
Research Article
J Dis Markers. 2014;1(2): 1010.
Red Cell Distribution Width as a Marker for Predicting In-Stent Rest Enosis after Percutaneous Coronary Intervention
Cheng-Gang Zhu1, Li-Feng Hong2, Xiao-Lin Li, Song-Hui Luo2, Yuan-Lin Guo1, Rui-Xia Xu1, Jing Sun1, Ping Qing1, Geng Liu1, Dong Qian1 and Jian- Jun Li1*
1Division of Dyslipidemia, Fuwai Hospital, China
2Department of Cardiology, Fifth Hospital of Wuhan City, China
*Corresponding author: Jian-Jun Li, MD, Division of Dyslipidemia, State Key Laboratory of Cardiovascular Disease, Fu Wai Hospital, National Center for Cardiovascular Disease, Chinese Academy of Medical Sciences and Peking Union Medical College, No 167 BeiLiShi Road, XiCheng District, Beijing, 100037, China
Received: July 18, 2014; Accepted: August 31,2014; Published: September 03, 2014
Abstract
Objective: Red Cell Distribution Width (RDW) has recently been considered as a predictor of a variety of cardiovascular diseases. However, no data is currently available with respect to the role of RDW in predicting In-Stent Rest enosis (ISR) after successful coronary stent implantation.
Methods: A cohort of 1733 consecutive patients was retrospectively enrolled who had a received coronary angiography follow-up at mean 7 months after stinting. Of them, 271 lesions were implanted with a Bare-Metal Stent (BMS) while 404 lesions with a Drug-Eluting Stent (DES). The relationship of admission RDW value and angiography-proven ISR at 7 months were evaluated.
Results: There were 106 patients with ISR (106/675, 15.7%; BMS, n=271, ISR=82, 30.3%; DES, n=404, ISR=24, 5.9%) and 569 patients without ISR at 7-month angiographic follow-up. Baseline RDW values were significantly higher in patients with ISR (RDW: 13.9±2.2 versus 12.5±2.0, p<0.001). In addition, the elevated RDW levels were found to be associated with ISR regardless of BMS or DES implantation. Moreover, there was a positive correlation between levels of RDW and CRP in patients with ISR (&ga= 0.661, p=0.000). Finally, in a Receiver Operating Characteristic (ROC) curve analysis, an RDW = 13.4% on admission had 71% sensitivity and 68% specificity in predicting the ISR after successful PCI.
Conclusion: The present study provides the first line of evidence for the use of RDW, an easy, inexpensive, routinely reported marker, as an independent predictor for ISR after successful coronary stent implantation.
Keywords: Red cell distribution width; Coronary artery disease; Stent; Instent rest enosis
Introduction
In-Stent Rest enosis (ISR) and Stent Thrombosis (ST) are major complications after Percutaneous Coronary Intervention (PCI) and coronary stent placement [1-3]. Compared with Bare-Metal Stent (BMS), Drug-Eluting Stent (DES) are associated with a further reduction in ISR and repeat revascularization with similar rates of death, reinfarction, and ST [4]. Nonetheless, repeat revascularization is still performed in ~5-7% of patients treated with primary PCI and DES, and angiographic rest enosis occurs ~15% of lesions, especially in patients with ST-segment elevation myocardial infarction [5]. Besides technical factors, the other status of an individual is strong predictor of the risk of ISR. Therefore, identifying biomarkers and studying their differential diagnostic values are critical for a more efficient personalized intervention, patient management, and adverse effects reduction [6].
Red Blood Cell Distribution Width (RWD) is a measure of the variability in the size of circulating erythrocytes and it has been utilized in the differential diagnosis of anemia [7]. More recently, elevated RDW was reported to be a marker and independent predictor in a variety of cardiovascular diseases including acute and Table Coronary Artery Disease (CAD) [8,9], heart failure [10], peripheral vascular disease [11], stroke [12], slow coronary flow [13], cardiac syndrome X [14], even the thrombosis and bleeding after successfully Percutaneous Coronary Intervention (PCI) [15,16]. However, the study on role of RDW in patients ISR after PCI has currently not been investigated. Additionally, although the higher RDW level has been established by previous studies in several cardiovascular diseases, the underlying mechanism has not clearly been elucidated yet. It has been postulated that RDW may be a marker of underlying inflammation, malnutrition, older age, and underlying renal dysfunction predisposing patients with higher baseline RDW to increased morbidity and mortality [17]. ISR is not rare complication in patients undergoing PCI even in the era of drugeluting stent and patients who ISR tend to be similar characteristics to those with higher RDW values such as inflammation, older age and renal insufficiency [1]. Thus, we hypothesized that baseline RDW values might be a predictor of ISR after successful PCI.
In the present study, therefore, we retrospectively angiographic ally evaluate admission RDW level and its relations to ISR after successful PCI in patients. To exclude the stent types also influence the effects of RDW on predicting the ISR, we specially enrolled the all patients who have received either BMS or DES implantation.
Methods
Study population
The study population consisted of 675 consecutive patients who underwent PCI between April 2004 to December 2005 in our single center. These patients were selected from 5144 patients according to our inclusion and exclusion criteria. The study complied with the Declaration of Helsinki. The study protocol was approved by the Fuwai hospital ethnic's committee review board. Informed written consent was obtained from all patients included in this analysis.
The patients who fulfilled inclusion criteria were enrolled in the study: (1) patients who received no more than one stent implantation regardless of BMS or DES in a pericardial coronary artery including Left Main artery (LM) or Left Anterior Descending (LAD) or Left Cyclotron Branch (LCX) or Right Coronary Artery (RCA) or their major branches; (2) patients who had angiographic follow-up at mean 7 months with a range 6-9 months after the stent implantation due to a variety of indications; (3) patients who had entire demographic, clinical angiographic, and stenting-related data. Primary angioplasty and/or stent implantation more than one arteries and/or lesions were not included. Exclusion criteria were acute myocardial infarction, renal dysfunction, left ventricular ejection fraction <45%. All patients received an optimal secondary prevention treatment after stent implantation according to currently available guidelines.
In addition, all subjects enrolled in this study had normal hepatic function. The hyperlipidemia was defined as low-density lipoprotein cholesterol 160mg/dl and/or Triglyceride (TG) =200 mg/dl. Patients with history or evidence of valvular heart disease, congestive heart failure, echocardiographic ally proven left ventricular hypertrophy, a history of dysphasia, swallowing as well as intestinal motility disorders, untreated thyroid disease, sinus node dysfunction or conduction disturbance, estrogen replacement therapy, carcinoma, poorly controlled hypertension (systolic blood pressure >160 mmHg or diastolic blood pressure >105 mmHg), recent major operation (<3 months), autoimmune disease were also excluded from the study.
Finally, patients who have had previous history of anemia, have received previous red blood cell transfusion or were on treatment for anemia, such as supplemental iron, foliate or an erythropoiesisstimulating agent were not included in this study. Patients with known hematological disease such as hemolytic anemia, neoplastic metastases to the bone marrow, iron replacement therapy that could increase plasma RDW levels were excluded. Patients who were lost to follow-up were also excluded.
Determination of RDW
Hemoglobin, RDW, and White Blood Cell (WBC) counts were determined by the automated hematology analyzer XE-1200 (Sysmex, Kobe, Japan) before PCI. The normal range of RDW (%) in our laboratory was 10-15% [13,14]. The other biochemical measurements were performed using a molecular analyzer (Roche Diagnostics, Manheim, Germany).
Stent implantation
Coronary stent implantations were performed by ten principle operators affiliated to our center, and indications were according to the operator deemed appropriate at the time. There was no strict protocol on how or which intervention list should perform the procedure, and there was no restriction on the choice or kind or the numbers of stents deployed. Routine use of intravascular ultrasound was deemed unnecessary unless there was a specific indication. Balloon pre-dilatation was performed followed by stent implantation using conventional technique for almost all of our patients. Postdilatation was used only if the primary angiographic result was not satisfactory. Pre-procedural intravenous heparin was given to maintain an activated clotting time =250 seconds, and all patients received aspirin (at least 75 mg) and clopidogrel (300 mg loading dose followed by 75 mg once daily for at least 12 months).
Angiographic analysis and ISR definition
The cineangiography on all eligible patients were reviewed and analyzed by two independent experienced interventional cardiologists. Quantitative Coronary Angiographic (QCA) analysis was performed on all patients in pre-percutaneous transluminal coronary stent and immediate post-stent as well as angiographic follow-up at approximately 7 months.
ISR was defined as >50% diameter steno sis by QCA within a previously stinted vessel segment, and was classified as focal (<10mm long), diffuse (>10mm long), proliferative (>10mm long and extending outside the stent edges), or totally occluded according to previously reported [5]. Two coronary segments (in-stent and in-segment) were subjected to QCA. The in-stent analysis comprised only the segment of the lesion encompassing the stent. The in-segment was defined as the in-stent segment plus segments 5 mm proximal and distal to the edge of the stent. Minimal Luminal Diameter (MLD) and percentage of diameter steno sis were measured for each segment. In-segment and in-stent late Lumen Loss (LL) was calculated as post-procedure MLD minus follow-up MLD. ISR was classified according to a modified Mehran classification [10].
Statistical analysis
Categorical variables were expressed as percent frequencies and continuous variables as mean±SD. χ2 or Fisher test was used to compare categorical variables; Student t test was performed for comparison of continuous variables. The univariate and multivariate analysis was used for baseline clinical characteristics, serum markers, and RDW. Association between RDW and CRP was test using Spearman correlation coefficient. Receiver Operating Characteristic (ROC) curve analysis was performed to define the optimal CADspecific cut-off points. A P value <0.05 was considered statistically significant.
Results
Baseline clinical characteristics
Between the periods of the study, a total of 5144 patients had a successful PCI, while 675 consecutive patients fulfilled our enrolled criteria. There were 106 patients with ISR (106/675, 15.7%) including 82 patients with a BMS implantation from a total of 271 patients (82/271, 30.3%) and 24 with a DES from a total of 404 (24/404, 5.9%) and 569 patients without ISR at 7-month angiographic follow-up.
Baseline clinical characteristics of patients with or without ISR were showed in Table 1. There was a predominance of males (87.0%). The average age was 57.2±10.8 years with a range of 34-82 years. More than half of them had at least one of risk factors for CAD. 514 patients (514/675, 76.1%) of them were diagnosed as sTable angina. Most baseline clinical characteristics were not significantly different, except for the age and rate of prior myocardial infarction, current smoker, and diabetes mellitus. In addition, there were no significantly differences in previous medications except for the lower percentage of calcium channel blocker administration in patients with ISR.
All (N=675)
ISR (n=106)
No-ISR
(n=569)
P value
Age (years)
57.2±10.8
58.3±11.2
56.7±10.5
0.04
Male, n (%)
587 (87.0)
91 (85.8)
496 (87.2)
0.49
BMI (kg/m2)
24.7±10.8
24.7±11.2
24.7±10.4
0.57
LVEF (%)
58±13
57±12
58±13
0.69
Prior MI, n (%)
317 (47.0)
59 (55.7)
258 (45.3)
0.02
Prior PCI, n (%)
155 (23.0)
27 (25.5)
128 (22.5)
0.38
Prior CABG, n (%)
24 (3.6)
3 (2.8)
21 (3.7)
0.13
Diabetes mellitus, n (%)
138 (20.4)
34 (32.1)
104 (18.3)
0.01
Hypertension, n (%)
375 (55.6)
55 (51.9)
320 (56.2)
0.25
Hyperlipidemia, n (%)
237 (35.1)
42 (39.6)
195 (34.3)
0.17
CAD family history, n (%)
29 (4.3)
4 (3.8)
25 (4.4)
0.38
Stroke history, n (%)
15 (2.2)
2 (1.9)
13 (2.3)
0.34
Smoking
Current smoker, n (%)
259 (38.4)
54 (50.9)
205 (36.0)
0.01
Former smoker, n (%)
113 (16.7)
18 (17.0)
95 (16.7)
0.71
Non-smoker, n (%)
303 (44.9)
48 (45.3)
255 (44.8)
0.92
Angina pectoris
0.36
Stable angina, n (%)
514 (76.1)
80 (75.5)
434 (76.3)
0.85
Unstable angina, n (%)
127 (18.8)
22 (20.8)
105 (18.5)
0.62
Previous Medications
Aspirin, n (%)
221 (32.7)
30 (28.3)
191 (33.6)
0.57
Beta-blocker, n (%)
72 (10.1)
15 (14.2)
57 (10.0)
0.60
CCB, n (%)
49 (7.3)
2 (1.9)
47 (8.3)
0.03
ACEI/ARB, n (%)
145 (21.5)
28 (26.4)
117 (20.6)
0.12
statins, n (%)
109 (16.2)
21 (19.8)
88 (15.5)
0.45
ISR: In-Stent Rest enosis; BMI: Body Mass Index; LVEF: Left Ventricular Ejection Farction; MI: Myocardial Infarction; PCI: Percutaneous Coronary Intervention; CABG: Coronary Artery Bypass Graft; CAD: Coronary Artery Disease; CCB: Calcium Channel Blocker; ACEI/ARB: Angiotensin Converting Enzymes Inhibitor/Angiotensin Receptor Blocker
Table 1: Baseline Clinical Characteristics.
Laboratory findings
Laboratory data of patients with or without ISR were showed in Table 2. As showed in Table 2, there were no differences between the two groups regarding lipid profile, erythrocyte count, hemoglobin, mean corpuscular volume, uric acid, creatinine, and white cell count levels. However, the levels of RDW were significantly higher in patients with ISR than that in patients without ISR (13.9±2.2 vs. 12.5±2.0, p=0.00). In addition, the levels of CRP were also higher in patients with ISR compared with that without ISR (0.27±0.15 mg/L vs. 0.23±0.11 mg/L p=0.00). There were also higher levels of serum hemoglobin A1C in patients with ISR compared with that in patients without ISR.
All (N=675)
ISR (n=106)
No-ISR
(n=569)
P value
Total cholesterol (mg/dl)
4.2±1.1
4.3±1.3
4.2±1.0
0.06
LDL-C (mg/dl)
2.5±0.9
2.6±1.0
2.5±0.8
0.12
HDL-C (mg/dl)
1.1±0.3
1.0±0.4
1.1±0.3
0.53
Triglyceride (mg/dl)
4.2±1.1
4.3±1.2
4.2±1.1
0.10
Hs-CRP (mg/L)
2.5±1.3
2.7±1.5
2.3±1.1
0.04
Hemoglobin A1c (%)
6.6± 0.9
7.1±1.0
6.3± 0.5
0.00
Creatinine (mg/dl)
76.8±16
76.9±15
76.2±16
0.18
Uric acid (mg/dl)
335.2±64
339.0±71
334.7±63
0.52
Hemoglobin (g/dl)
138.3±15
136.3±17
137.9±14
0.66
Platelet (mm3)
207.5±53
207.7±53
207.4±54
0.83
Mean platelet volume (fl)
10.5±0.9
10.4±1.0
10.5±1.1
0.90
WBC (109/L)
6.5±1.5
6.9±0.9
6.4±0.8
0.07
RDW
13.1±2.1
13.9±2.2
12.5±2.0
0.00
ISR: In-Stent Restenosis; LDL-C: Low-Density Lipoprotein Cholesterol; HDL-C: High-Density Lipoprotein Cholesterol; Hs-CRP: High-Sensitivity C-Reactive Protein; Hemoglobin A1c: Hemoglobin A1c; WBC: White Cell Count; RDW: Red Cell Distribution Width
Table 2: Laboratory findings and previous medications.
Procedure characteristics
As showed in Table 3, under our condition, the data showed that angulated lesion, total occlusion, lesion length, stent types, and postprocedural Minimal Luminal Diameter (MLD) used were higher in patients with ISR compared with that without ISR.
All (N=675)
ISR (n=106)
No-ISR
(n=569)
P value
Target vessel
LMT, n (%)
20 (3.0)
2 (1.9)
18 (3.2)
0.77
LAD, n (%)
297 (44.1)
45 (42.4)
252 (44.3)
0.60
LCX, n (%)
145 (21.4)
18 (17.0)
127 (22.3)
0.82
RCA, n (%)
213 (30.5)
41 (38.7)
172 (30.2)
0.23
Lesion type
A, n (%)
80 (11.9)
13 (12.2)
67 (11.8)
0.91
B1, n (%)
115 (17.0)
15 (14.2)
100 (17.6)
0.88
B2, n (%)
346 (51.2)
54 (50.9)
292 (51.3)
0.94
C, n (%)
134 (19.9)
24 (22.7)
110 (19.3)
0.65
Lesion morphology
Angulated, n (%)
152 (22.5)
52 (49.1)
100 (17.8)
0.00
Total occlusion, n (%)
56 (8.3)
27 (25.5)
29 (5.1)
0.00
Ostial lesion, n (%)
67 (9.9)
19 (17.9)
51 (9.0)
0.03
Stent
BMS
271 (40.2)
82 (77.4)
189 (32.2)
0.00
SES
340 (53.3)
18 (17.0)
322 (56.6)
0.00
PES
64 (6.5)
6 (5.6)
58 (10.2)
0.03
Diameter (mm)
3.00 ± 0.34
2.94 ± 0.31
3.03 ± 0.36
0.13
Length (mm)
26.23 ± 13.14
27.02± 13.05
26.17 ± 14.11
0.02
Max Deployment
Pressure (atm)
14.44 ± 2.55
14.47± 2.18
14.07± 3.14
0.82
Post-dilatation, n (%)
207 (30.1)
30 (28.3)
177 (31.1)
0.78
Post-PCI MLD (mm)
2.58 ± 0.34
2.44 ±0.31
2.63 ± 0.34
0.00
TIMI III flow, n (%)
657 (97.3)
102 (96.2)
545 (95.8)
0.21
ISR: In-Stent Restenosis; LMT: Left Main Truck; LAD: Left Anterior Descending; LCX: Left Circumflex Artery; RCA: Right Coronary Artery; BMS: Bare-Metal Stent; SES: Sirolimus-Eluting Stent; PES: Paclitaxel-Eluting Stent; TIMI: Thrombolysis In Myocardial Infarction; PCI: Percutaneous Trnasluminal Intervention; MLD: Minimal Luminal Diameter
Table 3: Procedure-related characteristics.
Differences of main variables in ISR patients with stents
To identify the impact of stent types on ISR, we examined the difference of major variables in patients with between BMS and DES. As showed in Table 4, our data showed that there were significantly differences between ISR and non-ISR group in the percentage of diabetes mellitus and the levels of HA1C and RDW regardless of patients who have received either BMS or DES implantation. However, ISR patients who have received BMS were older, longer stent length and hs-CRP levels compared with on-ISR, while patients with DES implantation had more angulated lesions (Table 4).
BMS
Non-ISR (n=189)
ISR
(n=82)
P value
DES
Non-ISR (n=380)
ISR (n=24)
P value
Age
56.73±10.1
59.2±12.4
0.01
56.3±10.3
57.6±11.1
0.17
DM, n (%)
16 (8.5)
18 (19.5)
0.00
89 (23.4)
15 (62.5)
0.00
Smoking, n (%)
40 (21.2)
14 (17.1)
0.61
193 (50.1)
12 (50.0)
0.87
Hs-CRP (mg/L)
2.2±1.2
2.9±1.8
0.00
2.4±1.4
2.5±1.6
0.15
HA1c (%)
6.8±0.8
7.3±1.0
0.03
6.0± 0.8
7.2±1.1
0.00
HB (g/dl)
136.0±17
135.9±14
0.92
137.0±15
136.7±15
0.70
WBC (109/L)
6.7±0.8
6.8±0.9
0.68
6.7±0.8
6.5±0.8
0.20
RDW
12.5±2.2
13.8±2.2
0.00
12.5±2.0
14.1±2.2
0.00
Angulated, n (%)
34 (17.9)
23 (28.1)
0.24
84 (22.1)
11 (45.8)
0.01
Stent length (mm)
24.1± 11.4
28.2± 13.3
0.00
26.9± 14.4
27.3± 13.1
0.88
ISR: In-Stent Restenosis; DM: Diabetes Mellitus; Hs-CRP: High-Sensitivity C-Reactive Protein; HA1c: Hemoglobin A1c; HB: Hemoglobin; WBC: White Cell Count; RDW: Red Cell Distribution Width
Table 4: Comparison of major variables in ISR patients with stents.
Univariate and multivariate logistic analysis
A total of 14 variables associated with ISR including age, prior myocardial infarction, diabetes mellitus smoking, hs-CRP, hemoglobin A1c, white cell count, RDW, BMS, PES, ostial lesion, total occlusion, stent length, and post-PCI MLD with p<0.05 in univariate analysis are presented in Table 3. And then, we put those 14 variables into the multivariate analysis. Not surprisingly and Interestingly, we found that diabetes mellitus, hs-CRP, RDW and BMS were the independent variables most strongly associated with ISR (diabetes mellitus: odds ratio 1.77, 95% confidence interval 1.21 to 3.50, p=0.031; hs-CRP: odds ratio 1.92, 95% confidence interval 1.17 to 3.07, p=0.004; RDW: odds ratio 2.01, 95% confidence interval 1.74 to 4.22, p=0.000; BMS: odds ratio 2.88, 95% confidence interval 2.13 to 6.04, p=0.000; Table 5).
Univirate OR
95%CI
P value
Multivariate
OR
95% CI
P value
Age
1.03
0.62-1.06
0.42
-
-
-
Prior MI
0.82
0.37-2.20
0.62
-
-
-
DM
1.21
1.09-3.23
0.00
1.77
1.21-3.50
0.031
Smoker
1.23
0.62-3.02
0.71
-
-
-
Hs-CRP
1.05
1.08-2.51
0.00
1.52
1.17-3.07
0.004
HemoglobinA1c
0.72
0.25-1.90
0.53
-
-
-
WBC
1.04
0.28-2.33
0.37
-
-
-
RDW
1.81
1.24-2.30
0.00
2.01
1.74-4.22
0.000
BMS
2.50
1.78-4.47
0.00
2.88
2.13-6.04
0.000
PES
1.09
0.28-2.05
0.82
-
-
-
Ostial lesion
0.91
0.47-2.11
0.63
-
-
-
Occlusion
0.89
0.52-1.83
0.59
-
-
-
Stent length
1.22
1.04-1.54
0.02
-
-
-
Post-PCI MLD
0.82
0.42-2.04
0.64
-
-
-
CI: Confidence Interval; MI: Myocardial Infarction; DM: Diabetes Mellitus; Hs-CRP: High-Sensitivity C-Reactive Protein; WBC: White Cell Count; RDW: Red Cell Distribution Width; BMS: Bare-Metal Stent; PES: Paclitaxel-Eluting Stent; PCI: Percutaneous Trnasluminal Intervention; MLD: Minimal Luminal Diameter
Table 5: Impacts of multiple variables on the ISR in univariate and multivariate logistic analysis.
Correlations of RDW with hs-CRP in patients with ISR
We also examined the correlation between the levels of RDW and hs-CRP in patients with ISR (n=106). As shown in figure 1, there was marked positive correlation between levels of RDW and hs-CRP in patients with ISR (γ=0.661, p=0.000).
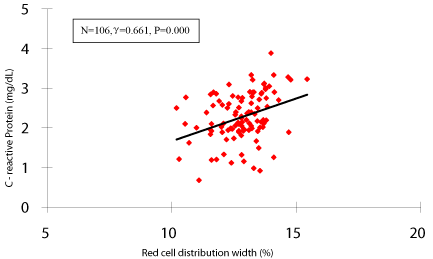
Figure 1: Correlation between admission RDW levels and hs-CRP values in
patients with ISR (n=106, RDW=Red Cell Distribution Width; hs-CRP=high
sensitivity C - reactive protein; ISR=In-Stent Restenosis).
ROC analysis
Finally, in a ROC curve analysis with Area Under Curve (AUC)= 0.67, 95%CI: 0.63-0.71, we found that an RDW value of 13.4% was used as an effective cut-point in the segregation of the presence or absence of ISR, a sensitivity of 71% and a specificity of 68% were obtained ( figure 2).
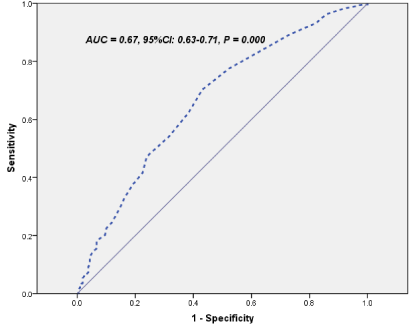
Figure 2: The Receiver-Operating Characteristics (ROC) curve of RDW
predicting ISR (ISR=In-Stent Rest enosis).
Discussion
The major findings of the present study, for the first time, are that (1) the RDW levels were significantly higher in patients with ISR regardless of stent used; (2) the baseline RDW level was an independent predictor for the future ISR; (3) In a ROC curve analysis, a RDW value of 13.4% was identified as an effective cut-point in the segregation of the follow-up ISR with a sensitivity of 71% and a specificity of 68%; (4) there was a positive correlation between levels of RDW and CRP in patients with ISR. These data, therefore, provided novel information regarding the link of RDW to ISR after successfully coronary stent implantation.
Coronary stent implantation has almost replaced plain old balloon angioplasty as a revascularization method given its much lower rate of need for revascularization and its lower need to emergency coronary artery bypass graft surgery [18]. Although coronary stenting is known for its few adverse reactions than plain old balloon angioplasty, ISR and ST remain among its most serious complications [3]. Although the ISR after stent implantation is caused by multifactorial process, including neointima formation, smooth muscle proliferation, inflammation, and thrombus, previous studies has suggested individual response to stent placement are relevant factors in ISR and ST apart from technical and mechanical factors related to stent implantation [1,19,20]. Therefore, the early detection of a subgroup of patients at high risk to develop ISR can be important to target specific pharmacologic or alternative therapies to reduce the risk of ISR [6].
Previous studies have demonstrated that inflammation response plays a critical role in the pathogenesis of ISR [1,21]. A number of inflammatory biomarkers have been associated with risks and clinical outcomes of coronary stent implantation. Of these, CRP is the best studied [6]. Our previous observation has also showed that the levels of pre-PCI hs-CRP were strongly associated with ISR after successfully coronary stent placement, whereas diabetes, and lipid profile were significantly correlated with coronary lesion progression [22,23]. It has also been reported that inflammatory status, as assessed by hs-CRP levels, predicts the risk of ISR after BMS implantation, although it does not predict the risk of ST. Conversely, hs-CRP levels fail to predict the risk of ISR after DES implantation, although they appear to predict the risk ST [1]. In our present study, we found that baseline hs-CRP levels were significantly higher in patient with ISR compared that without ISR after BMS implantation (2.9±1.8 mg/L vs. 2.2±1.2 mg/L, p=0.00) but not in patients with DES placement (2.5±1.6 mg/L vs. 2.4±1.4 mg/L, p=0.15). More interesting, baseline RDW levels were significantly higher in patients with ISR regardless of different stents implanted (BMS: 13.8±2.2 vs. 12.5±2.2, p=0.00; DES: 14.1±2.0 vs. 12.5±2.0, p=0.00). This novel finding may have important role in the early detection of patients of a high risk of rest enosis after coronary stent placement.
In fact, RDW is a numerical measure of the variability in size of circulating erythrocytes. Generally, it is used, in combination with mean corpuscle volume, as an indicator of differential diagnosis of anemia [17]. Factors that contribute to increased erythrocyte size heterogeneity include iron or vitamin B12/foliate deficiency, decreased erythrocyte life-span, impaired erythropoiesis and factors that contribute to erythrocyte fragmentation including increased fragility and destruction of red cell [17]. Recently, RDW has been shown to be a strong predictor for the presence of a variety of cardiovascular diseases and adverse outcomes in multiple settings [8-17]. Although those studies demonstrated the role of RDW in a variety of cardiovascular disease, the role of RDW in ISR, especially in association between RDW and ISR after either BMS or DES are lacking. Apparently, in the present study we extended previous studies and provides the first line evidence that baseline RDW levels is an independent predictor for patients with ISR after coronary stent placement.
The physiological mechanisms that the correlation between higher levels of RDW and ISR is not understood. It has been hypothesized that the systemic factors, such as inflammation and oxidative stress, might be the potential contributions. Previous studies suggested that higher RDW was associated with inflammatory markers such as soluble tumor necrosis factor receptors and CRP in the setting of atherosclerosis and other chronic diseases [24-26]. A strong association between RDW and inflammatory markers was also found in a large cohort of unselected adult outpatients, as well as patients with inflammatory bowel disease [27]. Pro-inflammatory cytokines may also contribute to the heterogeneity of erythrocyte population through other pathway such as oxidative stress [24]. Red blood cells have huge antioxidant capacity and serve as a primary oxidative sink, but they are prone to oxidative damage, which reduces cell survival and induces the release of juvenile erythrocytes into circulation [26,27]. Interestingly, we have previously proposed that the inflammation may play a pathogenic role in ISR after coronary stent implantation [22]. This hypothesis has also been demonstrated by our clinical observations in patients with isolated CAE [23]. In the present study, the data further demonstrated a positive correlation between RDW and plasma CRP, suggesting an inflammatory mechanism may be involved in higher RDW levels in patients withISR after coronary stent implantation.
In conclusion, in the present study, our data firstly demonstrated that RDW levels were higher, an independent predicting for ISR, and positive correlated with inflammatory marker, CRP in patients with ISR, suggesting that RDW, an easy, inexpensive, routinely reported test, whose assessment might allow the acquisition of significant diagnostic and prognosis information in patients with cardiovascular disorders [15] may be a useful marker and independent predictor for patients with ISR. Larger prospective trials to confirm and validate the utility of RDW in ISR after coronary stent placement are warranted.
Study limitations
Although the association the increased admission RDW with the follow-up ISR at mean 7 months has been established in the present study, whether RDW has a causal role contributing to isolated CAE or a marker of the disease process needs further investigation. Additionally, retrospective, relative small sample size may limit the significance of the present study. Moreover, the relation of RDW level to the ST after stinting was not evaluated due to hard to identification. The fact that the levels of foliate, iron, and vitamin B12 were not evaluated was another limitation.
Authors' Contributions
Li X-L collected and analyzed the data and wrote the manuscript. Hong L-F contributed to collect and analyzed the data. Li J-J designed the study, interpreted the data and contributed to review and edit the manuscript. The other co-authors for this manuscript contributed to collect the data.
Acknowledgement
The authors thank the staff at the hospitals for their assistance throughout the study. The work was partly supported by National Natural Scientific Foundation (81070171, 81241121), Specialized Research Fund for the Doctoral Program of Higher Education of China (20111106110013), Capital Special Foundation of Clinical Application Research (Z121107001012015), Capital Health Development Fund (2011400302), and Beijing Natural Scientific Foundation (7131014) awarded to Dr. Jian-Jun Li.
References
- Niccoli G, Montone RA, Ferrante G, Crea F. The evolving role of inflammatory biomarkers in risk assessment after stent implantation. J Am Coll Cardiol. 2010; 56: 1783-1793.
- Wessely R, Kastrati A, Schömig A. Late restenosis in patients receiving a polymer-coated sirolimus-eluting stent. Ann Intern Med. 2005; 143: 392-394.
- Khouzam RN, Shaheen M, Aziz RK, Ibebuogu UN. The important role of inflammatory biomarkers pre and post bare-metal and drug-eluting stent implantation. Can J Cardiol. 2012; 28: 700-705.
- Brar SS, Leon MB, Stone GW, Mehran R, Moses JW, Brar SK, et al. Use of drug-eluting stents in acute myocardial infarction: a systematic review and meta-analysis. J Am Coll Cardiol. 2009; 53: 1677-1689.
- De Luca G, Stone GW, Suryapranata H, Laarman GJ, Menichelli M, Kaiser C, et al. Efficacy and safety of drug-eluting stents in ST-segment elevation myocardial infarction: a meta-analysis of randomized trials. Int J Cardiol. 2009; 133: 213-222.
- Claessen B, Stone GW, Mehran R, Witzenbichler B, Brodie BR, Wöhrle J, et al. Relationship between biomarkers and subsequent clinical and angiographic restenosis after paclitaxel-eluting stents for treatment of STEMI: a HORIZONS-AMI substudy. J Thromb Thrombolysis. 2012; 34: 165-179.
- Saigo K, Jiang M, Tanaka C, Fujimoto K, Kobayashi A, Nozu K, et al. Usefulness of automatic detection of fragmented red cells using a hematology analyzer for diagnosis of thrombotic microangiopathy. Clin Lab Haematol. 2002; 24: 347-351.
- Dabbah S, Hammerman H, Markiewicz W, Aronson D. Relation between red cell distribution width and clinical outcomes after acute myocardial infarction. Am J Cardiol. 2010; 105: 312-317.
- Tonelli M, Sacks F, Arnold M, Moye L, Davis B, Pfeffer M. Cholesterol and Recurrent Events Trial Investigators. Relation between red blood cell distribution width and cardiovascular event rate in peoples with coronary disease. Circulation. 2008; 117: 163-168.
- Felker GM, Allen LA, Pocock SJ, Shaw LK, McMurray JJ, Pfeffer MA, et al. Red cell distribution width as a novel prognostic marker in heart failure: data from the CHARM Program and the Duke Databank. J Am Coll Cardiol. 2007; 50: 40-47.
- Ye Z, Smith C, Kullo IJ. Usefulness of red cell distribution width to predict mortality in patients with peripheral artery disease. Am J Cardiol. 2011; 107: 1241-1245.
- Ani C, Ovbiagele B. Elevated red blood cell distribution width predicts mortality in persons with known stroke. J Neurol Sci. 2009; 277: 103-108.
- Luo SH, Jia YJ, Nie SP, Qing P, Guo YL, Liu J,et al. Increased red cell distribution width in patients with slow coronary flow syndrome. Clinics (Sao Paulo). 2013; 68: 732-737.
- Qing P, Luo SH, Guo YL, Liu J, Xu RX, Zhu CG, et al. Evaluation of red blood cell distribution width in patients with cardiac syndrome X. Dis Markers. 2013; 34: 333-339.
- Karabulut A, Uyarel H, Uzunlar B, Çakmak M. Elevated red cell distribution level predicts worse postinterventional thrombosis in myocardial infarction flow reflecting abnormal reperfusion in acute myocardial infarction treated with a primary coronary intervention. Coron Artery Dis. 2012; 23: 68-72.
- Fatemi O, Torguson R, Chen F, Ahmad S, Badr S, Satler LF, et al. Red cell distribution width as a bleeding predictor after percutaneous coronary intervention. Am Heart J. 2013; 166: 104-109.
- Montagnana M, Cervellin G, Meschi T, Lippi G. The role of red blood cell distribution width in cardiovascular and thrombotic disorders. Clin Chem Lab Med. 2011; 50: 635-641.
- Stettler C, Wandel S, Allemann S, Kastrati A, Morice MC, Schömig A, et al. Outcomes associated with drug-eluting and bare-metal stents: a collaborative network meta-analysis. Lancet. 2007; 370: 937-948.
- Dangas GD, Claessen BE, Caixeta A, Sanidas EA, Mintz GS, Mehran R. In-stent restenosis in the drug-eluting stent era. J Am Coll Cardiol. 2010; 56: 1897-1907.
- Hwang CW, Levin AD, Jonas M, Li PH, Edelman ER. Thrombosis modulates arterial drug distribution for drug-eluting stents. Circulation. 2005; 111: 1619-1626.
- Chung IM, Gold HK, Schwartz SM, Ikari Y, Reidy MA, Wight TN. Enhanced extracellular matrix accumulation in restenosis of coronary arteries after stent deployment. J Am Coll Cardiol. 2002; 40: 2072-2081.
- Xu YL, Li JJ, Xu B, Zhu CG, Yang YJ, Chen JL, et al. Role of plasma C-reactive protein in predicting in-stent restenosis in patients with stable angina after coronary stenting. Chin Med J (Engl). 2011; 124: 845-850.
- Xu HY, Qiao SB, Zhang JF, Dong QT, Li JJ. Different impacts of C-reactive protein and lipid profile on coronary lesions following a percutaneous coronary intervention. Coron Artery Dis. 2012; 23: 181-187.
- Forhecz Z, Gombos T, Borgulya G, Pozsonyi Z, Prohászka Z, Jánoskuti L. Red cell distribution width in heart failure: prediction of clinical events and relationship with markers of ineffective erythropoiesis, inflammation, renal function, and nutrition state. Am Heart J. 2009; 158: 659-666.
- Papadaki HA, Kritikos HD, Valatas V, Boumpas DT, Eliopoulos GD. Anemia of chronic disease in rheumatoid arthritis is associated with increased apoptosis of bone marrow erythroid cells: improvement following anti-tumor necrosis factor-alpha antibody therapy. Blood. 2002; 100: 474-482.
- Lippi G, Targher G, Montagnana M, Salvagno GL, Zoppini G, Guidi GC. Relation between red blood cell distribution width and inflammatory biomarkers in a large cohort of unselected outpatients. Arch Pathol Lab Med. 2009; 133: 628-632.
- Cakal B, Akoz AG, Ustundag Y, Yalinkilic M, Ulker A, Ankarali H. Red cell distribution width for assessment of activity of inflammatory bowel disease. Dig Dis Sci. 2009; 54: 842-847.