Review Article
J Dis Markers. 2014;1(2): 1009.
Establishment of a Two Coronary Artery System in Anomalous Origin of Left Coronary Artery from Pulmonary Artery
Nguyen Luong Tan*, Thai Viet Tuan, Doan Duc Hoang, Nguyen Xuan Hung, Nguyen Thi Le and Bui Duc Phu
Cardiovascular and Thoracic Surgery Department, Hue Central Hospital, Vietnam
*Corresponding author: Nguyen Luong Tan, Cardiovascular and Thoracic Surgery Department, Hue Central Hospital, 6/72 Le Ngo Cat Street, Hue, Vietnam
Received: August 11, 2014; Accepted: August 30, 2014; Published: September 02, 2014
Abstract
Anomalous origin of the left coronary artery arising from the pulmonary artery is a relatively rare disease. Within 2 years, from August 2011 to June 2013, Cardiovascular Centre of Hue Central Hospital had received and treated 5 cases of this disease. The patients were from 4 weeks to 17 months old (median 4 months), and had an average weight of 6 kg (range 3-10 kg). All 5 cases were accurately diagnosed by echocardiography and operated successfully with reconstruction of left main coronary artery. Troponin T was used as a marker to identify the myocardial infarction after surgery. All patients had been supported with interlope after cardiac repair and in the postoperative period, the supporting time varied from several days to several weeks, depending on the hemodynamic and cardiac function, but none of the patients required postoperative extracorporeal membrane oxygenation or died. Ejection fraction returned to normal values in 4 patients after 3 months; however, in one patient with preoperative ejection fraction decreased to 18%, this figure was almost unimproved during 12 months of follow-up (20%). Early diagnosis and early surgery is to avoid chronic heart failure, requiring the surgeon to choose the most appropriate technique for each patient.
Introduction
Anomalous origin of Left Coronary Artery from Pulmonary Artery (ALCAPA) has an anomaly of coronary blood supply for the heart. The left coronary artery is connected to a low blood pressure of pulmonary artery and receives blood flow from the right coronary artery by collateral vessels. This abnormal coronary anatomy leads to blood steal syndrome of the left coronary artery to the pulmonary artery. This coronary-pulmonary artery steal syndrome may cause congenital myocardial ischemia at a very young age, consequently a left heart failure and further lesions such as left ventricular dilation, and severe mitral valve regurgitation [1,2]. Newborns are usually asymyomatic until physiologic pulmonary hypertension period ended, resulting in lower left coronary pressure. Natural evolution of this anomaly is frequently fatal with a mortality of 90% by 1 year of age [3]. The diagnostic of this anomaly is usually at the late stage of congestive heart failure, therefore the treatment becomes more difficult and complicated, the prognostic is poor in comparison with the repair at early stage when the heart is only at the onset of ventricular dysfunction without the consequences of myocardial infarction.
One of the traditional surgical repairs is ligation of the anomalous origin of the left coronary artery in order to avoid the steal phenomenon. However without enough collaterals between the two coronary systems. This technique may lead to a severe myocardial ischemia, high rate post operated morbidity and mortality [4]. Dual coronary system repair restores physiological ante grade flow to the ischemic left ventricular myocardium, which has improved surgical outcome and is currently the ideal treatment for this anomaly [5].
The origin of anomalous left coronary artery locates at different sinuses or high on the trunk of pulmonary artery; surgical techniques have to vary in every single individual anatomic form in order to reimplant the left coronary artery to the aortic root [6].
In addition, severe mitral valve regurgitation need to be repaired; although some surgeons hope this concomitant lesion will disappear after the coronary reimplantation. Because of severe conditions of the heart before surgery, after a cardiopulmonary bypass and cardioplegic period, early results are not easily predicted. Therefore, most ALCAPA repairs need an Extracorporeal Membrane Oxygenation (ECMO) reserved for an acute post operated heart failure.
Patients and Methods
Clinical history and operative technique
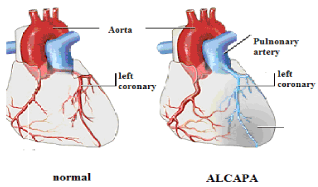
Between 8/2011 and 6/2013, 5 children were diagnosed with ALCAPA and referred for surgical repair. All of these patients delayed weight gain. Analysis of ECG showed myocardial ischemia with pathologic large and deep Q waves, elevated ST segment and inverted T waves in leads DI, AVL and V4-6. Preoperative diagnosis was established by Doppler echocardiography. 4 patients with left main coronary artery orifice come from the left sinus of the pulmonary artery root and 1 patient had left coronary artery orifice originating from the posterior sinus of pulmonary artery root. Right coronary artery is larger than normal and reflux blood flow from the left coronary artery into the pulmonary artery (Figure 1).
Figure 1: ALCAPA anatom y (umm.edu).
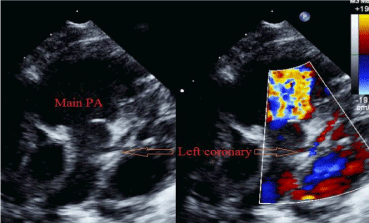
Reduced ejection fraction of the left ventricle occurred in 4 patients (18%, 28%, 32%, and 45%) and 1 patient had normal cardiac function with cardiac support drugs. Dilated left ventricle with increased left ventricular end-diastolic dimension was present in all patients (Figure 2).
Figure 2: Reflux blood flow from left coronary artery into pulmonary artery.
Mitral valvular regurgitation was graded moderate in 2 patients, severe in 3 patients, 1 among them had ischemic fibrosis papillary muscle of mitral valve with severe dilated left ventricle (diastolic diameter = 50mm, Zscore=6.93).
All patients were repaired ALCAPA in semi-urgent when hemodynamic and general conditions were stabilized.
Surgical techniques
Patients were on dorsal decubitis position, under general anesthesia. All patients had median sternotomy, Cardio Pulmonary Bypass (CPB) with bicaval cannulation. Left ventricular venting was through interatrial septum and moderate hypothermia (28°). Myocardial protection was achieved with blood cardioplegia, delivered simultaneously into the aortic root and pulmonary artery, and followed by intermittent retrograde cardioplegia into coronary sinus.
Reconstruction of the left coronary artery
One newborn patients, who had normalized ejection fraction under inotrope and high retrograde flow from left coronary artery to pulmonary artery, was legated the origin of the left coronary artery. An acute myocardial ischemia with increased troponin T up to 5 μg/L happened on the first postoperative 24 hours. The ALCAPA was urgently revacularized by an saphenous vein bypass, from ascending aorta to left anterior descending coronary. This patient recovered well and was discharge after 30 days in ICU.
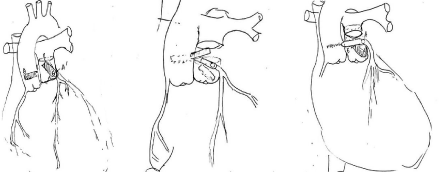
Four other patients had their left coronary artery reimplanted to the aortic root (Figure 3).
Figure 3: Repair of ALCAPA.
One patient, who had the anomalous left coronary ostium in the posterior-facing sinus of pulmonary artery was translocated the coronary button to the aortic root and repaired the pulmonary defect by an autologous pericardial patch.
Three other patients who had the anomalous left coronary ostium in the left sinus of pulmonary artery was reconstructed the left main coronary and anatomized into aortic root. The new ostium of the left coronary was identifying on the left wall of aortic root. Aorta was opened horizontally by two parallel lines about 3 mm apart from this site toward the posterior wall, so that a pedicle flap could be made to create the anterior wall of the new left main. Similarly, from the anomalous ostium of the left coronary artery, a flap was created by the tissue of the left sinus and posterior wall of the pulmonary artery for the haft anterior of the new left main. The length of each flap was estimated a haft of pulmonary artery circumference. Normally, the pulmonary needs to be temporarily transected in order to sew the two flaps of the new left main passed under pulmonary artery. The posterior opening of aorta was closed directly and pulmonary artery finally reconstructed with a pericardial patch.
Three severe mitral valve regurgitations were shortened the posterior wall by interrupted U stiches strengthened by an autologous pericardial strip.
All patients had ECMO been standby, in case CPB cannot wean due to low cardiac function.
Results
The mean cardiopulmonary bypass time was 71 minutes. The aortic cross clamp time varied from 32 to 105 minutes (mean=105 minutes).
All patients were successfully weaned from CPB after dual coronary repair without ECMO support, in operating room and in post operation. Inotrope were given to all patients in operating room and in ICU. Inotrope support lasted from several days to several weeks, depending on hemodynamic condition and cardiac function.
Three patients had temporary renal failure and needed a peritoneal dialysis in ICU. They recovered completely renal function without any complication of peritoneal cavity.
Echocardiography in ICU found one 4-week-old patient suspicious of an acute ischemia of myocardium during 24 hours after ligation of the origin of ALCAPA. This was confirmed by a high troponin T of 5 μg/Lwhile the normal value of this marker was under 0.08μg/L in our population. This patient immediately received a coronary artery bypass between ascending aorta and left anterior descending coronary artery by autologous saphenous vein graft. Hemodynamic and cardiac condition was improved after a second operation. The patient was extubated after 4 weeks of inotrope support.
Ejection fraction of left ventricle measured early after the repaired had not found any improvement in comparison with the one before surgery. Patients with very poor cardiac function before surgery might have temporarily lower EF immediately after the repair. On discharge from hospital, four patients had improved EF and normal EF at 3 months follow-up. However, the one with severe ischemia myocardium with fibro sic papillary muscle (EF=18% before surgery, 20% at 3 months).
Blood flow could be seen from ascending aorta to left coronary on their echocardiography without any steno sic of reconstructed left main coronary.
There were not any pulmonary artery stenoses.
All 5 patients improved mitral valve competence and are graded at trivial to mild regurgitation (Table).
Age
(months)
Weight
Kg
Preoperative
EF %
MR
repair technique
MV
repair
Postoperative
peritoneal
dialysis
3 months
EF %
case 1
6
6
28
severe
dual coronary repair
yes
yes
61
case 2
17
7
18
moderate
dual coronary repair
no
no
20
case 3
2
5
65
mild
ligation-bypass
no
yes
65
case 4
1
3
32
severe
dual coronary repair
yes
no
65
case 5
4
10
45
severe
coronary translocation
yes
no
65
Table 1: Outline of 5 operated patients. MR: Mitral valve Regurgitation; MV: Mitral Valve; EF: Ejection Fraction.
All 5 patients survived at 3 months follow-up. Hospital stay was from 12 to 72 days (mean=33 days).
Discussion
ALCAPA can be diagnosed very early in newborns before any irreversible myocardial ischemia occurs [2]. Most ALCAPA present a congestive heart failure, especially left heart failure is dominant. This anomaly must be differentiate from acute myocarditis and dilated cardiomyopathy by seeking the retrograde colored blood flow from left coronary artery into pulmonary artery.
Among 5 our patients, the oldest one was 17 months old who had severe heart failure with only 18% EF, important dilated left ventricle (diastolic left ventricle over 50 mm, Zscore=6.93), severe mitral valve regurgitation with fibrotic papillary muscle. Although this child survived after surgery but left ventricular function was only 20% at 3 months follow up. We suspect an irreversible heart failure on this patient. However Fratz et al [7] studying cardiac magnetic resonance including LV function analysis and late gadolinium enhancement magnetic resonance on 14 ALCAPA repairs have found that despite often severely compromised LV function and evidence of scarring be-fore corrective surgery of patients with ALCAPA, in long-term follow-up scar tissue is relatively scarce. Shivalkar et al [8] studied biopsy specimens taken from the region per fused by the anomalous artery showed a variable degree of fibrosis (51 +/- 32%). The ultra structure of the remaining myocytes revealed viable characteristics, but a substantial percent (46 +/- 26%) showed a markedly reduced fraction of contractile material. Their study suggest delayed sub cellular adaptive responses in the chronically hypoperfused myocardium of patients with ALCAPA syndrome.
There are many techniques to repair ALCAPA such as left coronary ligation [4], left coronary artery bypass, transposition of left coronary ostium with or without reconstruction of the left main [9-11]. Depending on the individual ALCAPA anatomy, the most suitable surgery is preferred. The best techniques must assure an enough, durable arterial blood flow for the left coronary artery. Direct reimplatation of the left coronary button to the ascending aorta is less complicated but in some ALCAPA which have origin from left sinus of pulmonary artery, there may be a tension on the anastomosis or the left main, especially during the acute heart failure episodes happening early after CPB, the reason of steno sis or occlusion of left coronary. Reconstruction of the left main avoids the tension on the left coronary artery, however this technique is very delicate and need to adjust the angle of the flaps so that the new left main has not torsion or kinking. The reconstruction of the aorta and pulmonary artery has to be very careful to prevent a bleeding from their posterior wall or steno sis.
One of the challenges of the ALCAPA repair is low cardiac output syndromes that need a mechanical support of Left Ventricular Assist Device (LVAD) or ECMO. Edwin et al [12] have found the prediction of an LVAD or ECMO is the fractional shortening and aortic cross clamp time. The fractional shortening and aortic cross clamp time together predict 80.9% of the variability in post repair left ventricular assist device implantation after repair of anomalous left coronary artery from the pulmonary artery. When preoperative left ventricular dysfunction is severe (fractional shortening < 20%), an aortic cross clamp time greater than 56 minutes is associated with a substantial risk of left ventricular assist device implantation after repair of ALCAPA. Our patients had not been implanted the circulatory support after their ALCAPA repair.
Conclusion
ALCAPA is rare congenital malformation and might confuse with other diseases. Early diagnosis and repair is necessary to prevent chronic heart failure and circulatory support installation after the repair. The surgeons have to choose the best technique for individual cardiac anatomy.
References
- Bakiler AR, Eliaclk K, Kose S, Atay Y. Anomalous origin of the left coronary artery from the pulmonary artery presenting as dilated cardiomyopathy. Turk Kardiyol Dern Ars. 2013; 41: 448-450.
- Bonnemains L, Lambert V, Moulin-Zinch A, Youssef D, Serraf A. Very early correction of anomalous left coronary artery from the pulmonary artery improves intensive care management. Arch Cardiovasc Dis. 2010; 103: 579-584.
- Amanullah MM, Hamilton JR, Hasan A. Anomalous left coronary artery from the pulmonary artery: creating an autogenous arterial conduit for aortic implantation. Eur J Cardiothorac Surg. 2001; 20: 853-855.
- Bunton R, Jonas RA, Lang P, Rein AJ, Castaneda AR. Anomalous origin of left coronary artery from pulmonary artery. Ligation versus establishment of a two coronary artery system. J Thorac Cardiovasc Surg. 1987; 93: 103-108.
- Vouhé PR, Tamisier D, Sidi D, Vernant F, Mauriat P, Pouard P, et al. Anomalous left coronary artery from the pulmonary artery: results of isolated aortic reimplantation. Ann Thorac Surg. 1992; 54: 621-626.
- Alsoufi B, Sallehuddin A, Bulbul Z, Joufan M, Khouqeer F, Canver CC, et al. Surgical strategy to establish a dual-coronary system for the management of anomalous left coronary artery origin from the pulmonary artery. Ann Thorac Surg. 2008; 86: 170-176.
- Fratz S, Hager A, Schreiber C, Schwaiger M, Hess J, Stern HC. Long-term myocardial scarring after operation for anomalous left coronary artery from the pulmonary artery. Ann Thorac Surg. 2011; 92: 1761-1765.
- Shivalkar B, Borgers M, Daenen W, Gewillig M, Flameng W. ALCAPA syndrome: an example of chronic myocardial hypoperfusion? J Am Coll Cardiol. 1994; 23: 772-778.
- El-Rassi I, Soueide A, Sweid O, Chabb B. Transfer technique of an anomalous coronary artery from the anterior pulmonary artery. Ann Thorac Surg. 2012; 93: 1738-1740.
- Barth MJ, Allen BS, Gulecyuz M, Chiemmongkoltip P, Cuneo B, Ilbawi MN. Experience with an alternative technique for the management of anomalous left coronary artery from the pulmonary artery. Ann Thorac Surg. 2003; 76: 1429-1434.
- Hoashi T, Kagisaki K, Okuda N, Shiraishi I, Yagihara T, Ichikawa H. Indication of Takeuchi technique for patients with anomalous origin of the left coronary artery from the pulmonary artery. Circ J. 2013; 77: 1202-1207.
- Edwin F, Kinsley RH, Quarshie A, Colsen PR. Prediction of left ventricular assist device implantation after repair of anomalous left coronary artery from the pulmonary artery. J Thorac Cardiovasc Surg. 2012; 144: 160-165.