
Research Article
J Dis Markers. 2015;2(3): 1029.
Predictors of Glomerular Filtration Rate Decline in Type 2 Diabetic Patients: Two-Years Follow-up Study
Čabarkapa V1,2*, Stošić Z1,2, Đerić M1,2, Bećarević M¹, Radosavkić I² and Eremić Kojić N1,2
¹Medical Faculty, University of Novi Sad, Serbia
²Clinical Centre of Vojvodina, Center of Laboratory Medicine, Novi Sad, Serbia
*Corresponding author: Čabarkapa V, Center of Laboratory Medicine, Clinical Centre of Vojvodina, Serbia
Received: July 07, 2015; Accepted: August 07, 2015; Published: August 10, 2015
Abstract
Background: Diabetic nephropathy is one of the leading causes of chronic renal failure. The aim was to investigate the importance of specific biomarkers and clinical features in prediction of glomerular filtration rate (GFR) decline in type 2 diabetic patients during two-year follow-up.
Methods: Patients (n = 113) were divided into the following groups: I-41 with GFR reduction >20% (in relation to the reference value for given sex and age) and urinary albumin excretion (UAE) >30 mg/day; II- 34 with GFR reduction ≤20% and UAE >30 mg/day, and III-38 with GFR reduction ≤20% and UAE ≤30 mg/day. The control group included 30 healthy subjects. We determined albuminuria (sandwich-immunometric method); proteinuria (pirogalol red); C reactive protein, apolipoprotein A-I and B, lipoprotein (a), cystatin C (immunoturbidimetry); homocysteine (FPIA); fibrinogen (Clauss); oxidized LDL (ELISA); lipid parameters, creatinine, urea and uric acid (standard biochemical methods). GFR was estimated via creatinine clearance. We also evaluated the presence of chronic complications of diabetes.
Results: GFR reduction >10% compared to baseline values was more frequent in group I (53.13%), than in group II (36.67%) and III (23.14%). Regression analysis revealed that proteinuria >1 g/day increases the risk of progression of renal dysfunction fourfold, homocysteinemia >10 μmol/L by 3.42, systolic blood pressure (SBP) >130 mmHg by 5.08, hemoglobin <130 g/L by 3.05 and the presence of macrovascular complications (MC) by 6.25 times.
Conclusion: Homocysteinemia >10 μmol/L, presence of macrovascular complications, hemoglobin <130 g/L, and SBP >130 mmHg were independent predictors of progression of renal dysfunction in patients with DM type 2.
Keywords: Diabetic Nephropathy; Type 2 Diabetes Mellitus; Glomerular Filtration Rate; Biochemical Markers; Macroangiopathy
Introduction
Diabetic nephropathy (DN) implies a wide range of renal dysfunctions that involve the development of microalbuminuria (MiA), proteinuria and progressive reduction in renal functional reserve (RFR) [1]. Besides hypertensive nephropathy and glomerulonephritis, DN is a leading cause of chronic renal failure and end-stage renal disease [2,3,4].
Mortality in DN patients is 5-8 times higher than in the general population, and it is influenced considerably by the high cardiovascular mortality that increases with progression of renal dysfunction [5].
Diabetic nephropathy is a consequence of interaction between a range of hemodynamic and metabolic factors (systemic and intraglomerular hypertension, activation of vasoactive substances, activation of alternative metabolic pathways with the formation of polyols and advanced glycation end products with increased oxidative stress). Combined action of these factors leads to increased renal albumin permeability as well as to an accumulation of extracellular matrix, which result in proteinuria, glomerulosclerosis and tubulointerstitial fibrosis [6,7].
However, there is significant individual variation in the rate of reduction in RFR, i.e., reduction in glomerular filtration rate (GFR), in patients with DN [8,9,10]. It is worrisome that despite all the measures currently in use in the treatment of subjects suffering from diabetes, a significant number of patients still experience a progressive decline in GFR [11]. This finding indicates the necessity of determining risk factors for progression of renal dysfunction, especially in the early stages of nephropathy.
Therefore, in this two-year follow-up study we examined the importance of specific biomarkers (i.e. total homocysteine (tHcy), oxidized LDL (oxLDL), C-reactive protein (CRP), fibrinogen, lipid status parameters, apolipoprotein (apo) A-I, apoB, lipoprotein(a) (Lp(a)), proteinuria, albuminuria, cystatin C, hemoglobin and the clinical features (the presence of chronic vascular complications of diabetes) in prediction of GFR decline in patients with diabetes mellitus type 2 with varying degrees of RFR reduction.
Materials and Methods
Study population
This two-year follow-up study was carried out in the Clinical Centre of Vojvodina, Novi Sad, Serbia. The study had previously been approved by an institutional ethics committee and all included subjects had approved their participation. The study was performed according to the principles of the Declaration of Helsinki.
The study included 113 subjects Caucasians with diabetes mellitus type 2, with no urinary tract disease or non-diabetic renal disease. Patients were divided into the three groups according to GFR and urinary albumin excretion (UAE): group I 41 subjects (29 men, 12 women) with UAE over 30 mg/day and GFR reduction ≥20% compared with the reference values for given sex and age; group II 34 subjects (20 men and 14 women) with UAE over 30 mg/day and GFR reduction ≤20% compared with the reference values for given sex and age; group III 38 subjects (17 men and 21 women) with and GFR reduction ≤20% compared with the reference values for given sex and age and UAE ≤ 30mg/day.
GFR was estimated through the calculation of creatinine clearance (CrCl), and the degree of reduction was assessed in relation to the reference value for a given sex and age.
The control group included 30 clinically healthy subjects (15 men and 15 women) matched for age, with serum glucose concentrations below 6.1 mmol/L, hemoglobin A1c (HbA1c) below 6.2%, CrCl within the normal range for given sex and age, UAE ≤30 mg/day and proteinuria ≤150 mg/day.
In the control group, blood samples were taken only once, while diabetic patients samples were taken three times: at the beginning of the study, after 12 months, and after 24 months. In all subjects blood was drawn after 12-hour fasting. We just followed the GFR during these 24 months, and other parameters have been doing at the beginning and the basis of the fall of GFR calculated their predictive value.
Analyses were performed immediately after sampling except for oxLDL (samples were kept frozen at -200C no longer than one month). In addition, 24-hour urine collection was performed in all subjects after the previously given instructions.
Smokers, chronic alcohol consumers and patients with acute infection, thyroid dysfunction, liver disease or malignancies were excluded from the study.
A relative reduction in GFR (rrGFR) was calculated as a ratio between the difference between GFR at baseline and GFR at the end (GFRb-GFRe) and GFRb. The value of 10% was an average of the reduction in GFR in all studied diabetic subjects. Subjects who after two-years follow-up had rrGFR of more than 10% were classified as progressors and those with rrGFR ≤10% as non-progressors.
Measurement of renal function parameters
Serum concentrations of creatinine, urea and uric acid were determined by standard biochemical methods (commercial kits- Beckman Coulter, Ireland on Olympus AU 400); cystatin C by the immunoturbidimetric method (commercial Dyazime kits, USA, on Olympus AU 400, reference range: 0.5-1.03 mg/L); albuminuria by the sandwich-immunometric method (commercial Nyco Card kits, Norvage, reference range ≤30 mg/day); proteinuria by pyrogalol red method (commercial Siemens kits, USA, on Advia 1800, reference range: ≤150 mg/day). Creatinine clearance (expressed as mL/ min/1.73 m2 of body surface area (BSA)) was calculated using the same 24-h urine used for proteinuria and albuminuria:
CrCl = (UCr x 24h urine volume (ml))/(SCr x 1440 (min/day))
UCr- urine creatinine (μmol/L), SCr– serum creatinine (μmol/L)
BSA was calculated by the formula [12]: BSA (m²) = 0.0235 x height (cm)0.42246 x weight (kg)0.51456.
Measurement of biochemical markers
Plasma tHcy concentration was determined by the fluorescence polarization immunoassay (FPIA) with commercial Abbott kits, USA, on AxSym analyzer (reference range: 5-12 μmol/L); lipid profile was measured by standard biochemical methods (commercial Siemens kits, USA, on Olympus AU 400); apo A-I and apoB (Beckman Coulter, Ireland), and Lp(a) serum concentrations (Sentinel, Italy) were determined by the immunoturbidimetric method on Olympus AU 400; oxLDL concentration was measured by enzyme-linked immunosorbent assay (ELISA) method (commercial Mercodia kits, Sweden); fibrinogen concentration were measured in citrate plasma by Claus method [13] on ACL system (Instrumentation Laboratory- Italy; reference range: 2.2-4.96 g/L); CRP levels were determined by immunoturbidimetric method (Beckman Coulter, Ireland, reference range 0-5 mg/L).
Assessment of glycemic control parameters
Serum concentration of glucose (hexokinase method, reference range 3.9-6.1 mmol/L) and glycated haemoglobin measured by immuno-inhibitory test as HbA1c (commercial Beckman-Coulter kits-Ireland, reference range <6.2%), were determined on Olympus AU 400.
Assessment of chronic complications of diabetes
Diabetic retinopathy was diagnosed after ophthalmological examination (standard fundus eye examination with presence of micro aneurysms, neovascularization, venous dilatation, cotton-wools spots or bleeding). Diabetic neuropathy was assessed by neurologic examination. Myocardial infarction (MI) was confirmed by a positive history of disease. Cerebrovascular disease was confirmed by cranial computerized tomography or magnetic resonance. Peripheral artery disease (PAD) was confirmed by vascular surgeon. Blood pressure values were determined with the patient sitting in an upright position after 10 minutes of rest.
Statistical analysis
Data was presented using descriptive statistical methods, such as mean values, standard deviation, median, percentage. Agreement with a normal distribution of data was tested by Kolmogorov Smirnov (n >50 by group) and Shapiro-Wilk’s test (n <50 by group).
Parametric (t-test, ANOVA) and non-parametric (Mann- Whitney, χ2 –test, Kruskal-Wallis test) statistical tests were used.
Univariate and multivariate logistic regression analyses were used to determine associations between a rrGFR of over 10% with baseline variables. A ROC curve and area under ROC curve were used for determining quality of the model obtained. P <0.05 was considered statistically significant.
Statistical analysis was performed using the Statistica 12 (Stat Soft Inc., Tulsa, OK, USA) software
Results
General characteristics and baseline laboratory parameters of all studied subjects are shown in Tables 1-3.
Group I ( ±SD)
Group II ( ±SD)
Group III ( ±SD)
CG ( ±SD)
Number (m/f)
41 (29/12)
34 (20/14)
38 (17/21)
30 (15/15)
Age (years)
63.6±5.7
59.7±8.3
61.2±9.5
61.1±5.5
BMI (kg/m2)
29.0±4.2a
29.2±3.9a
27.3±4.9
26.7±3.1
SBP (mmHg)
138±27.4
141.1±17.9
133.1±21.8
129.8±11.8
DBP (mmHg)
88.3±8.6
88.3±12.1
78.8±11.7
82.6±6.3
Duration of DM (years)
16.6±6.9
16.3±1.56
14.6±1.87
-
Fasting glucose (mmol/L)
10.3± 4.7b
9.9±2.7b
10.4±3.5b
5.6±0.5
HbA1c (%)
8.7±2.0b
9.2±1.6b
8.7±2.3b
5.5±0.4
Hemoglobin (g/L)
128.3±17.2b,c,d
138.5±10.2
139.3±12.5
141.9±8.7
Legend: Group I- patients with reduced glomerular filtration rate (GFR) over 20% and urinary albumin excretion (UAE) >30 mg/day; Group II-patients with reduced GFR ≤20% and UAE >30 mg/day; Group III- patients with reduced GFR ≤20% and UAE ≤30 mg/day; CG-control group; BMI-body mass index; SBP-systolic blood pressure; DBP- diastolic blood pressure; DM- diabetes mellitus; aP <0.05 compared to control group; bP <0.001 compared to control group; cP <0.001 compared to group II; dP <0.001 compared to group III.
Table 1: General characteristics of patients and control group at the beginning of study.
Group I ( ±SD)
Group II ( ±SD)
Group III ( ±SD)
CG ( ±SD)
Creatinine (µmol/L)
152.2±68.8a,b,e
(med. 125)
83.1±12.5
(med. 84)
83.7±11.5
(med. 82.5)
86.1±13.1
(med.89)
Urea
(mmol/L)
10.2±4.7a,b,e
( med. 8.7)
6.4±1.5
(med. 6.7)
6.4±1.3
(med. 6.2)
5.74±1.1
(med.5.4)
GFR (ml/min/
1.73m2)
53.6±19.7a,b,e
100.5±28.5d
(med. 99.5)
93.1±20.6
(med. 92.2)
88.3±16.0
(med.83)
Cystatin C (mg/L)
2.02±0.7a,b,e
(med. 1.84)
0.98±0.15
(med. 0.96)
0.98±0.17
(med. 0.94)
1.02±0.12
(med.0.98)
Uric acid
(µmol/L)
374.6±102.1a,b,e
(med. 349)
266.3±60.8
(med. 269.5)
240.8±60.0c
(med. 243)
294.7±72.5
(med.306)
Albuminuria (mg/dU)
302.6±247.1a,b,e
(med.252)
116.9±88.2c,f
(med. 86.5)
12.6±5.8
(med. 10)
12.9±6.6
(med.12.1)
Proteinuria (mg/dU)
1564.7±2091.2b,e,f
(med. 752)
343.5±239.2 b,e
(med. 247.5)
102.2±33.8
(103.5)
82.1±33.7
(med.74.2)
Table 2: Markers of renal function in patients and control group at the beginning of study ((), and/or median (med.
Group I
Group II
Group III
CG
Total cholesterol
(mmol/L)
5.5±1.67
(med. 5.16)
5.89±1.72
(med. 5.73)
5.68±1.12
(med. 5.55)
5.73±0.75
(med. 5.83)
Triglycerides (mmol/L)
2.08±1.65d
(med. 1.59)
2.44±2.74c
(med. 1.68)
1.76±1.5
(med.1.22)
1.24±0.51
(med.1.3)
HDL-C
(mmol/L)
1.12±0.34d,f
(med. 1.06)
1.18±0.24
(med. 1.16)
1.39±0.7
(med.1.29)
1.35±0.34
(med.1.34)
LDL-C
(mmol/L)
3.38±0.95
(med. 3.41)
3.64±1.03
(med. 3.49)
3.61±0.8
(med.3.75)
3.82±0.67
(med.3.86)
nonHDL-C (mmol/L)
4.38±1.54
(med. 4.02)
4.71±1.69
(med. 4.63)
4.35±1.02
(med.4.22)
4.38±0.69
(med.4.42)
apoA-I
(g/L)
1.38±0.24
1.4±0.17
1.49±0.23
1.5±0.21
apoB
(g/L)
1.05±0.32
(med. 0.92)
1.14±0.41c
(med. 1.08)
1.04±0.25
(med.1.04)
0.97±0.21
(med.1.01)
Lp(a)
(g/L)
0.29±0.34
(med. 0.2)
0.28±0.45
(med. 0.14)
0.25±0.40
(med.0.1)
0.22±0.29
(med.0.09)
oxLDL
(mU/L)
11.05±3.54d
(med. 10.29)
11.81±4.01c
(med. 11.2)
9.97±3.55
(med.9.66)
9.39±1.81
(med.8.79)
LDL/HDL-C
3.11±0.85
3.12±0.86
2.88±0.77
3.01±0.88
nonHDL/HDL-C
4.06±1.34
(med. 3.87)
4.11±1.56
(med. 3.73)
3.60±1.31
(med.3.48)
3.45±1.06
(med.3.41)
apoB/A-I
0.78±0.24c
(med. 0.75)
0.83±0.31d
(med. 0.76)
0.71±0.19
(med.0.71)
0.65±0.18
(med.0.64)
AIP
0.22±0.28c
(med. 0.21)
0.22±0.31c
(med. 0.21)
0.14±0.27
(med.0.08)
0.05±0.19
(med.0.11)
tHcy
(µmol/L)
16.5±4.25a,b,e
(med. 15.6)
10.7±2.87
(med. 10.2)
9.64±2.79
(med.8.88)
11.28±1.97
(med.11.1)
hsCRP
(mg/L)
11.9±17.5a,b,e
(med.6.7))
3.95±4.19
(med. 2.8)
3.4±4.66
(med. 2.2)
1.61±1.21
(med.1.3)
Fibrinogen
(g/L)
3.93±0.87
(med. 3.65)
3.22±0.86
(med. 3.15)
3.78±0.71
(med. 3.79)
3.02±0.35
(med.3.1)
Legend: Group I- patients with reduced glomerular filtration rate (GFR) over 20% and urinary albumin excretion (UAE) >30 mg/day; Group II-patients with reduced GFR ≤20% and UAE >30 mg/day; Group III- patients with reduced GFR ≤20% and UAE ≤30 mg/day; CG-control group; C-cholesterol; oxLDL-oxidized LDL; apoB/A-I-apolipoprotein B/apolipoprotein A-I; AIP-atherogenic index of plasma; CRP-C-reactive protein; tHcy-total homocysteine; aP <0.001 compared to group II; bP <0.001 compared to CG; cP <0.01 compared to CG; dP <0.05 compared to CG; eP <0.001 compared to group III; fP <0.01 compared to group III.
Table 3: Other or specific biomarkers in patients and control group at the beginning of study (, and/or median (med.)).
Hemoglobin concentrations were significantly lower in group I compared to other groups (P <0.001), and the other groups did not differ in mean hemoglobin concentrations. BMI values were significantly higher in group I and II compared to group III and control group (P <0.05). The parameters of glycemic control (glucose and HbA1c) were not significantly different between the investigated groups of patients.
The renal function markers: creatinine, urea, uric acid, cystatin C, albuminuria, and proteinuria were significantly higher and GFR was significantly lower in group I compared to other groups (P <0.01). In addition, albuminuria and proteinuria levels were significantly higher in group II compared to group III (P <0.001).
Baseline plasma tHcy and serum CRP concentrations were significantly higher in group I compared to other groups (P <0.001), whereas there were no significant differences in lipid parameters and bioindices between the studied groups.
Clinical characteristics of study subjects are shown in Table 4.
Group I
(%)
Group II
(%)
Group III
(%)
Macrovascular complications (MI, ICV, PAD)
63.4b,c
29.4
21
Rethinopathy
95.1b
70.5a
34.2
HTA
85.3
82.3
65.7
ACE-inhibitors
68.3
67.6
52.6
Antagonist AtR1
9.7
2.9
2.6
Insulin with/without OH
73.2
67.7
65.7
OH without insulin
26.8
32.3
34.3
Legend: Group I- patients with reduced glomerular filtration rate (GFR) over 20% and urinary albumin exrection (UAE) >30 mg/day; Group II-patients with reduced GFR ≤20% and UAE >30 mg/day; Group III- patients with reduced GFR ≤20% and UAE ≤30 mg/day; MI- myocardial infarction; ICV-cerebrovascular insult; PAD-perhipheral arterial disease; HTA- arterial hypertension; AtR1-angiotensin receptors 1; OH-oral hypoglycemics; aP <0.05; bP <0.01 compared to group II; cP <0.01 compared to group III.
Table 4: Clinical characteristics of patients at the begining of study.
Compared with diabetic patients without complications, diabetic patients with a macrovascular complication (MI, stroke, PAD) had significantly higher albuminuria (224.8±33.5 vs. 102.3±20.4 mg/ day, P = 0.002), proteinuria (1162.8±300.8 vs. 420.2±94 mg/dU, P = 0.02) and fibrinogen concentrations (4.44±0.16 vs. 3.99±0.14 g/L, P = 0.045).
Univariate logistic regression analysis showed that the following parameters predicted rrGFR >10% in diabetic patients: baseline renal functional status (risk of rrGFR >10% was 3.5 times higher in group I than in group III and 1.8 times higher in group II compared to group III); albuminuria (subjects with UAE >150 mg/day had 5.05 times higher the risk of rrGFR >10%); proteinuria (subjects with proteinuria >1 g/day had fourfold increased the risk of rrGFR >10%); BMI; hemoglobin levels; systolic blood pressure (SBP); tHcy; and presence of a macrovascular complication.
Prevalence of progressors is shown in Figure 1.
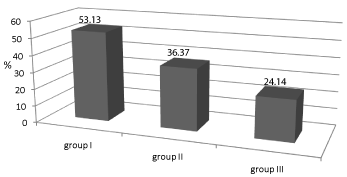
Figure 1: Incidence of patients (%) with relative reduction of GFR
(rrGFR)>10%.
Legend: Group I- patients with reduced GFR and pathological albumiuria,
Group II-patients with preserved functional status of kidney and pathological
albumiuria, Group III- patients with preserved functional status of kidney and
physiological albuminuria.
Group I had the highest percentage of progressors, compared with group III (P = 0.02) and group II (P = 0.151)
Degree of rrGFR in study subjects is shown in Figure 2.
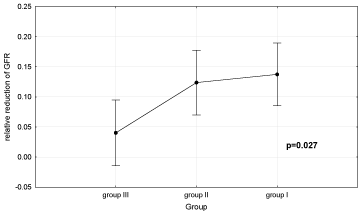
Figure 2: Relative reduction of glomerular filtration rate (GFR) (0.1 =10%)
in all groups of patients. Group I- patients with reduced GFR over 20% and
urinary albumin excretion (UAE) >30 mg/day; Group II-patients with reduced
GFR ≤20% and UAE >30 mg/day; Group III- patients with reduced GFR
≤20% and UAE ≤30 mg/day.
Analysis of variance showed that the study groups differed with regard to degree of rrGFR. Duncan’s test showed that there were significant differences in rrGFR between group I (13.8±2.6%) and group II (12.3±2.3%) compared to group III (4±2.3%) (P = 0.016 and P = 0.03, respectively), whereas there was no significant difference in rrGFR between groups I and II.
A significant percentage of participants (69.23%) with macroalbuminuria (MaA) at baseline had rrGFR >10%. Unlike that, rrGFR >10% had only 38.78% of patients with MiA, and only 24.14% of normoalbuminuria (NoA).
The chi-square test showed an association between the degree of albuminuria and the degree of rrGFR (P = 0.02). Duncan’s test showed that the degree of rrGFR was significantly higher in subjects with MaA (21.2±3.6%) compared to subjects with MiA (11.3±1.85%) and NoA (4.9±2.45%) (P = 0.023 and P <0.001, respectively). Diabetic patients with baseline proteinuria above 1 g/day had a more significant rrGFR (23%) over the observed period compared to diabetic patients with baseline proteinuria under 1 g/day (7.4%) (P = 0.009).
Subjects with baseline HbA1c >7% had a more significant rrGFR (11.6%) over the observed period compared to subjects with baseline HbA1c <7% (5.69%). The former subjects also had a higher relative change (increase) in proteinuria (62.8% vs. 35.9%).
As regards lipid and lipoprotein parameters, univariate regression analysis showed that subjects with serum triglyceride ≥2.3 mmol/L, then subjects in the group I with serum LDL-cholesterol >4.1 mmol/L, and women with increased apo B concentration (>0,9 g/L) had significantly higher rrGFR (P <0.05).
Multivariate logistic regression analysis (Table 5) showed SBP, tHcy, previous history of MI, stroke or PAD, and hemoglobin levels to be significant predictors of rrGFR.
p
Odds ratio (OR)
95% Confidence interval for OR
Lower
Upper
Hemoglobin (g/L)
Hemoglobin < 130
Reference value
Hemoglobin ³ 130
0.049
3.046
1.003
9.249
SBP (mmHg)
SBP ≤130
Reference value
SBP >130
0.006
5.08
1.59
16.206
Homocysteine (µmol/L)
Homocysteine ≤ 10
Reference value
Homocysteine > 10
0.049
3.428
1.002
11.792
MI, ICV, PAD
No
Reference value
Yes
0.002
6.25
1.914
20.423
Constant
0.000
0.032
Legend: MI- myocardial infarction; ICV-cerebrovascular insult; PAD-peripheral arterial disease; SBP-systolic blood pressure.
Table 5: Multivariate logistic regression analysis.
Subjects with tHcy >10μmol/L had significantly higher rrGFR compared to subjects with tHcy <10μmol/L (12.7± 2.3 vs. 4.7 ±2.0%, P = 0.014).
Hyperhomocysteinemia (HHcy) was found in 75% of subjects with UAE >30 mg/day and rrGFR >10% and 29.5% of subjects with UAE >30 mg/day and rrGFR <10% (P <0.001). Among subjects with NoA and rrGFR <10%, HHcy was found in 14.3%, and among subjects with NoA and rrGFR >10% HHcy was found in 21.7% of cases (P = 0.167).
Subjects with baseline SBP >130 mmHg had significantly higher rrGFR compared with subjects with baseline SBP <130 mmHg, as well as a significantly higher relative increase in proteinuria over the study period (76.9% vs. 29%).
Figure 3 shows frequency of rrGFR >10% in relation to coexistence of other risk factors.
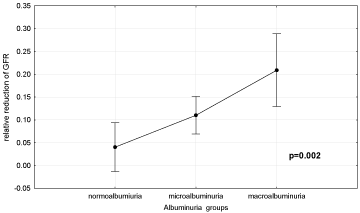
Figure 3: Relative reduction of GFR (0.1 = 10%) according to the basal
values of albuminuria in diabetic patients. Normoalbuminuria-urinary albumin
excretion (UAE) ≤30 mg/day; microalbumiuria- UAE = 30-300 mg/day;
macroalbuminuria- UAE >300 mg/day.
The chi-square test showed associations between the presence of proteinuria >1g/day and HHcy, and between the coexistence proteinuria >1 g/day, HHcy and anemia with the degree of rrGFR (P <0.009). In addition, there was an association between the coexistence of proteinuria >1 g/day, HHcy and serum oxLDL >9.72 mU/L with the degree of rrGFR (P = 0.006).
Discussion
The prevalence of DN at the time of diagnosing diabetes mellitus type 2 is 5-10% [14]. It develops in around 40% of all diabetic patients and represents one of the most prominent causes of end-stage renal failure [2,4], with significant individual variation in the degree of reduction in RFR [8,9,10,15].
Considering that our study fulfilled the criterion that assessment of the degree of reduction in GFR requires at least two years of followup and at least three measurements of GFR [16], this paper represents a cross-section after two-years follow-up of our patients, in which we examined the importance of specific biomarkers (i.e. tHcy, oxLDL, CRP, fibrinogen, lipid status parameters, apoA-I, apoB, Lp(a), proteinuria, albuminuria, cystatin C, hemoglobin) and the presence of chronic complications of diabetes in predicting GFR reduction in patients with diabetes mellitus type 2 with varying degrees of RFR reduction.
With respect to the physiological reduction of GFR which begins in the third decade of life, in this study we have taken into account the effect of age on the level of GFR. Therefore, we calculated the percentage deviation of GFR compared to reference values for age and sex. The coefficient of variation for CrCl reaches values up to 20%, so this value is taken as the limit of existence of RFR decline.
In this prospective study, the highest percentage of progressors (53.13%) was in the group of patients with baseline UAE over 30 mg/day and a reduction in GFR of over 20% compared to reference values (group I), then in group II (36.6%) (P = 0.11) and group III (24.14%) (P = 0.02). In addition, rrGFR was also the highest in group I (13.8%); similarly as in group II (12.4%), and significantly higher than in group III (3.8%, P = 0.016). Univariate logistic regression analysis showed that odds of progression of renal dysfunction were 3.56 times higher in group I compared with group III and 1.82 times higher than in group II. In the present study odds of progression of renal dysfunction were five times higher in patients with UAE >150 mg/day, and four times higher in patients with proteinuria >1 g/day.
Previous studies reported that the risk of progression of GFR decline was fourfold increase in MaA patients, and twofold increase in MiA patients compared with NoA patients [17], and showed proteinuria is one of the strongest predictors of renal dysfunction in diabetic patients [17,18]. However, in DM type 2 MiA does not necessarily precede renal disease [19]. Also, previous studies reported an average annual reduction in GFR in NoA patients in the range 1.3- 1.9 ml/min/1, 73 m2 i.e. 0.3%; in MiA patients 1.5-4.7 ml/min/1, 73 m2, i.e. 1.5%; and in MaA patients ~5,5 ml/min, i.e. 5.7% [20,21,22,23,24].
In our study multivariate regression analysis showed homocysteinemia to be an independent predictor of GFR reduction in diabetic patients, even with plasma concentrations that are in most laboratories within reference ranges. Thus, for a population of patients with diabetes mellitus, it is desirable to use more strict criteria for the upper limit of homocysteinemia. For this reason, in this paper we model the multivariate logistic regression analysis using different levels of homocysteine and found that odds of progression of DN was 3.42 times higher in subjects with tHcy >10 μmol/L than in subjects with tHcy <10 μmol/L (OR 3.428, P = 0.049). Looker et al. [25] also found that the incidence of DN was associated with homocysteinemia (OR 1,42, P = 0,01), and the lowest level of homocysteine in subjects who developed DN after eighteen years of follow-up was 10.09 μmol/L. Furthermore, some authors suggest a double increase in the risk for vascular damage associated with level of tHcy >10.2 μmol/L [26]. Other studies also indicate that diabetic patients with HHcy have significantly higher prevalence of renal dysfunction compared with patients with NHcy [27,28].
HHcy was characteristic of group I in our study (80%), significantly more prevalent than in group II (29.4%) and group III (18.4%) (P <0.001). In addition, homocysteinemia was in both group II (10.7±0.57 μmol/L) and group III (9.64±0.51 μmol/L) similar, even lower, compared to the control group (11.06±0.59 μmol/L), which is in agreement with the results of previous similar studies [29,30]. A most likely explanation is the existence of increased filtration at the level of glomeruli in these patients.
However, in addition to being a marker of renal dysfunction in diabetic patients, homocysteine has potential harmful effects that may lead to further progression of DN, as well as to other vascular complications [31]. Experimental studies suggest a possible role of HHcy in glomerular and interstitial renal impairment, which is proportional to plasma homocysteine concentration [32].
Due to non-enzymatic glycation of albumin, levels of free-form homocysteine are increased in diabetics, which may represent one of the more significant mechanisms of development and progression of vasculopathies in diabetes [33]. In addition, HHcy may represent one of the associations between DN and macrovascular complications.
In the present study, similar to previous studies, macrovascular complications were the most frequent in group I (63.4%), significantly more frequent than in groups II and III (29.4%, P = 0.005 and 21%, P = 0.002, respectively). Multivariate regression analysis showed that the presence of a macrovascular complication was a significant predictor of GFR decline (OR 6.25, P = 0.002). This is in accordance with the finding that the mechanism and changes that occur in glomerulosclerosis are similar to those in premature atherosclerosis [34], and also the risk factors are similar (e.g. dyslipidemia, hypertension, smoking). In addition, the process of tubulointerstitial damage also involves inflammation, proliferation and fibrosis [5,35,36].
Hypertension in DM type 2 is usually associated with insulin resistance and DN. In the present study, multivariate regression analysis showed that subjects with SBP above 130 mmHg had 5.08 times higher risk of GFR decline (OR=5.08, P = 0.006), whereas diastolic blood pressure (DBP) did not have a predictive role with regard to progression of renal dysfunction. Similar results were obtained in previous studies [15,37,38]. On the other hand, studies report that one of the independent predictive factors for progression of DN was mean arterial pressure, or DBP [17,39].
Anemia occurs in more than 20% of patients with DM type 2 [40] and its etiology is complex [41,42]. It appears earlier and is more severe in patients with diabetes and reduced RFR, regardless of the degree of GFR, compared to individuals with chronic kidney disease of other etiology [43,44]. Namely, anemia leads to renal hypoxia, which stimulates production of different growth factors (Transforming growth factor-β1, Vascular endothelial growth factor, Platelet-derived growth factor) and cytokines and contributes to scarring and thickening of the kidney [45,46]. In addition, hypoxia stimulates renal sympathetic activity, which with time leads to reduced renal blood flow and GFR [47]. However, despite the adverse effects of anemia, it is still under recognized and undertreated in diabetic population.
In our subjects hemoglobin concentrations at baseline were the lowest in group I (mean 128.3 g/L). Multivariate regression analysis showed hemoglobin to be an independent predictor of reduction in GFR, and subjects with hemoglobin levels ≤130 g/L had 3.04 times higher risk of progression of renal dysfunction (OR 3.04, P = 0.048). This result may be a consequence of the somewhat larger number of males involved in the study (66 male vs. 47 female). Other authors also suggest that anemia is an independent predictor of progression of kidney disease, particularly in diabetic patients [15,18,39,47].
Although previous studies report that dyslipidemia may lead to development as well as progression of DN [48,49,50], predictive value of specific lipid parameters is controversial [11,15,51]. Similarities between pathogeneses of atherosclerosis and glomerulosclerosis may provide an explanation for the implication of hyperlipidemia in the processes that lead to renal impairment. Furthermore, some studies demonstrated a reduction in the occurrence of MiA and in progression of renal dysfunction in diabetic patients using hypolipidemic agents [52,53].
In our study, the lipid parameter with the strongest effect on progression of kidney disease was serum total cholesterol levels (on the verge of significance as an independent predictor of reduction in GFR (P = 0.09)). In addition, multivariate analysis showed that relative change in serum total cholesterol level was an independent predictor of rrGFR.
Limitations of the study are relative short time of tracking GFR, and not use of gold clinical standard for measurement of GFR (DTPA or EDTA clearance).
In summary, in this study homocysteinemia >10 μmol/L, presence of macrovascular complications, hemoglobin <130 g/L, and SBP >130 mmHg were independent predictors of progression of renal dysfunction in patients with DM type 2.
Acknowledgment
The present work was supported by the Ministry of Science and Education of the Republic of Serbia on the basis of contract No III46005.
References
- Slade H, Williams SM, Manning PJ, Walker RJ. High-risk diabetic nephropathy patients: the outcome of evidence-based clinical practice in an outpatient clinic. Diabetes Res Clin Pract. 2011; 92: 356-360.
- Adler AI, Stevens RJ, Manley SE, Bilous RW, Cull CA, Holman RR; UKPDS GROUP. Development and progression of nephropathy in type 2 diabetes: the United Kingdom Prospective Diabetes Study (UKPDS 64). Kidney Int. 2003; 63: 225-232.
- Friedman EA, Friedman AL, Eggers P. End-stage renal disease in diabetic persons: is the pandemic subsiding? Kidney Int Suppl. 2006; : S51-54.
- Shikata K, Haneda M, Koya D, Suzuki Y, Tomino Y, Yamada K, et al. Diabetic Nephropathy Remission and Regression Team Trial in Japan (DNETT-Japan): Rationale and study design. Diabetes Res Clin Pract. 2010; 87: 228-232.
- Thomas MC, Burns WC, Cooper ME. Tubular changes in early diabetic nephropathy. Adv Chronic Kidney Dis. 2005; 12: 177-186.
- Soldatos G, Cooper ME. Diabetic nephropathy: important pathophysiologic mechanisms. Diabetes Res Clin Pract. 2008; 82 Suppl 1: S75-79.
- Cooper ME. Interaction of metabolic and haemodynamic factors in mediating experimental diabetic nephropathy. Diabetologia. 2001; 44: 1957-1972.
- Hovind P, Rossing P, Tarnow L, Smidt UM, Parving HH. Progression of diabetic nephropathy. Kidney Int. 2001; 59: 702-709.
- Nosadini R, Velussi M, Brocco E, Bruseghin M, Abaterusso C, Saller A, et al. Course of renal function in type 2 diabetic patients with abnormalities of albumin excretion rate. Diabetes. 2000; 49: 476-484.
- Trevisan R, Vedovato M, Mazzon C, Coracina A, Iori E, Tiengo A, et al. Concomitance of diabetic retinopathy and proteinuria accelerates the rate of decline of kidney function in type 2 diabetic patients. Diabetes Care. 2002; 25: 2026-2031.
- Vupputuri S, Nichols GA, Lau H, Joski P, Thorp ML. Risk of progression of nephropathy in a population-based sample with type 2 diabetes. Diabetes Res Clin Pract. 2011; 91: 246-252.
- Gehan EA, George SL. Estimation of human body surface area from height and weight. Cancer Chemother Rep. 1970; 54: 225-235.
- CLAUSS A. [Rapid physiological coagulation method in determination of fibrinogen]. Acta Haematol. 1957; 17: 237-246.
- Ibrahim HA, Vora JP. Diabetic nephropathy. Baillieres Best Pract Res Clin Endocrinol Metab. 1999; 13: 239-264.
- Rossing K, Christensen PK, Hovind P, Tarnow L, Rossing P, Parving HH. Progression of nephropathy in type 2 diabetic patients. Kidney Int. 2004; 66: 1596-1605.
- Levey AS, Gassman JJ, Hall PM, Walker WG. Assessing the progression of renal disease in clinical studies: effects of duration of follow-up and regression to the mean. Modification of Diet in Renal Disease (MDRD) Study Group. J Am Soc Nephrol. 1991; 1: 1087-1094.
- Bruno G, Biggeri A, Merletti F, Bargero G, Ferrero S, Pagano G, et al. Low incidence of end-stage renal disease and chronic renal failure in type 2 diabetes: a 10-year prospective study. Diabetes Care. 2003; 26: 2353-2358.
- Keane WF, Brenner BM, de Zeeuw D, Grunfeld JP, McGill J, Mitch WE, et al. The risk of developing end-stage renal disease in patients with type 2 diabetes and nephropathy: the RENAAL study. Kidney Int. 2003; 63: 1499-1507.
- Kramer HJ, Nguyen QD, Curhan G, Hsu CY. Renal insufficiency in the absence of albuminuria and retinopathy among adults with type 2 diabetes mellitus. JAMA. 2003; 289: 3273-3277.
- Nielsen S, Schmitz A, Rehling M, Mogensen CE. Systolic blood pressure relates to the rate of decline of glomerular filtration rate in type II diabetes. Diabetes Care. 1993; 16: 1427-1432.
- Friedman R, Gross JL. Evolution of glomerular filtration rate in proteinuric NIDDM patients. Diabetes Care. 1991; 14: 355-359.
- Gall MA, Nielsen FS, Smidt UM, Parving HH. The course of kidney function in type 2 (non-insulin-dependent) diabetic patients with diabetic nephropathy. Diabetologia. 1993; 36: 1071-1078.
- Murussi M, Gross JL, Silveiro SP. Glomerular filtration rate changes in normoalbuminuric and microalbuminuric Type 2 diabetic patients and normal individuals A 10-year follow-up. J Diabetes Complications. 2006; 20: 210-215.
- Hoefield RA, Kalra PA, Baker PG, Sousa I, Diggle PJ, Gibson MJ, et al. The use of eGFR and ACR to predict decline in renal function in people with diabetes. Nephrol Dial Transplant. 2011; 26: 887-892.
- Looker HC, Fagot-Campagna A, Gunter EW, Pfeiffer CM, Venkat Naryan KM, et al. Homocysteine as a risk factor for nephropathy and retinopathy in type 2 diabetes. Diabetologia. 2003; 46: 766-772.
- Wald DS, Law M, Morris JK. Homocysteine and cardiovascular disease: evidence on causality from a meta-analysis. BMJ. 2002; 325: 1202.
- Buysschaert M, Dramais AS, Wallemacq PE, Hermans MP. Hyperhomocysteinemia in type 2 diabetes: relationship to macroangiopathy, nephropathy, and insulin resistance. Diabetes Care. 2000; 23: 1816-1822.
- Cabarkapa V, Djeric M, Stosic Z, Sakac V, Davidovic S, Eremic N. Determining relationship between homocysteinemia and biomarkers of inflammation, oxidative stress, and functional kidney status in patients with diabetic nephropathy. J Med Biochem. 2013; 32: 131-139.
- Mazza A, Bossone E, Mazza F, Distante A. Reduced serum homocysteine levels in type 2 diabetes. Nutr Metab Cardiovasc Dis. 2005; 15: 118-124.
- Wollesen F, Brattström L, Refsum H, Ueland PM, Berglund L, Berne C. Plasma total homocysteine and cysteine in relation to glomerular filtration rate in diabetes mellitus. Kidney Int. 1999; 55: 1028-1035.
- Unlüçerçi Y, Bekpinar S, Gürdöl F, Seferoğlu G. A study on the relationship between homocysteine and diabetic nephropathy in rats. Pharmacol Res. 2002; 45: 249-252.
- Finocchiaro P, Zoccali C. [Hyperhomocysteinemia and progression of renal disease]. G Ital Nefrol. 2005; 22: 590-596.
- Mathai M, Radford SE, Holland P. Progressive glycosylation of albumin and its effect on the binding of homocysteine may be a key step in the pathogenesis of vascular damage in diabetes mellitus. Med Hypotheses. 2007; 69: 166-172.
- Meguid E, Nahas A, Bello AK. Chronic kidney disease: the global challenge. Lancet. 2005; 365: 331-340.
- Kobayashi T, Inoue T, Okada H, Kikuta T, Kanno Y, Nishida T, et al. Connective tissue growth factor mediates the profibrotic effects of transforming growth factor-beta produced by tubular epithelial cells in response to high glucose. Clin Exp Nephrol. 2005; 9: 114-121.
- Goestemeyer AK, Marks J, Srai SK, Debnam ES, Unwin RJ. GLUT2 protein at the rat proximal tubule brush border membrane correlates with protein kinase C (PKC)-betal and plasma glucose concentration. Diabetologia. 2007; 50: 2209-2217.
- Yokoyama H, Kanno S, Takahashi S, Yamada D, Itoh H, Saito K, et al. Determinants of decline in glomerular filtration rate in nonproteinuric subjects with or without diabetes and hypertension. Clin J Am Soc Nephrol. 2009; 4: 1432-1440.
- Bakris GL, Weir MR, Shanifar S, Zhang Z, Douglas J, van Dijk DJ, et al. Effects of blood pressure level on progression of diabetic nephropathy: results from the RENAAL study. Arch Intern Med. 2003; 163: 1555-1565.
- Ueda H, Ishimura E, Shoji T, Emoto M, Morioka T, Matsumoto N, et al. Factors affecting progression of renal failure in patients with type 2 diabetes. Diabetes Care. 2003; 26: 1530-1534.
- Thomas MC, MacIsaac RJ, Tsalamandris C, Molyneaux L, Goubina I, Fulcher G, et al. The burden of anaemia in type 2 diabetes and the role of nephropathy: a cross-sectional audit. Nephrol Dial Transplant. 2004; 19: 1792-1797.
- McGill JB, Bell DS. Anemia and the role of erythropoietin in diabetes. J Diabetes Complications. 2006; 20: 262-272.
- Craig KJ, Williams JD, Riley SG, Smith H, Owens DR, Worthing D, et al. Anemia and diabetes in the absence of nephropathy. Diabetes Care. 2005; 28: 1118-1123.
- Li Vecchi M, Fuiano G, Francesco M, Mancuso D, Faga T, Sponton A, et al. Prevalence and severity of anaemia in patients with type 2 diabetic nephropathy and different degrees of chronic renal insufficiency. Nephron Clin Pract. 2007; 105: c62-67.
- Ritz E, Haxsen V. Diabetic nephropathy and anaemia. Eur J Clin Invest. 2005; 35 Suppl 3: 66-74.
- Fine L, Bandyopadhyay D, Norman T. Is there a common mechanism for the progression of different types of renal diseases other than proteinuria? Towards the unifying theme of chronic hypoxia. Kidney Int. 2000; 57: 22-26.
- Mohanram A, Zhang Z, Shahinfar S, Keane WF, Brenner BM, Toto RD. Anemia and end-stage renal disease in patients with type 2 diabetes and nephropathy. Kidney Int. 2004; 66: 1131-1138.
- Denton KM, Shweta A, Anderson WP. Preglomerular and postglomerular resistance responses to different levels of sympathetic activation by hypoxia. J Am Soc Nephrol. 2002; 13: 27-34.
- Luk AO, So WY, Ma RC, Kong AP, Ozaki R, Ng VS, et al. Metabolic syndrome predicts new onset of chronic kidney disease in 5,829 patients with type 2 diabetes: a 5-year prospective analysis of the Hong Kong Diabetes Registry. Diabetes Care. 2008; 31: 2357-2361.
- Misra A, Kumar S, Kishore Vikram N, Kumar A. The role of lipids in the development of diabetic microvascular complications: implications for therapy. Am J Cardiovasc Drugs. 2003; 3: 325-338.
- Cardoso CR, Salles GF. Predictors of development and progression of microvascular complications in a cohort of Brazilian type 2 diabetic patients. J Diabetes Complications. 2008; 22: 164-170.
- van der Zijl NJ, Hanemaaijer R, Tushuizen ME, Schindhelm RK, Boerop J, Rustemeijer C, et al. Urinary matrix metalloproteinase-8 and -9 activities in type 2 diabetic subjects: A marker of incipient diabetic nephropathy? Clin Biochem. 2010; 43: 635-639.
- Ansquer JC, Foucher C, Rattier S, Taskinen MR, Steiner G. DIAS Investigators. Fenofibrate reduces progression to microalbuminuria over 3 yeras in a placebo-controled study in type 2 diabetes: results from Diabetes atherosclerosis and risk factors Study (DIAS). Am J Kidney Dis. 2005; 45: 485-493.
- Luk AO, Yang X, Ma RC, Ng VW, Yu LW, Lau WW, et al. Association of statin use and development of renal dysfunction in type 2 diabetes--the Hong Kong Diabetes Registry. Diabetes Res Clin Pract. 2010; 88: 227-233.