
Research Article
J Dis Markers. 2014;1(2): 1006.
The Effect of Glucose Loading on Adiponectin Levels in Diabetes Mellitus Type 2: Implications for Endothelial Dysfunction
Katerina Koniari1, Dimitris Tousoulis1, Charalambos Antoniades1, Aggeliki Nikolopoulou1, Evangelos Voltyrakis1, Nikolaos Papageorgiou1, Marina Noutsou2, Christodoulos Stefanadis1 and Konstantinos Makris3*
1Cardiology Department, Athens University Medical School, Greece
2Department of Medicine, University of Athens Medical School, Greece
3Department of Clinical Biochemistry, General Hospital, Greece
*Corresponding author: Konstantinos Makris, EurClinChem, Clinical Biochemist, Clinical Biochemistry, Laboratory of metabolic bone diseases, KAT General Hospital, 2 Nikis street, 14561 Kifissia Athens, Greece
Received: August 01, 2014; Accepted: Aug 19, 2014; Published: Aug 21, 2014
Abstract
Aim: Adiponectin levels are decreased in patients with type II Diabetes Mellitus (DM) but it is unclear whether Impaired Glucose Tolerance (IGT) affects adiponectin's release, or whether glucose intake modifies its release from adipocytes. We examined the effect of glucose loading on serum adiponectin and endothelial function in patients with newly diagnosed DM, IGT subjects and healthy individuals.
Methods: Seventy nine patients with newly diagnosed DM, eighteen with IGT, and sixteen healthy individuals were recruited. All participants received 75g oral glucose. Endothelium-Dependent Dilation (EDD) was evaluated by gaugestrain plethysmography before glucose loading (baseline) and every 1h until 3h post-loading. Blood samples were obtained at baseline and at 3 hours postloading and serum adiponectin and insulin levels were measured.
Results: Glucose loading significantly increased adiponectin levels in healthy and IGT but not in diabetic individuals. Although insulin was correlated with adiponectin both at baseline (r=-0.375, p=0.0001) and at 3h (r=-0.286, p=0.006), insulin variations did not follow the same pattern in our three groups. There was no association between the changes of insulin and those of adiponectin (p=NS). EDD was similarly decreased in healthy, in IGT and DM patients after glucose loading (p=NS for all).
Conclusion: Glucose intake increases adiponectin levels in healthy individuals and subjects with IGT, but not in those with DM. This effect is independent of insulin variations. On the contrary, endothelial function was similarly impaired after glucose intake in all study groups, suggesting that deregulation of adiponectin's expression after glucose loading precedes the development of endothelial dysfunction in diabetes mellitus type II.
Keywords: Adiponectin; Acute hyperglycemia; Diabetes mellitus; Impaired glucose tolerance; Endothelium
Abbreviations
DM: Diabetes Mellitus; TNF-a: Tumor Necrosis Factor-alpha; IGT: Impaired Glucose Tolerance; EDD: Endothelium Dependent Dilation; FBF: Forearm Blood Flow; ApoAI: Apolipoprotein AI; ApoB: Apolipoprotein B; ApoE: Apolipoprotein E; Lp (a): Lipoprotein (a); HbA1c: Glycosylated Haemoglobin A1c; HPLC: High-performance Liquid Chromatography; ELISA: Enzyme-Linked Immuno Sorbent Assay; HOMA: Homeostasis Model Assessment; AGEs: Advanced Glycation End Products; NO: Nitric Oxide; AMPK: Adenosine Monophosphate-activated Protein Kinase
Introduction
Diabetes Mellitus (DM) is nowadays recognized as a metabolic disorder which is characterized by endothelial dysfunction and accelerated atherosclerosis [1-5]. Evidence suggests that chronic lowgrade inflammation plays a key role in the process of atherogenesis in diabetes [6,7]. Inflammation begins with lipid per oxidation and secretion of chemo attractants, proinflammatory cytokines and growth factors, which then induce the expression of cell surface adhesion molecules on endothelial cells, mediate monocytes adhesion to the endothelium and lead to atherogenesis [8-10].
Adipose tissue plays an active role in the normal metabolic and vascular homeostasis as well as in the development of type 2 diabetes, dyslipidemia and atherosclerosis [11]. These actions are mediated by secreting many biologically active molecules referred to as adipokines, such as Tumour Necrosis Factor-alpha (TNF-a), lepton, resist in and Adiponectin. Adiponectin is an adipose tissue-specific protein with beneficial effects on vascular function. It inhibits the inflammatory processes of atherosclerosis by suppressing the expression of adhesion and cytokine molecules in vascular endothelial cells and macrophages, respectively. Adiponectin concentrations correlate negatively with glucose, insulin, triglyceride serum levels, liver fat content and body mass index and positively with high-density lipoprotein-cholesterol levels, hepatic insulin sensitivity and insulinstimulated glucose disposal. Adiponectin has been shown to increase insulin sensitivity and decrease plasma glucose by increasing tissue fat oxidation [12-14]. It is well-known that Adiponectin levels are decreased in patients with DM [11], however it is unclear whether Impaired Glucose Tolerance (IGT) affects adiponectin's release, or whether glucose intake modifies its release from adipocytes.
In the present study we examined whether glucose loading affects serum Adiponectin and insulin concentrations in subjects with impaired glucose tolerance, diabetes mellitus type 2 and normal individuals. We also evaluated the impact of acute hyperglycemia on endothelial function in the above three groups of our study population.
Materials and Methods
Patients
The protocol was approved by the Institutional Ethics Committee and an informed consent was given by each subject. All parts of the study were performed in accordance with the guidelines in the Declaration of Helsinki. One hundred and thirteen subjects (46 men and 67 women) were enrolled in this study. Eighteen patients had impaired glucose tolerance (IGT, age 55.4±3.3 years), 79 patients had newly diagnosed DM (receiving no treatment, aged 56.6±1.3 years, p=NS vs. IGT) and 16 healthy young subjects had normal glucose tolerance (aged 38.0±3.8 years). All subjects were selected from the registry of Cardiology and Diabetic Department in Hippokration Hospital of Athens. Diabetes mellitus and IGT were defined in accordance with the National Data Group Criteria [15]. The inclusion criteria were that they had never been treated with any anti-diabetic or hypolipidemic agent, and that they were receiving no cardiovascular medication. All subjects underwent glucose loading with 75g oral glucose. Endothelium-Dependent Dilation (EDD, measured by venous occlusion gauge-strain plethysmography) was evaluated before glucose loading and every 1 hour until 3 hours postloading. Blood samples were obtained before glucose loading (after a 12-hour overnight fast) and at 3 hours post-loading (just before the last EDD measurement). Blood glucose was measured 1 min before each new plethysmography using a finger stick and a One Touch Profile (Johnson & Johnson, Life scan Benelux). The demographic characteristics of the participants are presented in Table1.
Forearm blood flow measurements
All studies were performed between 08:00 and 12:00. All participants were asked to proceed after a 12 hour overnight fast. Vasoactive agents (such as caffeine, tobacco and alcohol) were also avoided for the last 12 hours before the examination. They were also asked to refrain from eating and drinking during the whole study. Subjects were rested in a supine position, in a dark quiet room under constant temperature 22-25oC, for 15 minutes prior to the study. Forearm Blood Flow (FBF) was measured using gauge-strain plethysmography (EC-400, D.E. Hokanson Inc) [16,17]. The FBF output signal was transmitted to a personal computer (Hokanson NIVP3 software). FBF was finally calculated as the % change of arm volume/100 ml tissue/minute. Forearm vasodilator response to reactive hyperaemia (endothelium-dependent dilation-EDD) induced by 5-minutes ischemic occlusion of the distal forearm, was defined as the percent change of FBF from baseline to the maximum flow during reactive hyperaemia [16,17]. EDD was determined before glucose loading and every 1 hour until 3 hours post-loading.
Biochemical measurements
Venous blood samples were obtained at baseline (before plethysmography and glucose loading were performed), as well as at 3 hours post-loading (just before the last EDD measurement). After centrifugation at 3500 rpm at 4o C for 15 minutes, plasma or serum was collected and stored at -80 °C until assayed. Routine biochemical methods were used to determine serum concentrations of total cholesterol, lipoproteins [ApoAI, ApoB, ApoE, Lp(a)], triglycerides and glucose on an automated clinical chemistry analyser (Architect-8200, Abbott, Il, USA). HbA1c was measured by an automated analyzer (Menarini-Akray HA8160; Menarini, Florence, Italy) using the HPLC technique, calibrated using standards traceable to National Glucosylation Standardization Program (NGSP). Adiponectin was measured by an enzyme linked immunosorbent assay (Mercodia Laboratory Medicine Inc, Sweden). Serum insulin levels were measured by a micro particle enzyme immunoassay technique on an automated immunoassay analyzer (Axsym, Abbott Diagnostics, Abbott Park, Il, USA). Free fatty acids were measured with an enzymatic method (Wako Diagnostics, Osaka, Japan) on an automated clinical chemistry analyser (Architect-8200, Abbott, Il, USA). All the laboratory measurements were performed blindly.
Statistical analysis
Variables were tested for normal distribution by Kolmokorov- Smirnov test. Comparisons of demographic characteristics between the 3 groups were performed by using Pearson's chi square test or one-way ANOVA for multiple comparisons as appropriate. Continuous variables are expressed as means±SEM. Comparisons between variables were performed by using bivariate analysis and Pearson's correlation coefficient was estimated.
The effect of glucose loading on each variable between the 3 groups was examined by two-way ANOVA for repeated measures, and as there were significant differences in age between the 3 groups, the statistical interactions of these effects with age were also tested. The statistical analysis was performed by using SPSS 20.0 statistical program.
Results
The baseline characteristics of our three study groups are shown in Table 1. There were significant differences in risk factor profile (BMI, W/H, hypercholesterolemia, hypertension). At baseline HOMAInsulin Resistance index was significantly lower in the normal group compared to IGT (p=0.045) and diabetes (p=0.0001) groups but there were not significant differences between IGT individuals and diabetic patients (p=0.641). At baseline (Table 1) serum Adiponectin levels were not significantly different between IGT and diabetes group (p=0.720). Although healthy controls exhibit higher baseline values compared to IGT and diabetes groups, these differences are not statistically significant (p=0.072 and 0.080 respectively). In addition, free fatty acid levels were significantly higher in the diabetic and IGT subjects compared to healthy controls (p=0.001 and 0.045 respectively), although there was no significant difference between the diabetic patients and the IGT subjects (p=0.238).
Diabetes Mellitus
IGT
Normal
P-value
Number of patients
79
18
16
Sex (male/female)
30/49
8/10
8/8
0.340
BMI (Kg/m2)
29.2±0.54
27.6±0.82
26.0±1.18
0.028
W/H
0.95±0.06
0.93±0.02
0.86±0.02
0.0001
Hypercholesterolemia (n)
37
6
1
0.0001
Family history of DM (n)
55
13
13
0.433
Smoking (n)
23
3
5
0.936
Hypertension (n)
37
6
1
0.009
HbA1c (%)
6.8±0.17
5.5±0.35
4.8±0.22
0.0001
HOMA-IR
3.8±0.33
3.4±0.82
1.5±0.17
0.014
Fasting glucose (mmol/L)
8.49±0.24
6.38±0.19
5.16±0.16
0.0001
Fasting cholesterol (mmol/L)
5.05±0.12
4.79±0.20
4.33±0.18
0.025
Fasting Triglycerides (mmol/L)
8.49±0.24
6.38±0.19
5.16±0.16
0.002
HDL (mmol/L)
1.11±0.03
1.17±0.04
1.11±0.05
0.656
Apolipoprotein A (g/L)
1.41±0.03
1.48±0.05
1.44±0.05
0.603
Apolipoprotein B (g/L)
1.01±0.03
0.94±0.06
0.86±0.3
0.022
Apolipoprotein E (mg/dL)
4.4±0.19
3.8±0.24
3.5±0.29
0.085
Resting FBF (ml/100 ml tissue/min)
5.6±0.2
5.1±0.4
6.4±0.5
0.238
Max. hyperemic FBF (ml/100 ml tissue/min)
10.0±0.4
9.7±0.6
11.0±0.8
0.502
Hyperemic %change of flow (%)
85.3±4.9
92.5±6.8
76.7±5.2
0.512
Insulin serum levels (IU/L)
10.1±0.8
11.9±2.8
6.5±0.7
0.125
Free fatty acids (mmol/L)
1.4±0.1
1.2±0.2
0.7±0.1
0.005
Adiponectin (µg/ml)
5.59±0.33
5.33±0.48
7.0±0.86
0.152
Abbreviations: BMI: Body Mass Index; W/H: Waist/Hip; HOMA-IR: Homeostasis Model Assessment of Insulin Resistance; FBF: Forearm Blood Flow
Table 1: Baseline demographic and biochemical characteristics of the participants.
Effects on serum glucose levels
Glucose loading induced a significant elevation of serum glucose levels in all groups at 1 (p<0.01) and 2 hours (p<0.05), while at 3 hours post-loading, glucose levels were still significantly (p<0.05) greater than baseline in diabetic patients but were significantly (p<0.05) lower than baseline in IGT and healthy individuals (Figure 1A). In two-way ANOVA analysis, the difference of the changes among the 3 groups was still significant (p=0.0001) after adjustment for age, with the changes in diabetes group being greater than both IGT and healthy control groups (p=0.0001 vs. both, after adjustment for age). Similarly, the change of glucose levels was significantly greater in IGT compared to healthy individuals (p=0.0001 after adjustment for age).
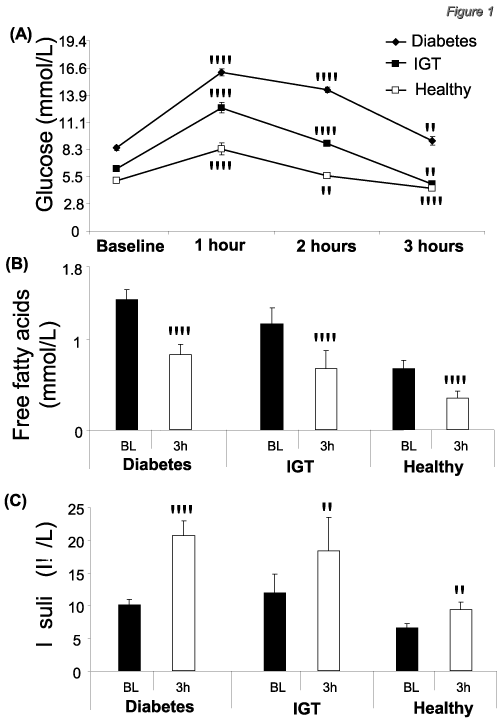
Figure 1: (A) Glucose levels were still elevated at 3 hours post-loading in
patients with diabetes mellitus, while they were significantly lower than
baseline in patients with impaired glucose tolerance (IGT) and healthy
individuals. (B) Glucose loading induced a significant reduction of free fatty
acids at 3 hours post-loading in patients with diabetes mellitus, IGT and
healthy individuals. (C) Insulin was significantly elevated at 3 hours post
glucose-loading compared to baseline, in all study groups. *p<0.05; **p<0.01
vs baseline (BL).
Effects on serum free fatty acid levels
Glucose loading induced a significant reduction (p<0.01) on the levels of free fatty acids at 3 hours post-loading in all three study groups (patients with diabetes mellitus, IGT and healthy individuals) compared to baseline (Figure 1B). When two-way ANOVA was used to examine the differences in the response among the 3 groups, the reduction of free fatty acids levels was significantly different across the 3 groups (p=0.028 after adjustment for age) with the change in IGT being significantly greater compared to healthy controls (p=0.017 after adjustment for age).
Effects on serum insulin levels
Insulin levels were significantly (p<0.05) increased at 3 hours post-loading in all study groups compared to baseline. When twoway ANOVA was performed, the increase of insulin levels was significantly greater in the diabetes group compared to healthy control group (p=0.046 after adjustment for age) (Figure 1c).
Effects on endothelial function
Hyperaemic blood flow and hyperemic % change of flow were not significantly different between the 3 groups, suggesting no differences in endothelial function in forearm resistance vessels among the three groups (Table 1). This could be explained by the early stage of diabetes mellitus in these subjects (newly diagnosed diabetes). Hyperaemic blood flow was significantly decreased (p<0.05) in patients with diabetes and impaired glucose tolerance (at 1 hour post-loading) and healthy individuals (at 2 hour post-loading) (Figure 2A). However, there were no significant differences in hyperaemic blood flow among the 3 groups when comparisons were performed by two-way ANOVA after adjustment for age (p=NS among the 3 groups).
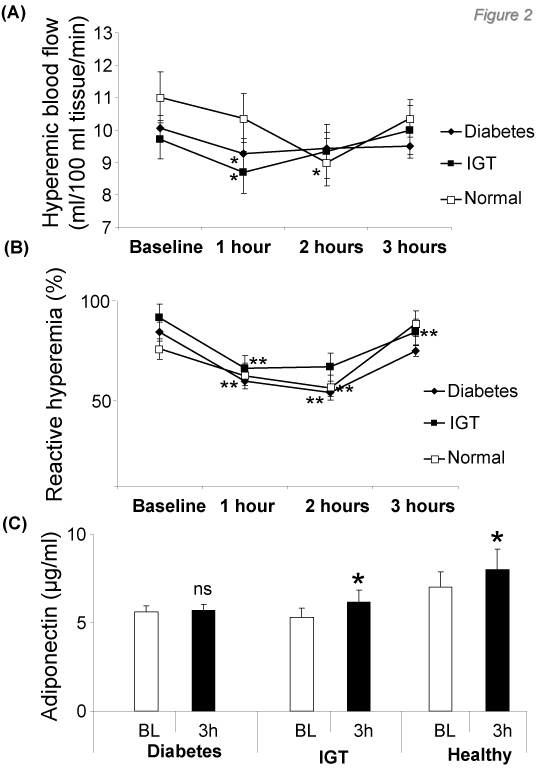
Figure 2: Overall survival
Endothelium-dependent dilatation of forearm resistance vessels (% change of forearm blood flow from baseline to maximum hyperaemic blood flow) was significantly (p<0.01) reduced in all study groups post-loading (Figure 2B).
Effects on serum adiponectin levels
Serum Adiponectin levels at 3 hours post-loading, were significantly increased (p<0.05) only in IGT and healthy individuals but not in patients with diabetes mellitus (Figure 2C). When two-way ANOVA was performed, the responses were significantly different across the 3 groups (p=0.007 after adjustment for age). When the changes of Adiponectin levels were compared between individual groups, there was a significantly lower response in diabetes group compared to healthy controls (p=0.006 after adjustment for age), while the differences between patients with diabetes and IGT did not reach statistical significance.
Although insulin was correlated with Adiponectin both at baseline (r=-0.375, p=0.0001) and at 3h (r=-0.286, p=0.006), there was no association between the changes of insulin and those of Adiponectin (r=-0.151, p=0.144), while there was a borderline association between the changes of Adiponectin and glucose levels (r=-0.175, p=0.09). Importantly, the change of insulin levels was significantly correlated with the change of free fatty acids at 3 hours post-loading (r=-0.244, p=0.017).
Discussion
In our study we examined the effect of glucose loading on serum Adiponectin and insulin levels and we evaluated the impact of acute hyperglycaemia on endothelial function. We also compared these effects among subjects with IGT, patients with DM and healthy individuals. We found that glucose intake induces a decrease in serum levels of free fatty acids in parallel to an increase of serum insulin levels, while it also blunts endothelial function in all study groups. However, glucose intake increases Adiponectin levels in healthy individuals and subjects with IGT, but not in those with DM, an effect independent of insulin variations. These findings suggest that Adiponectin may play a key role during the transition from IGT to diabetes mellitus.
Hyperglycemia and endothelial function in diabetes mellitus type 2
The vascular endothelium controls many important functions, including maintenance of blood circulation and fluidity, regulation of vascular tone, coagulation and inflammatory responses. Therefore, it plays an essential role in vascular homeostasis [18]. Endothelial cell dysfunction is considered an important early event in the process of atherogenesis and is consistently demonstrated in patients with DM [17,19-21]. Evidence suggests that hyperglycemia has a specific role in the increased risk of atherosclerosis in patients with DM and IGT [22,23], beyond dyslipidemia, insulin resistance and hyperinsulinemia.
It is well-known that chronic hyperglycemia is related to diabetic micro vascular complications by inducing the production of Advanced Glycation End Products (AGEs) [24]. On the other hand acute hyperglycemia induced e.g. by oral glucose loading leads to increased generation of oxygen free radicals, such as superoxide anion, through autoxidation and activation of protein kinase C pathway. The consequent increased oxidative stress causes endothelial cell injury, inactivation of endothelial-derived Nitric Oxide (NO) and finally acute impairment in endothelial function [25- 27]. Even if fasting blood glucose levels are within normal limits and hyperglycemia occurs only post-prandially, these repeated spikes of hyperglycemia may stimulate atherogenesis through acute oxidative stress and endothelial dysfunction, in healthy subjects with normal glucose tolerance [26,28,29].
In the present study we demonstrated that endothelial function at rest was similar among the 3 groups. This finding may be explained by the early stage of diabetes in diabetic patients (newly diagnosed diabetes) and by the normal fasting glucose levels in the IGT group. It is worth noting that in all study groups (diabetic, IGT and normal individuals) the endothelium-dependent vasodilation was rapidly attenuated in response to glucose loading and returned to the baseline levels after 3h, possibly through the activation of a defending mechanism. This finding agrees with the knowledge that acute hyperglycemia causes reversible abnormalities in blood flow and vascular permeability [26].
Adiponectin in atherosclerosis and diabetes mellitus type 2
In the past, adipose tissue was considered to be a passive depot for storing excess energy. Recent studies have demonstrated that adipocytes synthesize and secrete biologically active molecules, known as "adipocytokines" [12, 13,30,31]. Adiponectin is a key adipokine, with a critical role in atherogenesis [17].
Adiponectin is an adipose tissue-specific protein which acts as a key regulator of insulin sensitivity and tissue inflammation. It is a beneficial adipokine that exerts anti-atherogenic and anti-diabetic properties [32]. It has direct effects in liver, skeletal muscle and the vasculature, with prominent roles to improve hepatic insulin sensitivity, increase fuel oxidation [through the up-regulation of Adenosine Monophosphate-Activated Protein Kinase (AMPK) activity], decrease vascular inflammation [32,33] and induce endothelium-dependent nitric oxide-mediated vasorelaxation [34].
Unlike other adipocytokines, the plasma levels of Adiponectin as well as the adiponectin gene expression are reduced in obese individuals, subjects with type 2diabetes and coronary artery disease [11,35]. Adiponectin knockout mice exhibit various manifestations of the metabolic syndrome, such as insulin resistance, glucose intolerance, hyperlipidemia, impaired endothelium-dependent vasorelaxation and hypertension [36].
Hyperglycemia and adiponectin
As it has already been referred to, serum Adiponectin levels are reduced in states such as diabetes, insulin resistance and glucose intolerance. In our study we demonstrated that acute hyperglycemia increases adiponectin levels in healthy and IGT individuals, but not in the diabetics. A study by Szosland et al has shown that Adiponectin levels are increased during oral glucose tolerance test in insulin-resistant subjects [37]. Another study has demonstrated that hyperglycaemia prevents the suppressive effect of hyperinsulinemia on plasma Adiponectin levels in healthy humans [38]. These are two of the very few studies in the literature concerning the relationship between acute hyperglycaemia and serum Adiponectin levels. In our study, although insulin was correlated with Adiponectin both at baseline and at 3h, insulin variations did not follow the same pattern in our three groups and there was no association between the changes of insulin and those of Adiponectin.
Conclusion
This is the first study demonstrating that rapid elevation of serum glucose levels induces a parallel elevation of serum Adiponectin in healthy and IGT individuals but not in patients with newly diagnosed diabetes mellitus. This effect was independent of insulin variations. This finding supports the notion that acute hyperglycaemia is a stress factor that up regulates Adiponectin secretion in healthy individuals and IGT patients, while the onset of DM is accompanied by an inability to up-regulate Adiponectin expression post glucose intake. This partly explains the lower Adiponectin levels observed in patients with DM compared to healthy individuals, and supports the hypothesis that Adiponectin may be a key regulator of atherogenesis in diabetes mellitus.
In addition, as adiponectin's response to glucose loading is impaired in those patients with newly diagnosed DM, while endothelial dysfunction is not yet present, we could hypothesize that dysregulation of Adiponectin expression precedes the development of endothelial dysfunction in resistance vessels in patients with newly diagnosed diabetes mellitus type II. These findings provide new insights into the associations between insulin, Adiponectin and endothelial function in diabetes mellitus.
References
- Uusitupa MI, Niskanen LK, Siitonen O, Voutilainen E, Pyörälä K. Ten-year cardiovascular mortality in relation to risk factors and abnormalities in lipoprotein composition in type 2 (non-insulin-dependent) diabetic and non-diabetic subjects. Diabetologia. 1993; 36: 1175-1184.
- Stamler J, Vaccaro O, Neaton JD, Wentworth D. Diabetes, other risk factors, and 12-yr cardiovascular mortality for men screened in the Multiple Risk Factor Intervention Trial. Diabetes Care. 1993; 16: 434-444.
- McLenachan JM, Williams JK, Fish RD, Ganz P, Selwyn AP. Loss of flow-mediated endothelium-dependent dilation occurs early in the development of atherosclerosis. Circulation. 1991; 84: 1273-1278.
- Feener EP, King GL. Vascular dysfunction in diabetes mellitus. Lancet. 1997; 350: I9-13.
- Chowienczyk PJ, Watts GF. Endothelial dysfunction, insulin resistance and non-insulin dependent diabetes. Endocrinol Metab. 1997; 4: 225-232.
- Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest. 2005; 115: 1111-1119.
- Antoniades C, Tousoulis D, Tountas C, Tentolouris C, Toutouza M, Vasiliadou C, et al. Vascular endothelium and inflammatory process, in patients with combined Type 2 diabetes mellitus and coronary atherosclerosis: the effects of vitamin C. Diabet Med. 2004; 21: 552-558.
- Gonzalez MA, Selwyn AP. Endothelial function, inflammation, and prognosis in cardiovascular disease. Am J Med. 2003; 115: 99-106.
- Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999; 340: 115-126.
- Bastard JP, Maachi M, Lagathu C, Kim MJ, Caron M, Vidal H, et al. Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur Cytokine Netw. 2006; 17: 4-12.
- Paquot N, Tappy L. [Adipocytokines: link between obesity, type 2 diabetes and atherosclerosis]. Rev Med Liege. 2005; 60: 369-373.
- Nishida M, Funahashi T, Shimomura I. Pathophysiological significance of adiponectin. Med Mol Morphol. 2007; 40: 55-67.
- Bastard JP, Maachi M, Lagathu C, Kim MJ, Caron M, Vidal H, et al. Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur Cytokine Netw. 2006; 17: 4-12.
- Menzaghi C, Ercolino T, Di Paola R, Berg AH, Warram JH, Scherer PE, et al. A haplotype at the adiponectin locus is associated with obesity and other features of the insulin resistance syndrome. Diabetes. 2002; 51: 2306-2312.
- Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. National Diabetes Data Group. Diabetes. 1979; 28: 1039-1057.
- Tousoulis D, Antoniades C, Stefanadis C. Evaluating endothelial function in humans: a guide to invasive and non-invasive techniques. Heart. 2005; 91: 553-558.
- Tousoulis D, Antoniades C, Tountas C, Bosinakou E, Kotsopoulou M, Toutouzas P, et al. Vitamin C affects thrombosis/ fibrinolysis system and reactive hyperemia in patients with type 2 diabetes and coronary artery disease. Diabetes Care. 2003; 26: 2749-2753.
- Behrendt D, Ganz P. Endothelial function. From vascular biology to clinical applications. Am J Cardiol. 2002; 90: 40-48.
- Kannel WB, McGee DL. Diabetes and cardiovascular disease. The Framingham study. JAMA. 1979; 241: 2035-2038.
- Stamler J, Vaccaro O, Neaton JD, Wentworth D. Diabetes, other risk factors, and 12-yr cardiovascular mortality for men screened in the Multiple Risk Factor Intervention Trial. Diabetes Care. 1993; 16: 434-444.
- McLenachan JM, Williams JK, Fish RD, Ganz P, Selwyn AP. Loss of flow-mediated endothelium-dependent dilation occurs early in the development of atherosclerosis. Circulation. 1991; 84: 1273-1278.
- Gerstein HC. Glucose: a continuous risk factor for cardiovascular disease. Diabet Med. 1997; 14: 25-31.
- Balkau B, Shipley M, Jarrett JR, Pyörälä K, Pyörälä M, Forhan A, et al. High blood glucose concentration is a risk factor for mortality in middle-aged nondiabetic men: 20-year follow-up in the Whitehall Study, the Paris Prospective Study and the Helsinki Policemen Study. Diabetes Care. 1998; 21: 360-367.
- Vlassara H, Palace MR. Diabetes and advanced glycation endproducts. J Intern Med. 2002; 251: 87-101.
- Title L, Cummings P, Giddens K, Nassar B. Oral glucose loading acutely attenuates endothelium-dependent vasodilation in healthy adults without diabetes: An effect prevented by vitamins C and E. J Am Coll Cardiol. 2000; 36: 2185-2191.
- Kawano H, Motoyama T, Hirashima O, Hirai N, Miyao Y, Sakamoto T, et al. Hyperglycemia rapidly suppresses flow-mediated endothelium-dependent vasodilation of brachial artery. J Am Coll Cardiol. 1999; 34: 146-154.
- Williams SB, Goldfine AB, Timimi FK, Ting HH, Roddy MA, Simonson DC, et al. Acute hyperglycemia attenuates endothelium-dependent vasodilation in humans in vivo. Circulation. 1998; 97: 1695-1701.
- Akbari CM, Saouaf R, Barnhill DF, Newman PA, LoGerfo FW, Veves A. Endothelium-dependent vasodilatation is impaired in both microcirculation and macrocirculation during acute hyperglycemia. J Vasc Surg. 1998; 28: 687-694.
- Kim SH, Park KW, Kim YS, Oh S, Chae IH, Kim HS, et al. Effects of acute hyperglycemia on endothelium-dependent vasodilation in patients with diabetes mellitus or impaired glucose metabolism. Endothelium. 2003; 10: 65-70.
- Goldstein BJ. Insulin resistance as the core defect in type 2 diabetes mellitus. Am J Cardiol. 2002; 90: 3-10.
- Shuldiner AR, Yang R, Gong DW. Resist in, obesity and insulin resistance--the emerging role of the adipocyte as an endocrine organ. N Engl J Med. 2001; 345: 1345-1346.
- Antoniades C, Antonopoulos AS, Tousoulis D, Stefanadis C. Adiponectin: from obesity to cardiovascular disease. Obes Rev. 2009; 10: 269-279.
- Bastard JP, Maachi M, Lagathu C, Kim MJ, Caron M, Vidal H, et al. Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur Cytokine Netw. 2006; 17: 4-12.
- Chen H, Montagnani M, Funahashi T, Shimomura I, Quon MJ. Adiponectin stimulates production of nitric oxide in vascular endothelial cells. J Biol Chem. 2003; 278: 45021-45026.
- Alberti L, Gilardini L, Girola A, Moro M, Cavagnini F, Invitti C. Adiponectin receptors gene expression in lymphocytes of obese and anorexic patients. Diabetes Obes Metab. 2007; 9: 344-349.
- Beltowski J, towski J, Jamroz Wisniewska A, Widomska S. Adiponectin and its role in cardiovascular diseases. Cardiovasc Hematol Disord Drug Targets. 2008; 8: 7-46.
- Szosland K, Lewandowski K, Randeva H, Lewinski A. Adiponectin and resistin concentrations during oral glucose tolerance test (OGTT) in insulin-resistant subjects. Endocrine abstracts. 2006; 11: 219.
- Blümer RM, van der Crabben SN, Stegenga ME, Tanck MW, Ackermans MT, Endert E, et al. Hyperglycemia prevents the suppressive effect of hyperinsulinemia on plasma adiponectin levels in healthy humans. Am J Physiol Endocrinol Metab. 2008; 295: 613-617.