DCs from T1D patients acquired an immature phenotype [8,24]. This proves that expression of different levels of co-stimulatory molecules can be regulated by a variety of inflammatory cytokines during the process of diabetes in agreement with similar studies [25].
T cell priming is influenced by receptor-ligand interaction with mDCs which are potent antigen presenting cells, hence, a reduction in surface markers should result in a defective T cell response and supress antigen-driven inflammatory effector T cell activation. CD40 and MHC class II molecules trigger production of pro-inflammatory cytokine IL-12 in DCs [26] and further up-regulates CD80 and CD86 [27] which stimulate T cell activation [28]. Furthermore, downregulation of CD83 in mDC results in suppression of allogeneic T cell proliferation and IFN-gamma secretion by established T cells [29].
These findings were further supported by gene analysis. The significant upregulation in IL-6 in conditioned DCs supports the immunosuppressive role of PDLSCs as IL-6 has been shown to induce differentiation of monocytes into macrophages instead of DCs [30] and plays an immunosuppressive role in DCs. IL-6 has been [31] shown to suppress inflammation in animal models [32,33] through downregulating IL-1 and TNF production.
IL-6 is has also been associated with regulating insulin secretion [34] and preventing pancreatic islet cells from apoptosis. As mentioned earlier In vivo assessment of islet beta cells that are pre-incubated with IL-6 using mouse models of mouse model revealed IL-6 conferred significantly better blood glucose control and graft survival [34]. Conclusively, IL-6 protects pancreatic islets or β-cells from inflammatory cytokines-induced cell death and functional impairment both in vitro and in vivo.
As for IL-10 gene expression results, despite being insignificant, showed a four folds increase in upregulation which further provides proof for the immunosuppressive role of PDLSCs. IL-10 role as an anti-inflammatory cytokine in T1DM owes to it favouring the proliferation of Tregs and suppression of Th2 proinflammatory cytokines such as IL-2 and IFN.
As for TGF-b, no difference was noticed between mDCs and co-cultured mDCs. this comes in disagreement with what we were expecting which is a surge in its upregulation. On its own however, qPCR data is not sufficient to conclude whether in reality its gene was upregulated or not for many reasons and should be confirmed in conjunction with analysis of the concentration of TGF in the media culture. Perturbations in the gene expression of cytokines are not necessarily translated to a change in serum levels of the cytokines. This could be due to the fact that protein level cytokine results are long-lived in comparison with mRNA results that are short lived in comparison. In this study qPCR was done 48h after coculture, and results might have varied if done at 24h interval instead.
Surprisingly gene expression of IL-1b was also upregulated up to 15 folds, despite results being insignificant. It has been recognized that IL-1 is selectively cytotoxic to rodent and human beta-cells in vitro and anti-IL-1 therapies reduce diabetes incidence in animal prevention models [35]. However, as mentioned earlier change in gene transcriptions levels do not necessarily translate into an identical increase or decrease in cytokine production levels as measured at protein level due to multiple factors including the DNA posttranslational modification process. Furthermore, the complexity of IL-1b signalling pathway, which is secreted by an unconventional protein secretion pathway, including multiprotein complex that controls activation of IL-1b processing protease caspase-1, should also be taken into consideration [36].
As for the inflammatory cytokine TNF-a, the significant downregulation in its transcription provides further evidence to the immunomodulatory function of PDLSCs on dendritic cells, TNF-a was found to upregulate costimulatory molecules and accelerate apoptosis of beta cells [35] and hence its downregulation is vital in halting initiation of T1DM. Results of some studies support a diabetogenic role for TNF-a in the initiation of T1D and strengthen the targeting of TNF-a as a new therapeutic strategy [37].
This study hence confirms that PDLSC modulate mDCs-mediated antigen presentation through the induction of tolDCs which in turn can modulate antigen-specific T cell response and induce T cell anergy.
Recommendations
Safety of this method needs to first be established, then optimizing treatment and customizing it to match disease stage. Further recommendations include considering PDLSCs priming prior to administration as Human mesenchymal stromal cell priming, MSC licensing or cell activation has recently been proposed to overcome low expression of immunosuppressive factors by MSCs. This method involves exposing MSCs to pro-inflammatory cytokines such as IFN, TNF-, IL1-b, and IL-1a.
Increasing sample size to increase viability of results and include patients that have been diagnosed with T1DM within no more than 6 months as this has been shown to be the most receptive and reversible period for immune therapy.
Conclusion
Collectively, the results of this study support the role of PDLSCs as immunomodulatory agents that alter the morphology, phenotype and function of dendritic cells of type 1 diabetic patients.
This study paves the road to utilizing PDLSC-based stem cell therapy in T1DM context, further in vitro studies to analyse effect on T cell response and gene transcription profile of a wider range of immunomodulatory and immunostimulatory genes is indeed needed, since it’s the interaction between various cytokines that dictates the nature of the milieu.
Author Statements
Conflict of Interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
References
- Mobasseri M, Shirmohammadi M, Amiri T, Vahed N, Fard HH, Ghojazadeh M. Prevalence and incidence of type 1 diabetes in the world: A systematic review and meta-analysis. Health Promotion Perspectives. 2020; 10: 98–115.
- Polychronakos C, Li Q. Understanding type 1 diabetes through genetics: advances and prospects. Nat Rev Genet. 2011; 12: 781-92.
- Naushad N, Perdigoto AL, Rui J, Herold KC. Have we pushed the needle for treatment of Type 1 diabetes?. Curr Opin Immunol. 2017; 49: 44–50.
- Figueroa FE. Mesenchymal stem cell treatment for autoimmune diseases: a critical review. 2012; 45: 269-277.
- Jakubzick CV, Randolph GJ, Henson PM. Monocyte differentiation and antigen-presenting functions. Nature revies Immunology. 2017; 17: 349-362.
- Bluestone JA, Tang Q, Sedwick CE. regulatory cells in autoimmune diabetes: past challenges, future prospects. J Clin Immunol. 2008; 28: 677–684.
- Nepom GT, Ehlers M, Mandrup-Poulsen T. Anti-cytokine therapies in T1D: Concepts and strategies. Clin Immunol. 2013; 149: 279-85.
- Favaro E, Carpanetto A, Caorsi C, Giovarelli M, Angelini C, Cavallo-Perin P, et al. Human mesenchymal stem cells and derived extracellular vesicles induce regulatory dendritic cells in type 1 diabetic patients. Diabetologia. 2016; 59: 325–333.
- Wang Y, Chen X, Cao W, Shi Y. Plasticity of mesenchymal stem cells in immunomodulation: pathological and therapeutic implications. Nat Immunol. 2014; 15: 1009–1016.
- Stagg J. Immune regulation by mesenchymal stem cells: two sides to the coin. Tissue Antigens. 2007; 69: 1-9.
- Rasmusson I, Ringdén O, Sundberg B, Le Blanc K. Mesenchymal stem cells inhibit the formation of cytotoxic T lymphocytes, but not activated cytotoxic T lymphocytes or natural killer cells. Transplantation. 2003; 76: 1208-13.
- Artholomew A, Sturgeon C, Siatskas M, Ferrer K, McIntosh K, Patil S, et al. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol. 2002; 30: 42-8.
- Lymperi S, Ligoudistianou C, Taraslia V, Kontakiotis E, Anastasiadou E. Dental Stem Cells and their Applications in Dental Tissue Engineering. Open Dent J. 2013; 7: 76-81.
- Kaku M, Komatsu Y, Mochida Y, Yamauchi M, Mishina Y, Ko CC. Identification and characterization of neural crest-derived cells in adult periodontal ligament of mice. Arch Oral Biol. 2012; 57: 1668-75.
- Gay IC, Chen S, MacDougall M. Isolation and characterization of multipotent human periodontal ligament stem cells. Orthod Craniofac Res. 2007; 10: 149-60.
- Zhu W, Liang M. Periodontal Ligament Stem Cells: Current Status, Concerns, and Future Prospects. Stem Cells International. 2015; 2015: 972313.
- Liu Y, Zheng Y, Ding G, Fang D, Zhang C, Bartold PM, et al. Periodontal Ligament Stem Cell-Mediated Treatment for Periodontitis in Miniature Swine. STEM CELLS. 2008; 26: 1065-1073.
- Wang L, Shen H, Zheng W, Tang L, Yang Z, Gao Y, et al. Characterization of stem cells from alveolar periodontal ligament. Tissue Eng Part A. 2011; 17: 1015–1026.
- Ashour L, Al Habashneh RA, Al-Mrahelh MM, Abuarqoub D, Khader YS, Jafar H, et al. The modulation of mature dendritic cells from patients with type 1 diabetes using human periodontal ligament stem cells. an in-vitro study. Journal of Diabetes & Metabolic Disorders. 2020; 19: 1037–1044.
- Obermaier B, Dauer M, Herten J, Schad K, Endres S, Eigler A. Development of a new protocol for 2-day generation of mature dendritic cells from human monocytes. Biol Proced Online. 2003; 5: 197-203.
- Tran Hle B, Doan VN, Le HT, Ngo LT. Various methods for isolation of multipotent human periodontal ligament cells for regenerative medicine. In Vitro Cell Dev Biol Anim. 2014; 50: 597-602.
- Maleki M, Ghanbarvand F, Behvarz MR, Ejtemaei M, Ghadirkhomi E. Comparison of mesenchymal stem cell markers in multiple human adult stem cells. International Journal of Stem Cells. 2014; 7: 118–126.
- Mrahleh MA, Matar S, Jafar H, Wehaibi S, Aslam N, Awidi AS. Human Wharton’s jelly-derived mesenchymal stromal cells primed by tumor necrosis factor-α and interferon-γ modulate the innate and adaptive immune cells of type 1 diabetic patients. Frontiers in Immunology. 2021; 12: 732549.
- Jiang XX, Zhang Y, Liu B, Xi-Zhang S, Wu Y, Yu XD, et al. Human mesenchymal stem cells inhibit differentiation and function of monocyte-derived dendritic cells. Blood. 2005; 105: 4120–4126.
- Zhong JX, Chen J, Rao X, Duan L. Dichotomous roles of co-stimulatory molecules in diabetes mellitus. Oncotarget. 2017; 9: 2902–2911.
- Koch F, Stanzl U, Jennewein P, Janke K, Heufler C, Kampgen E, et al. High level IL-12 production by murine dendritic cells: upregulation via MHC class II and CD40 molecules and downregulation by IL-4 and IL-10. J Exp Med. 1996; 184: 741-6.
- Caux C, Dezutter-Dambuyant C, Schmitt D, Banchereau J. GM-CSF and TNF-alpha cooperate in the generation of dendritic Langerhans cells. Nature. 1992; 360: 258-261.
- Pletinckx K, Dohler A, Pavlovic V, Lutz MB. Role of Dendritic Cell Maturity/Costimulation for Generation, Homeostasis, and Suppressive Activity of Regulatory T Cells. Front Immunol. 2011; 2: 39.
- Aerts-Toegaert C, Heirman C, Tuyaerts S, Aerts JL, Bonehill A, Thielemans K, et al. CD83 expression on dendritic cells and T cells: correlation with effective immune responses. Eur J Immunol. 2007; 37: 686-95.
- Chomarat P, Banchereau J, Davoust J, Palucka AK. IL-6 switches the differentiation of monocytes from dendritic cells to macrophages. Nat Immunol. 2000; 1: 510–4.
- Pasare C, Medzhitov R. Toll pathway-dependent blockade of CD4+CD25+ T cell-mediated suppression by dendritic cells. Science. 2003; 299: 1033–6.
- Barton BE, Jackson J. Protective role of interleukin 6 in the lipopolysaccharide-galactosamine septic shock model. Infect Immun. 1993; 61: 1496-9.
- Ulich TR, Yin S, Guo K, Yi ES, Remick D, del Castillo J. Intratracheal injection of endotoxin and cytokines. II. Interleukin-6 and transforming growth factor beta inhibit acute inflammation. Am J Pathol. 1991: 138: 1097-101
- Wunderlich FT, Strohle P, Konner AC, Gruber S, Tovar S, Bronneke HS, et al. Interleukin-6 signaling in liver-parenchymal cells suppresses hepatic inflammation and improves systemic insulin action. Cell Metab. 2010; 12: 237–49.
- Lu J, Liu J, Li L, Lan Y, Liang Y. Cytokines in type 1 diabetes: Mechanisms of action and immunotherapeutic targets. Clinical & Translational Immunology. 2020; 9: e1122.
- Lopez-Castejon G, Brough D. Understanding the mechanism of il-1β secretion. Cytokine & Growth Factor Reviews. 2011; 22: 189–195.
- Akash MS, Rehman K, Liaqat A. Tumor necrosis factor‐alpha: Role in development of insulin resistance and pathogenesis of type 2 diabetes mellitus. Journal of Cellular Biochemistry. 2017; 119: 105–110.