
Special Article – Novel Markers in Heart Diseases
J Dis Markers. 2015;2(3): 1027.
Urinary NT-proBNP is Independently Associated with Long-term Prognosis of Mortality in Chronic Heart Failure
Carsten G Jungbauer*, Stefan Stadler, Christoph Birner, Markus Resch, Ekrem Ücer, Sabine Fredersdorf, Lars S Maier and Andreas Luchner
Klinik und Poliklinik fuer Innere Medizin II, Universitätsklinikum Regensburg, Regensburg, Germany
*Corresponding author: Dr. Carsten Jungbauer, Klinik und Poliklinik für Innere Medizin II – Kardiologie, Universitätsklinikum Regensburg, 93042 Regensburg, Germany
Received: May 24, 2015; Accepted: July 13, 2015; Published: July 15, 2015
Abstract
Aims: Plasma NT-proBNP is the established heart failure marker. Recently, several studies showed that NT-proBNP may have potential as a urinary marker due to its renal arterio-venous clearance. The objective of this study was to assess the prognostic capacity of urinary NT-proBNP for patients with chronic heart failure.
Methods: NT-proBNP (Elecsys proBNP®, Roche) was assessed simultaneously in fresh spot urine and plasma from 149 patients with chronic heart failure. During a 5-year-follow-up, data was obtained regarding allcause mortality (n= 47) and a combined endpoint of all-cause-mortality and rehospitalisation due to congestive heart failure (n= 67).
Results: Urinary and plasma NT-proBNP were both significantly elevated in patients suffering from an event compared to patients without event (each p< 0.05). Urinary NT-proBNP above the 75th percentile incorporated significant prognostic information regarding all-cause mortality and the combined endpoint (each p<0.05). In cox-regression analysis, urinary NT-proBNP as well as plasma NT-proBNP were both independent predictors for all-cause mortality (each p< 0.05), beside age, diabetes and ejection fraction (EF). Only plasma NT-proBNP was a significant predictor for the combined endpoint (p <0.05; urinary NTproBNP p= n.s.), beside age, male gender, diuretic use and EF. The combination of urinary NT-proBNP and plasma NT-proBNP showed additive prognostic value compared with plasma NT-proBNP alone.
Conclusions: Urinary NT-proBNP incorporated significant and independent predictive value, especially regarding all-cause mortality. Measurement of urinary NT-proBNP seems to be a promising method for heart failure prognostication.
Keywords: Urinary NT-proBNP; Heart failure; Cardiac markers; Natriuretic peptides
Abbreviations
AUC: Area Under the Curve; CI: Confidence Interval; EF: Ejection Fraction; GFR: Glomerular Filtration Rate; JVP: Jugular Venous Pressure; LVD: Left Ventricular Dysfunction; NT-proBNP: N-Terminal pro-Brain Natriuretic Peptide; OR: Odds Ratio; ROC: Receiver Operating Characteristic.
Introduction
N-terminal pro-brain natriuretic peptide (NT-proBNP) is an established heart failure biomarker. NT-proBNP incorporates strong predictive value in heart failure, but also in other cardiac conditions [1-7].
NT-proBNP undergoes predominantly renal clearance–opposite to BNP, which is cleared by enzymatic degradation through neutral endopeptidase and receptor-mediated clearance beside renal extraction [8-10]. NT-proBNP can be detected in the urine and seems to have important potential as a urinary marker of heart failure. Investigation of urine is simple and non-invasive, which may be very attractive under special conditions, e.g. in medical practices without own laboratory. However, studies of urinary NT-proBNP are very sparse [11-15], particularly studies using fresh, unfrozen samples [16,17]. Even less data is available regarding the prognostic capacity of urinary NT-proBNP [13,18].
It was therefore our aim to assess urinary NT-proBNP for the first time in fresh urine regarding its long-term predictive value in comparison with plasma NT-proBNP levels. We analysed plasma and urinary NT-proBNP simultaneously from fresh samples in a cohort of patients with chronic heart failure and performed a 5-years-followup.
Methods
Study procedure
Between January 2008 and June 2008, 150 patients with structural heart disease participated in the study. Patients were recruited from the heart failure outpatient’s clinic of university hospital Regensburg after presenting with stable disease over 6 months. According to the ESC guidelines criteria, heart failure was diagnosed in patients with typical signs and symptoms and objective evidence of a structural or functional abnormality of the heart at rest [19]. Patients with acute myocardial infarction, pulmonary embolism or stroke in the last 6 months were not included. In addition to that also patients with severe chronic pulmonary disease and severe or end stage chronic renal disease were excluded (KDOQI stage 5 with eGFR < 15 ml/ min/1.73m2 or on dialysis). Patients between 18 and 80 years who were able to sign the consent form and suffering from ischemic or dilated cardiomyopathy were included into the study.
Every participant was interviewed (NYHA stage, drugs, especially diuretic dose) and physically examined (edema, pulmonary rales, elevated JVP). Ejection fraction (EF) was echocardiographically evaluated by Simpson´s method. EGFR was calculated according to the CKD EPI formula from plasma creatinine, sex and age [20].
Survival confirmation and date of death were obtained from hospital or death registries or by confirmation of relatives. Further, the composite of rehospitalisation for congestive heart failure and allcause mortality was used as combined endpoint.
The study was approved by the institutional ethics committee (vote 07/152) and was performed in accordance with good clinical practice guidelines and with the standards established for human experimentation by the Declaration of Helsinki.
Sample processing and biochemical analyses: To gain the requested results, blood samples and fresh spot morning urine samples were sent to the central laboratory immediately after collection on the same day. The urine samples were collected into standard urine collection tubes without the addition of degradation inhibitors. Blood was collected into a serum tube according to our local laboratory protocol. The Elecsys 2010 NT-proBNP assay (Roche Diagnostics, Mannheim, Germany) was used for analysis of both urinary and plasma NT-proBNP immediately upon receipt of the samples. The analytical range was 5-35000 pg/ml. The feasibility of the Elecsys 2010 NT-proBNP assay to accurately determine urinary NT-proBNP was shown previously [16]. All urinary biomarkers were normalized to urinary creatinine in order to minimize dilutional bias, especially in a blended heart failure collective prescribed with diverging diuretic dosis.
Statistics
Descriptive data are presented as mean (+/-SEM), medians (IQR) or percentages. Normally distributed values were evaluated with Student´s unpaired two-sided T-test. The Mann-Whitney-U-test was used for continuous variables. For follow-up analysis, we constructed Kaplan-Meier survival curves reflecting the relationship between the time of follow-up and probability of reaching the endpoints. Patients were followed for a mean duration of 53 months (IQR 49 – 66 months); only one patient was lost to follow-up. Upon analysis, in 149 patients the following endpoints were observed: a total of 47 allcause mortalities and 67 combined events of rehospitalisation(s) due to congestive heart failure and all-cause mortality. The median of the patients collective and dichotomization for < and >= 75th percentile were each used as binary cut point for all markers and the Kaplan- Meier curves were compared by log-rank test. Multivariable Cox proportional hazard analyses were performed as stepwise regressions with backward elimination to evaluate possible associations between each marker and both endpoints. Age, male gender, BMI, ischemic cardiomyopathy, history of hypertension, diabetes or stroke, diuretic use were included into cox regression analysis. Biomarkers (urinary and plasma NT-proBNP, eGFR, urinary albumin) and EF were used as continuous variables in Cox regression analysis. Further, the combination of plasma and urinary NT-proBNP was analysed to discriminate an additional value of the combined use of both markers (Figure 2a).
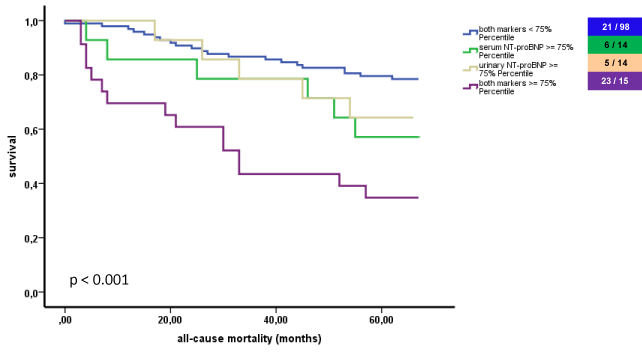
Figure 2a: Kaplan-Meier curves according to marker stratification for urinary
and serum NT-proBNP.
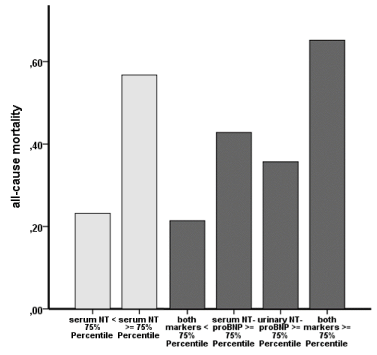
Figure 2b: Mortality rate after stratification for serum NT-proBNP (left) and
the combination of urinary and serum NT-proBNP (right).
Data was analysed using commercially available statistical software packages (SPSS 22.0, SPSS Inc., Chicago, Illinois and MedCalc 8.0, MedCalc Software, Mariakerke, Belgium).
Results
Study population
Patients were followed for a mean duration of 53 months (IQR 49 – 66 months). One patient was lost to follow up. In 149 patients, 47 deaths from all causes occurred. The combined endpoint of allcause mortality and rehospitalisation due to congestive heart failure was evident in 67 patients.
The clinical characteristics of the study population are shown in Table 1. The majority of patients were male. 61.1% suffered from ischaemic cardiomyopathy. EGFR was 71.8 ± 24.0 ml/min/1.73m2.
Heart Failure (n = 149)
deceased patients (n=47)
alive patients (n=102)
p (between deceased and alive patients)
male
130 (87.2%)
45 (95.7%)
85 (83.3%)
age, years
62.2 ± 11.6
64.7 ± 11.9
61.1 ± 11.4
n.s.
BMI, kg/m2
28.4 ± 4.8
27.5 ± 4.4
28.3 ± 5.1
n.s.
Ischemic CMP
91 (61.1%)
30 (63.8%)
62 (60.8%)
n.s.
EF
30.0 ± 9.3%
28.6% ± 8.5
34.2% ± 9.1
0.001
NYHA 1 / 2 / 3 / 4
12 (8.1%) / 66 (44.3%) / 62 (41.6%) / 9 (6%)
1 (2.1%) / 11 (23.4%) / 29 (61.7%) / 6 (12.8%)
11 (10.8%) / 55 (53.9%) / 33 (32.4%) / 3 (2.9%)
<0.001
Diabetes
45 (30.2%)
24 (51.1%)
21 (20.6%)
<0.001
Hypertension
116 (77.9%)
37 (78.7%)
79 (77.5%)
n.s.
Stroke
18 (12.1%)
7 (14.9%)
11 (10.8 %)
0.007
plasma creatinine mg/dL
1.17 (0.91; 1.44)
1.28 (IQR 1.08; 1.68)
1.04 (IQR 0.90; 1.37)
0.001
eGFR ml/min/1.73m2
71.8 (IQR 49.9; 86.1)
57.5 (IQR 42.7; 73.3)
78.4 (IQR 50.8; 89.7)
<0.001
urinary creatinine mg/dL Cr
105.0 (IQR 49.7; 160.0)
97.0 (IQR 55.0; 153.1)
107.5 (IQR 48.8; 162.8)
n.s.
urinary albumin mg/g Cr
23.1 (IQR 8.6; 79.8)
46.0 (IQR 11.8; 123.9)
18.3 (IQR 7.7; 41.3)
0.007
plasma NT-proBNP pg/mL
1500 (IQR 600; 2820)
2470 (IQR 1690; 5100)
870 (IQR 430; 2270)
<0.001
urinary NT-proBNP pg/g Crea
0.51 (IQR 0.23; 1.05)
0.63 (IQR 0.36; 1.70)
0.49 (IQR 0.20; 0.90)
0.006
drug therapy
ACE-I / ATRB
129 (86.5%)
39 (83.0%)
90 (88.2%)
n.s.
beta-blocker
122 (81.9%)
37 (78.8%)
85 (83.3%)
n.s.
digitalis
26 (17.4%)
9 (19.1%)
17 (16.7%)
n.s.
calcium channel blocker
10 (6.7%)
3 (6.4%)
7 (6.9%)
n.s.
loop diuretic
107 (71.8%)
43 (91.5%)
64 (62.7%)
<0.001
thiazide diuretic
37 (24.8%)
12 (25.5%)
25 (24.5%)
n.s.
aldosterone antagonist
91 (61.1%)
31 (66.0%)
60 (58.8%)
n.s.
Table 1: Clinical characteristics.
Deceased Patients were significantly more often male, showed worse EF, worse renal function parameters (plasma creatinine and eGFR) and elevated urinary albumin (each p< 0.05, Table 1).
Urinary and plasma NT-proBNP were significantly higher in deceased patients as well as in patients with occurrence of the combined endpoint compared to surviving patients (each p<0.05). Both were significantly correlated (r= 0.50, p<0.001).
Predictive value of urinary and plasma NT-proBNP
Using the urinary NT-proBNP median as binary cut-off Kaplan-Meier analysis yielded only a predictive trend for above median concentrations regarding both endpoints (each p= n.s.). After dichotomization for below and above 75th percentile values, urinary NT-proBNP was a significant predictor for both endpoints (each p<0.05, Table 2a and 2b, Figure 1). Plasma NT-proBNP was also a significant predictor for both endpoints (each p<0.05, for dichotomization according to median as well as to 75th percentile). Each urinary and plasma NT-proBNP was investigated as continuous variable with Cox regression analysis. Thereby, urinary NT-proBNP as well as plasma NT-proBNP were independent predictors for allcause mortality, beside age, diabetes and EF (each p<0.05, Table 3a). Regarding the combined endpoint, plasma NT-proBNP was also shown to be an independent predictor (p<0.05), but not urinary NTproBNP (p= n.s.).
all-cause mortality
urinary NT-proBNP
plasma NT-proBNP
< median
19 / 75
10 / 74
>= median
28 / 74
37 / 75
p
n.s.
<0.001
< 75th percentile
27 / 112
26 / 112
>= 75th percentile
20 / 37
21 / 37
p
<0.001
<0.001
Table 2a: Kaplan-Meier analysis according to urinary and plasma NT-proBNP regarding all-cause mortality (47 events).
combined endpoint
urinary NT-proBNP
plasma NT-proBNP
< median
29 / 75
21 / 74
>= median
38 / 74
46 / 75
p
n.s.
<0.001
< 75th percentile
45 / 112
41 / 112
>= 75th percentile
22 / 37
26 / 37
p
=0.017
<0.001
Table 2b: Kaplan-Meier analysis according to urinary and plasma NT-proBNP regarding combined endpoint of rehospitalisation due to congestive heart failure and all-cause mortality (67 events).
all-cause mortality
urinary NT-proBNP
plasma NT-proBNP
< median
19 / 75
10 / 74
>= median
28 / 74
37 / 75
p
n.s.
<0.001
< 75th percentile
27 / 112
26 / 112
>= 75th percentile
20 / 37
21 / 37
p
<0.001
<0.001
Table 3a: Urinary and plasma NT-proBNP: Cox regression analysis regarding all-cause mortality and the combined end-point.
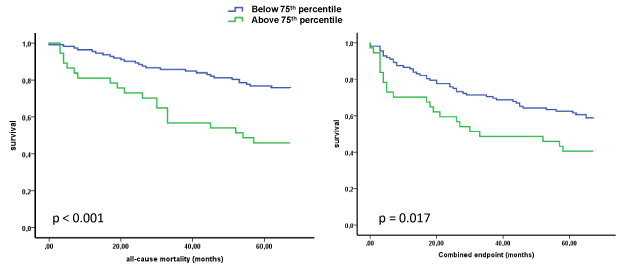
Figure 1: Urinary NT-proBNP: Kaplan-Meier curves regarding all-cause
mortality (left) and the combined endpoint (right); blue: urinary NT-proBNP <
75th percentile, green: urinary NT-proBNP >= 75th percentile
Combined prognostic value of urinary and plasma NTproBNP
Urinary and plasma NT-proBNP were included in a combined analysis regarding all-cause mortality (Table 3b). The patients were subdivided in 4 groups: group 1 with both markers below 75th percentile, group 2 and 3 with one marker above 75th percentile, the other below 75th percentile and vice versa, group 4 with both markers above 75th percentile. Thereby, the event rate increased stepwise and significantly. Of note, the highest event rate could be seen in the group with both markers above the 75th percentile: the event rate increased to 65.2% from 54.1% (urinary NT-proBNP) and 56.8% (plasma NTproBNP), respectively (Figure 2).
combined endpoint
urinary NT-proBNP
plasma NT-proBNP
< median
29 / 75
21 / 74
>= median
38 / 74
46 / 75
p
n.s.
<0.001
< 75th percentile
45 / 112
41 / 112
>= 75th percentile
22 / 37
26 / 37
p
=0.017
<0.001
Table 3b: Combined use of urinary and plasma NT-proBNP (stratified according to 4 groups: both markers < 75th percentile, one marker >= 75th percentile, the other marker < 75th percentile and vice versa, both markers >= 75th percentile): Cox regression analysis regarding all-cause mortality and the combined endpoint.
Discussion
In the current study, the predictive value of urinary NT-proBNP was evaluated head-to-head to plasma NT-proBNP. For the first time, the present study investigated the prognostic capacity of urinary NTproBNP in a long-term follow-up of 5 years. Thereby, urinary NTproBNP was shown as significant and independent predictor for all-cause mortality. Opposite to plasma NT-proBNP, urinary NTproBNP was no independent predictor for the combined endpoint. Further, urinary NT-proBNP offered additional predictive value to plasma NT-proBNP. Therefore, the current data suggests urinary NT-proBNP as simple and non-invasive method for heart failure prognostication.
Urinary NT-proBNP and prognosis in chronic heart failure
So far, only sparse data is available regarding the potential role of urinary NT-proBNP as prognostic factor in heart failure. In the respective studies urinary NT-proBNP was analysed from deepfrozen specimen and not from fresh urine. Cortés et al investigated chronic heart failure patients, but they didn´t use Kaplan-Meier analysis or Cox regression analysis and therefore they weren´t able to assess censored observations adequately. Nevertheless, they described a prognostic value of urinary NT-proBNP according to ROC-analysis [13]. Mazano-Fernández et al estimated endpoint data according to Kaplan-Meier method and Cox regression analysis and weren´t able to detect relevant prognostic information of urinary NT-proBNP. But there were relevant differences regarding patients collective and methods between both studies. The follow-up duration of the study by Mazano-Fernández was only one year. Their patient´s collective was an acute heart failure collective with a high rate of heart failure with preserved ejection fraction. Mazano-Fernández et al weren´t able to detect a relevant association between urinary NT-proBNP and adverse outcomes [18]. Opposite, the current study investigated chronic heart failure patients with reduced ejection fraction in stable condition. Further, we performed a very long follow-up with a mean duration of 53 months and used the 75th percentile for dichotomization. Also, diminishing potential bias due to degradation processes, urinary NT-proBNP was measured promptly in fresh urine. In view of serious methodical differences between both studies, direct comparisons seem not to be feasible and further studies are necessary to determine the predictive value of urinary NT-proBNP. The current study proposes in chronic heart failure patients a prognostic longterm value of urinary NT-proBNP measured in fresh urine. Especially patients with very high values of urinary NT-proBNP, e.g. above the 75th percentile, are prone to suffer from adverse events.
Urinary and plasma NT-proBNP
Both, urinary and plasma NT-proBNP, incorporate a relevant prognostic information. Plasma NT-proBNP seems to have more prognostic power, especially regarding the combined endpoint. Nevertheless, the 54.1% mortality rate of urinary NT-proBNP was quite similar to the 56.8% of plasma NT-proBNP. Further, patients with high values of urinary NT-proBNP (above the 75th percentile) had a higher risk of adverse clinical events than patients with urinary NT-proBNP below the 75th percentile. In patients with concentrations above the 75th percentile, similar numbers of patients died according to urinary and plasma NT-proBNP. Therefore, the predictive value of urinary NT-proBNP seems to be relevant in patients with high urinary NT-proBNP concentrations. Dichotomization according to the median showed only a non-significant predictive trend for urinary NT-proBNP, opposite to plasma NT-proBNP. Interestingly, plasma and urinary NT-proBNP, which are not well correlated, seem to incorporate relevant additive information. Therefore, it should be further evaluated if both markers show different aspects of chronic heart failure.
Potential role of urinary NT-proBNP
The role of urinary NT-proBNP is still unsettled, especially in consideration of the small number of studies dealing with this marker. Regarding diagnosis urinary NT-proBNP incorporates some potential, but plasma NT-proBNP seems to be superior [11-16]. The current study is the first to investigate the predictive value of urinary NT-proBNP from fresh urine in a long-term follow-up.
Relevance of heart failure increases steadily incorporating a major burden for health systems. Heart failure diagnosis depends on echocardiography or a blood test for BNP or NT-proBNP [21-23] and therefore on a specialized health care environment, e.g. facilities with a possibility for venepuncture or echocardiography. To investigate urine, for example with a urine test strip, instead of blood seems to be very attractively, especially under consideration of cost-effectiveness. Further, the possibility of a urine NT-proBNP test would allow in an easy way remote testing, self-testing or repeated testing. But nevertheless, plasma NT-proBNP is superior to urinary NT-proBNP. Opposite to plasma NT-proBNP, urinary NT-proBNP seems to be prognostic mainly in patients with severe heart failure.
The present data are promising regarding the prognostic capability of urinary NT-proBNP, but further prospective studies are necessary to affirm the current data and to define cut-off values for urinary NT-proBNP to use it in daily clinical practice. Especially the combined value of urinary and plasma NT-proBNP should be further evaluated.
Conclusion
Urinary NT-proBNP was assessed prospectively from fresh urine in a cohort of patients with chronic heart failure. One single measurement of urinary NT-proBNP in patients with stable chronic heart failure seems to incorporate relevant predictive value in a longterm follow-up, especially regarding all-cause mortality. Therefore, urinary NT-proBNP has a clear potential as promising and simple urinary cardiac marker.
References
- Nielsen LS, Svanegaard J, Klitgaard NA, Egeblad H. N-terminal pro-brain natriuretic peptide for discriminating between cardiac and non-cardiac dyspnoea. Eur J Heart Fail. 2004; 6: 63-70.
- Richards M, Nicholls MG, Espiner EA, Lainchbury JG, Troughton RW, Elliott J, et al. Comparison of B-type natriuretic peptides for assessment of cardiac function and prognosis in stable ischemic heart disease. J Am Coll Cardiol. 2006; 47: 52-60.
- Hunt PJ, Richards AM, Nicholls MG, Yandle TG, Doughty RN, Espiner EA. Immunoreactive amino-terminal pro-brain natriuretic peptide (NT-PROBNP): a new marker of cardiac impairment. Clin Endocrinol (Oxf). 1997; 47: 287-296.
- Hammerer-Lercher A, Ludwig W, Falkensammer G, Müller S, Neubauer E, Puschendorf B, et al. Natriuretic peptides as markers of mild forms of left ventricular dysfunction: effects of assays on diagnostic performance of markers. Clin Chem. 2004; 50: 1174-1183.
- Luchner A, Hengstenberg C, Löwel H, Trawinski J, Baumann M, Riegger GA, et al. N-terminal pro-brain natriuretic peptide after myocardial infarction: a marker of cardio-renal function. Hypertension. 2002; 39: 99-104.
- Luchner A, Hengstenberg C, Löwel H, Buchner S, Schunkert H, Riegger GA, et al. NT-ProBNP in outpatients after myocardial infarction: interaction between symptoms and left ventricular function and optimized cut-points. J Card Fail. 2005; 11: S21-27.
- Luchner A, Hengstenberg C, Löwel H, Riegger GA, Schunkert H, Holmer S. Effect of compensated renal dysfunction on approved heart failure markers: direct comparison of brain natriuretic peptide (BNP) and N-terminal pro-BNP. Hypertension. 2005; 46: 118-123.
- Andreassi MG, Del Ry S, Palmieri C, Clerico A, Biagini A, Giannessi D. Up-regulation of 'clearance' receptors in patients with chronic heart failure: a possible explanation for the resistance to biological effects of cardiac natriuretic hormones. Eur J Heart Fail. 2001; 3: 407-414.
- Goetze JP, Jensen G, Møller S, Bendtsen F, Rehfeld JF, Henriksen JH. BNP and N-terminal proBNP are both extracted in the normal kidney. Eur J Clin Invest. 2006; 36: 8-15.
- Hall C. Essential biochemistry and physiology of (NT-pro)BNP. Eur J Heart Fail. 2004; 6: 257-260.
- Ng LL, Geeranavar S, Jennings SC, Loke I, O'Brien RJ. Diagnosis of heart failure using urinary natriuretic peptides. Clin Sci (Lond). 2004; 106: 129-133.
- Ng LL, Loke IW, Davies JE, Geeranavar S, Khunti K, Stone MA, et al. Community screening for left ventricular systolic dysfunction using plasma and urinary natriuretic peptides. J Am Coll Cardiol. 2005; 45: 1043-1050.
- Cortés R, Portolés M, Salvador A, Bertomeu V, García de Burgos F, Martínez-Dolz L, et al. Diagnostic and prognostic value of urine NT-proBNP levels in heart failure patients. Eur J Heart Fail. 2006; 8: 621-627.
- Cortés R, Rivera M, Salvador A, Bertomeu V, de Burgos FG, Roselló-Lletí E, et al. Variability of NT-proBNP plasma and urine levels in patients with stable heart failure: a 2-year follow-up study. Heart. 2007; 93: 957-962.
- Linssen GC, Damman K, Hillege HL, Navis G, van Veldhuisen DJ, Voors AA. Urinary N-terminal prohormone brain natriuretic peptide excretion in patients with chronic heart failure. Circulation. 2009; 120: 35-41.
- Jungbauer CG, Buchner S, Birner C, Resch M, Heinicke N, Debl K, et al. N-terminal pro-brain natriuretic peptide from fresh urine for the biochemical detection of heart failure and left ventricular dysfunction. Eur J Heart Fail. 2010; 12: 331-337.
- Toufan M, Namdar H, Abbasnezhad M, Habibzadeh A, Esmaeili H, Yaraghi S, et al. Diagnostic Values of Plasma, Fresh and Frozen Urine NT-proBNP in Heart Failure Patients. J Cardiovasc Thorac Res. 2014; 6: 111-115.
- Manzano-Fernández S, Januzzi JL, Boronat García M, Bonaque-González JC, Muñoz-Esparza C, Albaladejo-Otón MD, et al. [Comparative prognostic value of plasma and urinary N-terminal pro-B-type natriuretic peptide in patients with acute destabilized heart failure]. Rev Esp Cardiol. 2011; 64: 365-372.
- Dickstein K, Cohen-Solal A, Filippatos G, McMurray JJ, Ponikowski P, Poole-Wilson PA, et al; ESC Committee for Practice Guidelines (CPG). ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the Task Force for the diagnosis and treatment of acute and chronic heart failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM). Eur J Heart Fail. 2008; 10: 933-989.
- Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009; 150: 604-612.
- Redfield MM. Heart failure--an epidemic of uncertain proportions. N Engl J Med. 2002; 347: 1442-1444.
- Hunt SA. ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure). J Am Coll Cardiol. 2005; 46: e1-82.
- Jessup M, Brozena S . Heart failure. N Engl J Med. 2003; 348: 2007-2018.