
Research Article
J Fam Med. 2014;1(1): 2.
The Biomarker Risk Prediction Score in Chronic Heart Failure
Alexander E Berezin1*, Alexander A Kremzer2, Yulia V Martovitskaya3, Tatyana A Samura2 and Tatyana A Berezina4
1Internal Medicine Department, State Medical University, Ukraine
2Clinical Pharmacology Department, State Medical University, Ukraine
3Pathology Department, State Medical University, Ukraine
4Private Medical Center, Zaporozhye, Ukraine
*Corresponding author: Alexander E Berezin, Cardiology Unit, Internal Medicine Department, State Medical University, 26, Mayakovsky av, Zaporozhye, UA- 69035, Ukraine
Received: July 21, 2014; Accepted: July 29, 2014; Published: July 29, 2014
Abstract
The study aim was to evaluate whether biomarker risk prediction score is powerful tool for risk assessment of three-year fatal and non-fatal cardiovascular events in CHF patients.
Methods: It was studied prospectively the incidence of fatal and non-fatal cardiovascular events, as well as the frequency of occurrence of death from any cause in a cohort of 388 patients with CHF during 3 years of observation. Circulating levels of NT-pro Brain Natriuretic Peptide (NT-pro-BNP), galectin-3, high-sensitivity C - reactive protein (hs-CRP), osteoprotegerin and its soluble receptor sRANKL, osteopontin, osteonectin, adiponectin, Endothelial Apoptotic Micro Particles (EMPs) and Mononuclear Progenitor Cells (MPCs) were measured at baseline.
Results: Median follow-up of patients included in the study was 2.76 years. There were 285 cardiovascular events determined, including 43 deaths and 242 readmissions. Independent predictors of clinical outcomes in patients with CHF were NT-pro-BNP, galectin-3, hs-CRP, osteoprotegerin, CD31+/annexin V+ EMPs and EMPs / CD14+CD309+ MPCs ratio. Index of cardiovascular risk was calculated by mathematical summation of all ranks of independent predictors,which occurred in the patients included in the study. The findings showed that the average value of the index of cardiovascular risk in patients with CHF was 3.17 points (95% CI = 1.65 - 5.10 points.). Kaplan-Meier analysis showed that patients with CHF and the magnitude of the risk of less than 4 units have an advantage in survival when compared with patients for whom obtained higher values of ranks cardiovascular risk score.
Conclusion: biomarker risk score for cumulative cardiovascular events, constructed by measurement of circulating NT-pro-BNP, galectin-3, hs-CRP, osteoprotegerin, CD31+/annex in V+ EMPs and EMPs / CD14+CD309+ MPCs ratio, allowing reliably predict the probability survival of patients with CHF, regardless of age, gender, state of the contractile function of the left ventricle and the number of co morbidities.
Keywords: Chronic Heart Failure; Biomarkers; Cardiovascular Outcomes; Predictive Value
Abbreviations
BMI: Body Mass Index; BMP: Brain Natriuretic Peptide; CI: Confidence Interval; CHF: Chronic Heart Failure; EMPs: Endothelial-Derived Apoptotic Micro particles; Gal-3: Galectin-3; GFR: Glomerular Filtration Rate; LVEF: Left Ventricular Ejection Fraction; MPCs: Mononuclear Progenitor Cells; NYHA: New York Heart Association; OR: Odds Ratio; TNF: Tumor Necrosis Factor.
Introduction
Chronic Heart Failure (CHF) remained leading cause of cardiovascular death worldwide [1]. As expected, significant improvements in survival have occurred for patients with CHF, with an increasing array of therapeutic options sharing quite varied properties of cost, invasiveness, and impact on life expectancy [2,3]. Contemporary risk models allow patients and physicians to achieve a better understanding of prognosis than is possible through unstructured holistic assessment [4]. Recent clinical studies have been shown that short-term and long-term prognosis among heart failure persons may be reappraised and recalculated using biological marker models demonstrated to be credible in clinical practice and useful predictable tool for physicians [5-7]. Natriuretic peptides, galectin-3 (Gal-3), high sensitive C - reactive protein (hs-CRP) were positively associated with all-cause and cardiovascular mortality and were discussed useful for estimating prognosis in persons with chronic stable heart failure [8-10]. Therefore, wide spectrum of biomarkers reflected immune status, pro inflammatory activation, endothelial function, was tested in predictive models for CHF patients [11-14]. However, no ideal biomarkers with optimal decremented potency were found that leads to prompting of use a multi marker approach in risk modeling for heart failure persons. Although several multivariate risk scores have shown significant utility in predicting patient outcomes in acute and acutely decompensate heart failure, contemporary models, such asSeattle Heart Failure Model, substantially underestimated the absolute risk of death in ambulatory CHF patients [15].
The study aim was to evaluatewhether biomarker risk prediction score is powerful tool for risk assessment of three-year fatal and nonfatal cardiovascular events in CHF patients.
Methods
Study population
The study population consisted of 388 consecutive patients with CHF who underwent angiography or PCI between April 2010 to June 2014, as well as were referred as post-myocardial infarction subjects within this period in our five centers participated in this investigation. All these patients were selected from 1427 patients according to our inclusion and exclusion criteria. The study protocol was approved by the Zaporozhe State medical University Ethnics committee review board. The study complied with the Declaration of Helsinki and voluntary informed written consent was obtained from all patients included in this study.
We analyzed cumulative survival related to CHF, and additionally all-cause mortality was examined.Prognosis was assessed by the composite endpoint all-cause death, CHF-related death or CHF hospitalization, censored at 3 years.
Methods for visualization of coronary arteries
Multispiral computed tomography angiography and/or angiographic study have been carried out to verify the ischemic nature of the disease in patients. Multispiral computed tomography angiography has been carried out for all the patients prior to their inclusion in the study. When atherosclerotic lesions of the coronary arteries were verified, patients were subjected to conventional angiographic examination provided indications for revascularization were available. CAD was considered to be diagnosed upon availability of previous angiographic examinations carried out not later than 6 months ago provided no new cardiovascular events occurred for this period, and the procedure are available for assay. The coronary artery wall structure was measured by means of contrast spiral computed tomography angiography [16] on Soma tom Volume Zoom scanner (Siemens, Erlangen, Germany) with two detector rows when holding patients breathe at the end of breathing in. After preliminary native scanning, non-ionic contrast omnipaque (Amersham Health, Ireland) was administered for the optimal image of the coronary arteries.
Echocardiography and tissue Doppler imaging
Transthoracic B-mode echocardiography and tissue Doppler imaging were performed according to a conventional procedure on ACUSON scanner (SIEMENS, Germany) using phased transducer of 5 ?Hz. Left Ventricular End-Diastolic and End-Systolic Volumes, and Ejection Fraction (LVEF) were measured by modified Simpson's plan metric method [17,18]. Peak systolic (Sm), early diastolic (Em), and late diastolic (?m) myocardial velocities were measured in the mitral annulus area, followed by calculating velocity of early diastolic left ventricular filling (E) to ?m (?/?m) ratio and to Em (?/Em) ratio. Inter- and intraobserver variability coefficients for LVEF were 3.2% and 1.1% respectively.
Glomerular filtration rate measurement
Calculation of Glomerular Filtration Rate (GFR) was calculated by CKD-EPI formula [19].
Biomarker determination
All biomarkers were determined at baseline. To measurement of biological marker concentrations, blood samples were drawn in the morning (at 7-8 a.m.) into cooled silicone test tubes. Samples were processed according to the recommendations of the manufacturer of the analytical technique used. They were centrifuged upon permanent cooling at 6,000 rpm for 3 minutes. Then, plasma was refrigerated immediately to be stored at a temperature -70?? until measurement.
Circulating NT-pro-BNP level was measured by immune electro chemo luminescent assay using sets produced by R&D Systems (USA) on Elecsys 1010 analyzer (Roche, Mannheim, Germany). Serum concentrations of Tumor Necrosis Factor alpha (TNF-alpha), solubilized Fas (sFas), sFas ligand, galectin-3, and adiponectine were determined in duplicate with commercially available enzyme-linked immunosorbent assay kits (Bender Med Systems GmbH, Vienna, Austria).
Circulatingbone-related proteins (osteoprotegerin, osteonectine, and osteopontine) were determined in duplicate by ELISA method using kits produced by IBL (Immunochemie und Immunobiologie Gmb, Gewmany).
The high-sensitivity C-Reactive Protein (hs-CRP) levels were measured by using nephelometric technique on AU640 analyzer manufactured by Diagnostic Systems Group (Japan).
Concentrations of Total Cholesterol (TC) and cholesterol of High-Density Lipoproteins (HDLP) were measured by fermentation method. Concentration of cholesterol of Low-Density Lipoproteins (LDL-C) was calculated according to the Friedewald formula (1972).
A total of 100 μl of serum samples was assayed in parallel to known standard concentrations for each biological marker. The mean intra-assay coefficients of variation were <10% of all cases.
Identifying fractions of mononuclear and endothelial progenitor cells
Mononuclear cells populations were phenotype by flowcytofluorimetry by means of monoclonal antibodies labeled with FITC fluorochromes (fluoresce in isothiocyanate) or double-labeled with FITC/PE (phycoerythrin) (BD Biosciences, USA) to CD45, CD34, CD14, Tie-2, and ?D309(VEGFR2) antigens as per HD-FACS (High-Definition Fluorescence Activated Cell Sorter) methodology, with red blood cells removed obligatory with lysing buffer according to gating strategy of International Society of Hematotherapy and Graft Engineering sequential (ISHAGE protocol of gating strategy) [20]. For each sample, 500 thousand events have been analyzed. Circulating Mononuclear Progenitor Cells (MPCs) have been identified as CD45- CD34+ cells. Proangiogenic phenotype for endothelial MPCs was determined as CD14+?D309 (VEGFR2)+Tie-2+ antigens. Obtained when laser beam is scattered in longitudinal and transversal directions in the flowcytofluorimeter, the scatter gram results were analyzed by using Boolean principles for double or triple positive events.
Endothelial-derived apoptotic microparticles determination
Endothelial-derived apoptotic micro particles were phenotyped by flow cytofluorimetry by Phycoerythrin (PE)-conjugated monoclonal antibody against CD31 (BD Biosciences, USA) followed by incubation with Fluoresce in Isothiocyanate (FITC)-conjugated annex in V (BD Biosciences, USA) per HD-FACS (High-Definition Fluorescence Activated Cell Sorter) methodology. The samples were incubated in the dark for 15 min at room temperature according to the manufacturer's instructions. The samples were then analyzed on a FC500 flow cytometer (Beckman Coulter) after 400 L annex in-V binding buffer was added. For each sample, 500 thousand events have been analyzed. EMPs gate was defined by size, using 0.8 and 1.1 mm beads (Sigma, St Louis, MO, USA). CD31+/annex in V+ micro particles were defined as EMPs positively labeled for CD31 and annex in V (CD31+/annex in V+) [21,22].
Statistical analysis
Statistical analysis of the results obtained was carried out in SPSS system for Windows, Version 22 (SPSS Inc, Chicago, IL, USA) and Graph pad Prism for Windows, Version 5 (Graph Pad Software Inc, La Jolla, CA , USA). The data were presented as Mean (?) and Standard Deviation (±SD) or 95% Confidence Interval (CI); Median (??) and Inter Quartile Range (IQR), as well as numerous (n) and frequencies (%) for categorical variables. To compare the main parameters of patients' groups (subject to the type of distribution of the parameters analyzed), two-tailed Student t-test or Mann-Whitney U-test were used. To compare categorical variables between groups, Chi2 test (?2) and Fisher F exact test were used. The circulating EMPs, MPCs, and NT-pro-BNP level in the blood failed to have a normal distribution, while distribution of the hs-CRP, bone-related proteins, adiponectine, total cholesterol and cholesterol fractions had a normal character (estimated by means of Kolmogorov-Smirnov test) and was not subjected to any mathematical transformation. The factors, which could be associated potentially with clinical outcomes, were determined by Cox regression analysis. Receive Operation Characteristic (ROC) curves were constructed for assessment of optimal balanced cut-off points that were suitable for independent predictors of clinical outcomes. Areas under curves were compared using method provided by DeLong et al (1988) [23]. Reclassification methods (C-statistics) were utilized for prediction performance analyses. The Kaplan-Meyer curves were constructed depending categories of the Biomarker risk prediction score. A calculated difference of P<0.05 was considered significant.
Results
Study patient population
The characteristics of the patients participated in the study are depicted in Table 1. At baseline, mean age in box sexes was 58.34 years. The prevalence of II (37.9%) and III (21.4%) NYHA class was determined. At least 55.5% of the subjects enrolled in the study were hypertensive. Likewise, cardiovascular risk factors, such as dyslipidemia, type two diabetes mellitus and obesity, were reported 66.0%; 37.6%; and 44.3% respectively. Mean left ventricular ejection fraction was decreased slightly. Regarding biomarker levels, increased Gal-3, NT-pro-BNP, hs-CRP, bone-related proteins (osteoprotegerin, osteopontin, osteonectin), sRANKL and adiponectin were found. Depletion of ccirculating levels of MPCs labeled as CD14+CD309+ and CD14+CD309+Tie2+ were determined. Increased CD31+/annex in V+ EMPs were found.
Entire patient cohort (n=388)
Age, years
58.34±9.60
Male, n (%)
207 (53.3%)
I NYHA class, n (%)
77 (19.8%)
II NYHA class, n (%)
147 (37.9%)
III NYHA class, n (%)
83 (21.4%)
IV NYHA class, n (%)
81 (20.9%)
Hypertension, n (%)
214 (55.5%)
Dyslipidemia, n (%)
256 (66.0%)
Type two diabetes mellitus, n (%)
146 (37.6%)
Obesity, n (%)
172 (44.3%)
Adherence to smoke, n (%)
76 (19.6%)
BMI, kg/m2
24.1 (95% CI = 21.6 - 28.7)
Systolic BP, mm Hg
130.90±8.41
Diastolic BP, mm Hg
77.90±5.12
Heart rate, beat per min.
70.52±3.34
LVEF, %
42.80±0.76
GFR, 1,73 ml/ min/ m2
82.3 (95% CI = 68.7 - 102.6)
Creatinine, μmol/L
72.3 (95% CI = 58.7 - 92.6)
Fasting glucose, mmol/L
5.20 (95% CI =3.3-9.7)
HbA1c, %
6.8 (95% CI =4.1-9.5)
Hemoglobin, g/L
132.4 (95% CI = 125.5 - 140.1)
Total cholesterol, mmol/L
5.1 (95% CI = 3.9 - 6.1)
Cholesterol HDL, mmol/L
0.91 (95% CI = 0.89 - 1.12)
Cholesterol LDL, mmol/L
3.23 (95% CI = 3.11 - 4.40)
Uric acide, mmol/L
3.5 (95% CI = 25.3 - 40.1)
NT-pro-BNP, pg/mL
153.6 (95% CI = 644.5 - 2560.6)
Galectin-3, ng/mL
1.58 (95% CI = 15.90 - 18.65).
hs-CRP, mg/L
7.34 (95% CI =6.77-7.95)
Osteoprotegerin, pg/mL
554.3 (95% CI =5306.4-5782.1)
Osteopontin, ng/mL
99.5 (95% CI = 57.7 - 142.7 )
Osteonectin, ??/??
788.54 (95% CI = 665.12-912.30)
sRANKL, ??/??
2206.50 (95% CI =2057.2-2355.8)
sRANKL / osteoprotegerin ratio, unit
0.39 (95% CI =0.22-0.45)
Adiponectin, μg/mL
10.61 (95% CI =4.83-17.35)
MPCs with phenotype CD14+CD309+ ×10-4, %
29.18 (95% CI = 15.00 - 34.50)
MPCs with phenotype CD14+CD309+Tie2+×10-4, %
0.67 (95% CI = 0.21 - 1.10)
CD31+/annexin V+ EMPs, cells/mL
0.48 (95% CI = 0.29-0.64)
EMPs / CD14+CD309+ ???, ??. ×10
6.59 (95% CI = 4.10 - 8.96)
Notes: CI - 95% Confidence Interval; NYHA - New York Heart Association; GFR - Glomerular Filtration Rate; BMP - Brain Natriuretic Peptide; BP - Blood Pressure; LVEF - Left Ventricular Ejection Fraction; BMI - Body Mass Index; sRANKL - serum Receptor Activator Of Nuclear Factor-Kappa B Ligand; EMPs - Endothelial-Derived Apoptotic Microparticles; MPCs - Mononuclear Progenitor Cells; HbA1c - Glycated Hemoglobin; HDL - High-Density Lipoprotein; LDL - Low-Density Lipoprotein.
Table 1: The characteristics of participants.
The majority patients with CHF were treated with ACE inhibitors or ARAs, beta-adrenoblockers, I/f blocker ivabradine, mineralocorticoid receptor antagonists, and antiplatelet drugs Table 2. Adding loop diuretics was done when fluid retention was determined. Dihydropyridine calcium channel blockers were added when elevated was uncontrolled by previous treatment scheme. Metformin and / or sitagliptin were used in type two diabetes patients as a component of contemporary treatment of CHF.
Entire patient cohort (n=388)
ACE inhibitors or ARAs, n (%)
388 (100%)
Aspirin, n (%)
305 (78.6%)
Other antiplatelet drugs, n (%)
83 (21.4%)
Beta-adrenoblockers, n (%)
324 (83.5%)
Dihydropyridine calcium channel blockers, n (%)
63 (16.2%)
Ivabradine, n (%)
137 (35.3%)
Mineralocorticoid receptor antagonists, n (%)
152 (39.2%)
Loop diuretics, n (%)
311 (80.1%)
Statins, n (%)
294 (75.7%)
Metformin, n (%)
146 (37.6%)
Sitagliptin, n (%)
48 (12.4%)
Notes: ACE - Angiotensin-Converting Enzyme; ARAs - Angiotensin-2 Receptors Antagonist.
Table 2: Treatment strategy in CHF patients enrolled in the study.
Clinical event determination
Median follow-up was of 2.76 years (IQR=1.8-3.4). During follow-up, 285 cardiovascular events (including 43 fatal cases) were determined. Thirty five patients were died due to advance of CHF, and eight cases of death were related with suddenly death, fatal myocardial infarction, and systemic thromboembolism. No other causes of death were defined. Additionally, 206 subjects were hospitalized repetitively due to worsening CHF and also 36 subjects were readmitted in the hospital due to other cardiovascular reasons.
Biomarker predictors of cumulative cardiovascular events
The independent biomarker predictors of cumulative cardiovascular events in CHF patients obtained by multivariable Cox regression analyses were NT-pro-BNP, galectin-3, hs-CRP, osteoprotegerin, sRANKL / osteoprotegerin ratio, MPCs labeled CD14+CD309+Tie2+, and EMPs / CD14+CD309+ MPCs ratio Table 3. ROC curves analysis have shown that there were significant difference between AUCs for independent variables and AUC for standard model (LVEF less 40%) Table 4. Therefore, the best discriminate value was found for EMPs / CD14+CD309+ MPCs ratio and CD14+CD309+Tie2 MPCs.
Variances
Univariate analysis
Multivariate analysis
OR
95% CI
? value
OR
95% CI
? value
Creatinine per 30 μmol/L
1.06
1.01-1.11
0.001
1.02
0.87-1.06
0.001
Fasting glucose per 3 mmol/L
1.04
0.96-1.09
0.002
HbA1c per 1%
1.05
1.01-1.07
0.002
Total cholesterol per 1 mmol/L
1.08
1.01-1.09
0.001
Uric acid per 10 mmol/L
1.08
1.03-1.09
0.001
1.03
0.92-1.08
0.001
NT-pro-BNP per 400 pg/mL
1.97
1.25-3.06
0.001
1.37
1.08-2.10
0.001
Galectin-3 per 2.5 ng/mL
2.16
1.78-3.77
0.001
1.46
1.22-1.89
0.003
hs-CRP per 1 mg/L
1.42
1.22-1.87
0.001
1.12
1.03-1.25
0.001
Osteoprotegerin per 325 pg/mL
1.34
1.18-1.62
0.006
1.19
1.12-1.33
0.001
Osteopontin per 65 ng/mL
1.16
1.03-1.36
0.002
0.95
0.87-1.11
0.003
Osteonectin per 50 ng/mL
1.19
1.07-1.28
0.001
1.06
0.91-1.19
0.002
sRANKL per 100 pg/mL
1.08
1.02-1.15
0.001
1.02
0.86-1.07
0.001
sRANKL / osteoprotegerin per 0.15 units
1.56
1.23-1.72
0.002
1.17
1.04-1.25
0.003
Adiponectin per 3.5 μg/mL
1.05
1.01-1.09
0.006
1.03
0.89-1.07
0.001
CD14+CD309+ MPCs per 10×10-4%
1.12
1.05-1.27
0.001
1.05
1.00-1.11
0.001
CD14+CD309+Tie2+ MPCs per -0,2×10-4%
1.15
1.03-1.29
0.006
1.06
1.01-1.09
0.001
CD31+/annexin V+ EMPs per 0.2 cells/mL
1.18
1.10-1.27
0.001
1.07
1.02-1.13
0.001
EMPs / CD14+CD309+ MPCs per 2.5 ×10 units
2.14
1.18-3.55
0.001
1.19
1.12-1.27
0.001
Notes: CI - Confidence Interval; OR - Odds Ration; HbA1c - Glycated Hemoglobin; BNP - Brain Natriuretic Peptide; sRANKL - Serum Receptor Activator of Nuclear Factor-Kappa B Ligand; EMPs - Endothelial-Derived Apoptotic Microparticles; MPCs - Mononuclear Progenitor Cells.
Table 3: Univariate and multivariate Cox regression analysis.
Models
AUC
95% CI
P values
Standard Model: LVEF
0.646
0.612 - 0.661
-
NT-pro-BNP
0.683
0.644 - 0.703
0.045
Galectin-3
0.731
0.711 - 0.754
0.013
hs-CRP
0.656
0.634 - 0.687
0.068
Osteoprotegerin
0.722
0.707 - 0.739
0.012
sRANKL / osteoprotegerin ratio
0.734
0.723 - 0.752
0.001
CD14+CD309+Tie2 MPCs
0.785
0.755 - 0.794
0.001
EMPs / CD14+CD309+ MPCs ratio
0.834
0.805 - 0.861
0.001
Abbreviations: AUC - Area Under Curve; LVEF - Left Ventricular Ejection Fraction; BNP - Brain Natriuretic Peptide; hs-CRP - high sensitive C-Reactive Protein; sRANKL - serum Receptor Activator Of Nuclear Factor-Kappa B Ligand; EMPs - Endothelial-Derived Apoptotic Microparticles; MPCs - Mononuclear Progenitor Cells.
Table 4: Comparison of AUCs characterized biomarker models to standard model calculated for LFEV less 40%. The results of ROC curves analysis.
C-statistic of the model with continuous variable shown that Cox regression model contains eight categorized predictors that did not differ from ABC model (C-statistic 0.81; 95% CI = 0.79 - 0.95; ?=0.001), whether C-statistic of the model with binary predictors containing sRANKL / osteoprotegerin ratio, MPCs labeled CD14+CD309+Tie2+, and EMPs / CD14+CD309+ MPCs ratio did distinguish from ABC model (C-statistic 1.04; 95% CI = 1.01 - 1.06; ?=0.001).
Biomarker risk prediction score for cumulative cardiovascular events
For Biomarker risk prediction score construction we enrolled six biomarkers: NT-pro-BNP, galectin-3, hs-CRP, osteoprotegerin, CD31+/annex in V+ EMPs and EMPs / CD14+CD309+ MPCs ratio. Each independent predictor was assigned the value of 1 or 0 when present or absent respectively. The sum of number of the independent predictors was ranged from 0 to 6 points, and then was used for Biomarker risk prediction score grading. The entire cohort of the CHF patients the Biomarker risk prediction score averaged 3.17 point (95% CI = 1.65 - 5.10 points). The distribution of the Biomarker risk prediction score in the CHF patients is Figure 1.
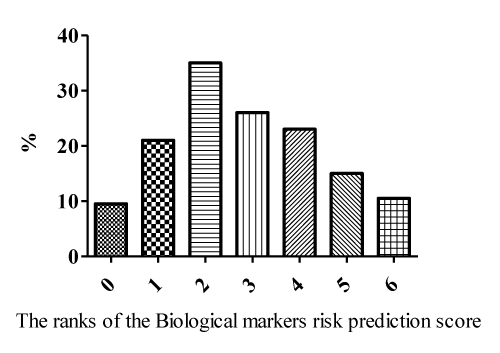
Figure 1: The distribution of various ranks of Biomarker risk prediction score
in patients with ischemic CHF.
The analysis of obtained results have shown that there is a significant association between rank of Biomarker risk prediction score and numerous of cumulative cardiovascular events in CHF patients (r=0.72; Wald ?2=11.9; ?=0.001). Therefore, Odds ratio calculated for cumulative cardiovascular events steadily increases related with up of Biomarker risk prediction score rank per 1 point Figure 2. We suggested that ranks of Biomarker risk prediction score ≤ 4 points reflect low risk of cumulative cardiovascular events in CHF patients, whether ranks ≥ 5 points of prediction score show high cardiovascular risk.
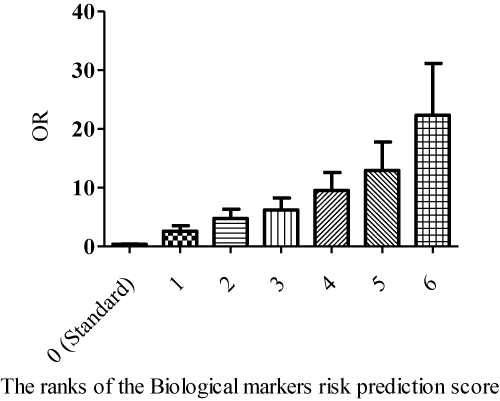
Figure 2: Stratification of CHF patients depending on the Odds Ratio (OR) of
cumulative cardiovascular events.
Figure 3 shows the Kaplan-Meyer survival curves for CHF patients stratified according to low and high cumulative cardiovascular risk. The accumulation of clinical event determined within observation period leads to a significant divergence (?<0.001) of survival curves constructed for two patient cohorts stratified depending low (≥ 4 points) and high (≥ 5 points) risk.

Figure 3: The Kaplan-Meyer survival curves for CHF patients stratified according to low and high cumulative cardiovascular risk.
Discussion
The results of the present study shown that the rank of the Biomarker risk prediction score was associated with cumulative clinical outcomes in CHF patients and that score system constructed biological markers may be capable to accurately identify patients at high-risk irrespective metabolic co morbidities. We included in the analysis several biological markers reflected different aspects and faces of the pathogenesis of CHF. Thus, in addition routinely measured biomechanical stress markers such as NT-pro-BNP, phenotypic marker at high risk of galectin-3 and the proinflammatory marker hs-CRP we have used multi-functional markers such as osteoprotegerin and its soluble receptor sRANKL, osteopontin, osteonectin, adiponectin , CD31+/ annex in V+ EMPs and MPCs with angiopoetic potency. The positive side of the multi marker approach is low dependence from demographic, metabolic co morbidities, and renal clearance that is crucial for CHF patients [24]. Earlier attempts to create new risk scores of CHF were based on isolated criteria such as clinical data or echocardiographic parameters, as well as levels of certain biomarkers, mainly natriuretic peptides and galectin-3 [7,25]. However, this approach proved to be more successful in a population of patients with acute or acutely decompensate heart failure than in those with stable chronic heart failure [26]. In addition, for the most scores such variables as age, gender, metabolic conditions (obesity, type 2 diabetes), renal clearance, and anemia were already established critical for reliability of prediction [5,6,27]. We have tried to incorporate these data in order to minimize the influence of additional factors on reliability prediction model to include in the biomarkers identified those that do not depend on renal clearance (MPCs and EMPs), were not associated with myocardial dysfunction (sRANKL / osteoprotegerin ratio), reflected the severity of endothelial dysfunction and coagulation (osteopontin, osteonectin). Although both biomarkers NT-pro-BNP and galectin-3 remained as the main biological indicators reflecting biomechanical / overload response and phenotypic risk of heart failure, they have limitation related with age, sex, kidney function, obesity, and diabetes [8, 28]. On the other hand, there are novel biomarkers, such as ST2 protein, that as expected may overcome the limitations suitable for Natriuretic peptides [29]. However, lack of data reflected surpassing ST2 protein to galectin-3 and other proinflammatory cytokines in turn of prediction of outcomes in CHF patient population [30]. Moreover, results of PRIDE study have been shown that NT-proBNP was superior to ST2 protein for primary diagnosis of acute or acutely decompensate heart failure [31]. Taken together these data are clarified that significant distinguishes in predictive value between several biomarkers were found and that no necessary to expect the appearance of one ideal biomarker for CHF patients. In fact, future perspective, probably, should affect the creation of multi marker models that would be more powerful tools to be rest ratified the patients at risk. Over all, we suggested that the Biomarker risk prediction score may reflect negative evolution of CHF and looks optimistic in terms of reliability evaluation system as a whole, although it requires a comparison with already established systems such as the Seattle Heart Failure Model Scores, appear that the purpose of further research in this direction. More investigations are needed to be recognizing optimal combination of biomarkers incorporated in the novel predictive score.
Conclusion
we suggested that biomarker risk score for cumulative cardiovascular events, constructed by measurement of circulating NT-pro-BNP, galectin-3, hs-CRP, osteoprotegerin, CD31+/annex in V+ EMPs and EMPs / CD14+CD309+ MPCs ratio, allowing reliably predict the probability survival of patients with CHF, regardless of age, gender, state of the contractile function of the left ventricle and the number of co morbidities.
Acknowledgment
We thank all patients for their par icipation in the investigation, staff of the Regional Zaporozhye Hospital (Ukraine), and the doctors, nurses, and administrative staff in Regional Cardiology Center (Zaporozhye, Ukraine) and City hospital # 6 (Zaporozhye, Ukraine), general practices, and site-managed organizations that assisted with the study.
Ethical principles
All the patients have given their voluntary written informed consent for participation in the study. The study was approved by the local ethics committee of State Medical University, Zaporozhye, Ukraine. The study was carried out in conformity with the Declaration of Helsinki.
Study Limitations
This study has some limitations. It is necessary to note that a large pool of nanoparticles might be produced after blood sampling due to destruction of platelets and blood cells. Therefore, preparation of isolates of micro particles in samples is the most sophisticated step for further examination. Venous citrated blood drawn from the fistula-free arm was performed obligatorily. We believe that these risks are systemic, and to minimize them, we refused to freeze the blood samples before measurement of micro particles. Although HD-FACS methodology is widely used, theoretically overlap between two or more fluorochromes might reflect some obstacles for further interpretation of obtained results. Another limitation of the present study is that a specific role of EMPs and PMCs is also possible and has not been characterized in depth in CHF patients. However, the authors suppose that these restrictions might have no significant impact on the study data interpretation. Additionally, retrospective, relative small sample size may limit the significance of the present study. However, this was not a randomized and controlled study. The authors believe that a greater cohort of patients with more incidences detected is desirable to improve the credibility of the study.
Authors' Contributions
Alexander E Berezin initiated the hypothesis and designed the study protocol, contributed to collect, analyze and interpret the data, performed statistical analysis, and wrote the manuscript. Alexander A. Kremzer contributed to enroll the patients, collected and analyzed the data, checked clinical events and reviewed the source documents. Yulia V. Martovitskaya contributed circulating biomarker determination, preformed preparation of isolates of micro particles in samples with further phenotyping by flowcytofluorimetry, and interpreted the obtained results. Tatyana A. Samura preformed visualization procedures and analyzed the results of examinations. Tatyana A. Berezina contributed to enroll the patients in the study and collect the data.
References
- Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, et al. Heart disease and stroke statistics--2010 update: a report from the American Heart Association. See comment in Pub Med Commons below Circulation. 2010; 121: e46-46e215.
- Ketchum ES, Levy WC. Multivariate risk scores and patient outcomes in advanced heart failure. See comment in PubMed Commons below Congest Heart Fail. 2011; 17: 205-212.
- Sartipy U, Goda A, Mancini DM, Lund LH. Assessment of a University of California, Los Angeles 4-variable risk score for advanced heart failure. See comment in PubMed Commons below J Am Heart Assoc. 2014; 3: e000998.
- Maisel A. Biomonitoring and biomarker-guided therapy: the next step in heart failure and biomarker research. See comment in PubMed Commons below J Am Coll Cardiol. 2011; 58: 1890-1892.
- Chyu J, Fonarow G.C, Tseng C.H, Horwich T.B. Four-variable risk model in men and women with heart failure. Circ. Heart Fail. 2014; 7: 88-95.
- Alba AC, Agoritsas T, Jankowski M, Courvoisier D, Walter SD, Guyatt GH, et al. Risk prediction models for mortality in ambulatory patients with heart failure: a systematic review. See comment in PubMed Commons below Circ Heart Fail. 2013; 6: 881-889.
- Carluccio E, Dini FL, Biagioli P, Lauciello R, Simioniuc A, Zuchi C, et al. The 'Echo Heart Failure Score': an echocardiographic risk prediction score of mortality in systolic heart failure. See comment in PubMed Commons below Eur J Heart Fail. 2013; 15: 868-876.
- Oremus M, Don-Wauchope A, McKelvie R, Santaguida PL, Hill S, Balion C, et al. BNP and NT-proBNP as prognostic markers in persons with chronic stable heart failure. See comment in PubMed Commons below Heart Fail Rev. 2014.
- Carrasco-Sánchez FJ, Páez-Rubio M1. Review of the Prognostic Value of Galectin-3 in Heart Failure Focusing on Clinical Utility of Repeated Testing. See comment in PubMed Commons below Mol Diagn Ther. 2014.
- Rajendiran KS, Ananthanarayanan RH, Satheesh S, Rajappa M. Elevated levels of serum sialic acid and high-sensitivity C-reactive protein: markers of systemic inflammation in patients with chronic heart failure. See comment in PubMed Commons below Br J Biomed Sci. 2014; 71: 29-32.
- Rajendiran KS, Ananthanarayanan RH, Satheesh S, Rajappa M. Elevated levels of serum sialic acid and high-sensitivity C-reactive protein: markers of systemic inflammation in patients with chronic heart failure. See comment in PubMed Commons below Br J Biomed Sci. 2014; 71: 29-32.
- DeBeradinis B, Januzzi JL. Use of biomarkers to guide outpatient therapy of heart failure. See comment in PubMed Commons below Curr Opin Cardiol. 2012; 27: 661-668.
- Berezin AE, Kremzer AA. Circulating endothelial progenitor cells as markers for severity of ischemic chronic heart failure. See comment in PubMed Commons below J Card Fail. 2014; 20: 438-447.
- Berezin AE, Kremzer AA., Samura TA., Martovitskaya YuV. Circulating Endothelial-Derived Apoptotic Microparticles in the Patients with Ischemic Symptomatic Chronic Heart Failure: Relevance of Pro-Inflammatory Activation and Outcomes. Int Cardiovasc Res J.2014; 8(3): 116-123.
- Laszczynska O, Severo M, Friões F, Lourenço P, Silva S, Bettencourt P, et al. Validity of the Seattle Heart Failure Model for prognosis in a population at low coronary heart disease risk. See comment in PubMed Commons below J Cardiovasc Med (Hagerstown). 2014.
- Bluemke DA, Achenbach S, Budoff M, Gerber TC, Gersh B, Hillis LD, et al. Noninvasive coronary artery imaging: magnetic resonance angiography and multidetector computed tomography angiography: a scientific statement from the American Heart Association Committee on Cardiovascular Imaging and Intervention of the Council on Cardiovascular Radiology and Intervention, and the Councils on Clinical Cardiology and Cardiovascular Disease in the Young. Circulation. 2008; 118: 586-606.
- Schiller NB, Shah PM, Crawford M, DeMaria A, Devereux R, Feigenbaum H, et al. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-Dimensional Echocardiograms. J Am Soc Echocardiogr. 1989; 2: 358-367.
- Pellerin D, Sharma R, Elliott P, Veyrat C. Tissue Doppler, strain, and strain rate echocardiography for the assessment of left and right systolic ventricular function. Heart. 2003; 89: iii9-17.
- Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, Feldman HI, et al. A new equation to estimate glomerular filtration rate. See comment in PubMed Commons below Ann Intern Med. 2009; 150: 604-612.
- Tung JW, Parks DR, Moore WA, Herzenberg LA, Herzenberg LA. New approaches to fluorescence compensation and visualization of FACS data. See comment in PubMed Commons below Clin Immunol. 2004; 110: 277-283.
- Lacroix R, Judicone C, Mooberry M, Boucekine M, Key NS, Dignat-George F. The ISTH SSC Workshop. Standardization of pre-analytical variables in plasma microparticle determination: results of the International Society on Thrombosis and Haemostasis SSC Collaborative workshop. J Thromb Haemost. 2013 Apr 2. doi: 10.1111/jth.12207. [Epub ahead of print]
- Orozco AF, Lewis DE . Flow cytometric analysis of circulating microparticles in plasma. See comment in PubMed Commons below Cytometry A. 2010; 77: 502-514.
- DeLong ER, DeLong DM, Clarke-Pearson DL . Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. See comment in PubMed Commons below Biometrics. 1988; 44: 837-845.
- Giallauria F, Fattirolli F, Tramarin R, Ambrosetti M, Griffo R, Riccio C, et al; ISYDE-2008 Investigators of the Italian Association for Cardiovascular Prevention and Rehabilitation (GICR-IACPR). Cardiac rehabilitation in chronic heart failure: data from the Italian SurveY on carDiac rEhabilitation (ISYDE-2008). J. Cardiovasc. Med. (Hagerstown) 2014; 15: 155-163.
- Li Y, Neilson MP, Whellan DJ, Schulman KA, Levy WC, Reed SD . Associations between Seattle Heart Failure Model scores and health utilities: findings from HF-ACTION. See comment in PubMed Commons below J Card Fail. 2013; 19: 311-316.
- Scrutinio D, Ammirati E, Guida P, Passantino A, Raimondo R, Guida V, et al. The ADHF/NT-proBNP risk score to predict 1-year mortality in hospitalized patients with advanced decompensated heart failure. See comment in PubMed Commons below J Heart Lung Transplant. 2014; 33: 404-411.
- Vakil KP, Dardas T, Dhar S, Moorman A, Anand I, Maggioni A, et al. Impact of renal dysfunction on the Seattle Heart Failure Model. See comment in PubMed Commons below J Heart Lung Transplant. 2014; 33: 163-169.
- McMurray J.J, Adamopoulos S, Anker S.D, Auricchio A, Böhm M, Dickstein K, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012. Eur Heart J. 2012; 33: 1787-1847.
- Ciccone MM, Cortese F, Gesualdo M, Riccardi R, Di Nunzio D, Moncelli M, et al. A novel cardiac bio-marker: ST2: a review. See comment in PubMed Commons below Molecules. 2013; 18: 15314-15328.
- Bhardwaj A, Januzzi JL. ST2: a novel biomarker for heart failure. See comment in PubMed Commons below Expert Rev Mol Diagn. 2010; 10: 459-464.
- Januzzi JL, Peacock WF, Maisel AS, Chae CU, Jesse RL, Baggish AL, et al. Measurement of the interleukin family member ST2 in patients with acute dyspnea: results from the PRIDE (Pro-Brain Natriuretic Peptide Investigation of Dyspnea in the Emergency Department) study. J Am Coll Cardiol. 2007; 50: 607-13.