
Research Article
J Dis Markers. 2021; 6(1): 1043.
Simple Hepatic Cysts as Markers of Thoracic Aortic Disease
Kim C¹, Ziganshin BA², Zafar MA², Buntin J² and Elefteriades J²*
¹Department of Biostatistics, Yale School of Public Health, New Haven, Connecticut, United States of America
²Aortic Institute at Yale-New Haven Hospital, Yale University School of Medicine, New Haven, Connecticut, United States of America
*Corresponding author: John Elefteriades, Aortic Institute at Yale-New Haven Hospital, Yale University School of Medicine, New Haven, Connecticut, United States of America
Received: June 08, 2021; Accepted: June 29, 2021; Published: July 06, 2021
Abstract
Objective: Thoracic Aortic Disease (TAD) is potentially lethal, yet difficult to detect as most patients are asymptomatic until the aneurysm dissects and becomes life threatening. Several clinical markers for TAD have been identified such as: bicuspid aortic valve, intracranial aortic aneurysm, bovine aortic arch, positive family history, and simple renal cysts. The aim of this study was to investigate the prevalence of Simple Hepatic Cysts (SHC) among individuals diagnosed with TAD in order to assess whether they can be used as a predictor of TAD.
Methods: In this retrospective study, the prevalence of SHC for (n=1244) hospital patients treated for TAD was evaluated and compared to a control group of (n=809) patients. TAD patients were divided into four subgroups: ascending aneurysm (788; 63.3%; descending aneurysm (123; 9.9%); type A dissection (137; 11%); type B dissection (196; 15.8%). The presence of SHC was determined based on either computed tomography, magnetic resonance imaging, or ultrasound imaging of these patients.
Results: Prevalence of SHC was 14.8%, 11.4%, 12.4%, and 14.8% in patients with ascending aneurysm, descending aneurysm, type A dissection, and type B dissection, respectively. Prevalence of SHC in the control group was 3.8% (p<0.001). The prevalence of SHC was not significantly different between males and females among the TAD patients as well as the control population.
Conclusion: Individuals with TAD have an increased prevalence of SHC compared to individuals without TAD. SHC can potentially be used as a clinical marker to detect patients at risk for TAD.
Keywords: Hepatic cysts; Thoracic aortic disease; Aortic aneurysms; Aortic dissections
Abbreviations
SHC: Simple Hepatic Cysts; TAD: Thoracic Aortic Disease; TAA: Thoracic Aortic Aneurysms; PCLD: Polycystic Liver Disease; CT: Computerized Tomography; US: Ultrasound; MRI: Magnetic Resonance Imaging
Introduction
According to the most recent statistics from the Centers for Disease Control and Prevention, aortic aneurysm and dissection has been responsible for taking the lives of more than 110,000 individuals in all age groups annually in the last decade [1]. Ruptured aortic aneurysms have almost a 90% mortality rate, and aneurysm disease is the 15th most common cause of death for individuals aged 65 and over [2]. The incidence of Thoracic Aortic Aneurysms (TAA) is estimated to be at least 5-10 per 100,000 person-years; surprisingly, TAD causes more deaths than the Human Immunodeficiency Virus (HIV) [3,4]. Death from acute aortic dissection often causes pre-hospital sudden death, yet its incidence is grossly underestimated with reports estimating that about 50% of type A dissections remain undetected as it resembles other disorders such as myocardial infarction [5]. Recent studies have reported a staggering 7% of all sudden deaths in the outof- hospital cardiopulmonary arrest due to type A dissection [6].
Given the lethality of thoracic aortic aneurysms, a timely detection and application of surgical therapy is critical. However, detection is often difficult given that only 5% of thoracic aortic aneurysms are symptomatic, the majority of which are diagnosed minutes before death-earning TAA the appropriate nickname of being a ‘silent killer’ [7]. Fortunately, recent studies have identified clinical markers, or ‘associates’ of TAA that can detect individuals at risk of having or developing an aneurysm in their chest. Such guilty suspects that have been associated with Thoracic Aortic Disease (TAD) include intracranial aneurysms, bovine aortic arch, bicuspid aortic valve, a family history of thoracic aortic disease, and renal cysts [7].
A “Simple Hepatic Cyst” (SHC) refers to a solitary, benign, and non-parasitic cyst of the liver that may contain a clear yellow fluid [8]. These are thought to arise due to abnormal development of congenitally aberrant intrahepatic bile ducts that slowly dilate later in life [8]. Most SHC are rarely symptomatic and do not require immediate clinical attention: 80% to 95% of hepatic cysts have reported to remain asymptomatic until an acute event such as upper abdominal pain, rupture, infection, or hemorrhage [9]. Several autopsy and imaging-based studies have tried to evaluate the prevalence of SHC in the general population, with great variability in results. Estimates of hepatic cyst prevalence have been reported to be as low as 0.1% or to be as high as 29%, largely depending on factors such as the imaging modality and population studied (Table 1). The majority of the literature reports higher prevalence rates of SHC among females (Table 1). The prevalence of SHC also increases with age [8].
Source
Modality
No. of Subjects
Mean Age (Range)
Overall Prevalence of SHC
Male-to-Female Ratioa
Sanfelippo et al, 1973 [9]
Surgically
88,000
59 (3 mo-82)
0.10%
0.62
Gaines et al, 1989 [10]
US
1,695
0-80+
2.50%
0.54
Jones et al, 1992 [11]
CT
1,454
35-85
17.50%
-
Caremani et al, 1993 [12]
US
26,514
19-91
2.90%
0.8
Huang et al, 1995 [13]
US
3,600
0-60+
3.70%
1.64
Kreft et al, 2001 [14]
MRI
628
-
28.50%
-
Carrim et al, 2003 [15]
CT
617
64.2 (17-92)
17.80%
1.39
Larssen et al, 2005 [16]
US
1,541
59.6 (Female) 55.4 (Male)
10.40%
0.6
Victor et al, 2013 [17]
MRI
246
53.3
13.80%
-
Horta et al, 2015 [18]
CT
1,184
61 (48-74)
24.40%
0.63
Kaltenbach et al, 2016 [19]
US
45,319
64.7
5.80%
0.78
aMale-to-female ratio in the prevalence of SHC.
Table 1: Previous reports in the literature on the prevalence of Simple Hepatic Cysts (SHC) in the general population.
Previous studies have found an increased prevalence of both renal and hepatic cysts among Marfan syndrome patients [20]. Furthermore, another recent study has found a positive association between renal cysts and thoracic aneurysms [21]. At the present, however, the association between hepatic cysts and thoracic aortic aneurysms has not, to the best of our knowledge, been explored. Thus, the purpose of our study is to determine whether simple hepatic cysts are associated with thoracic aortic aneurysms. We evaluated the prevalence of SHC among patients seen at the Aortic institute at Yale New Haven with thoracic aortic aneurysm or dissection and compared that to the prevalence of SHC in a control group of patients without thoracic aortic aneurysm or dissection. If an association exists between hepatic cysts and TAD (aneurysm and dissection), we anticipate that hepatic cysts may be an important screening marker for TAD.
Methods
Patient population
All consecutive patients treated for TAD between the years of 2000 and 2015 at the Aortic Institute of Yale-New Haven Hospital were retrospectively reviewed for the present study. The Aortic Database query revealed a total of 1983 patients treated for TAD. Among this number, a detailed radiological imaging report containing information regarding the liver was available for 1244 patients (62.7%). Patients treated for TAD but who had no imaging information regarding the liver (739 patients (37.2%)) were excluded from this study. Of the remaining 1244 patients who comprised our study population, there were 830 males (65.0%) and 445 females (34.9%). The mean age of the total study population was 63.4±14.3 and the age range was 8 to 93 years old.
Subgroup population
Thoracic Aortic Disease (TAD) is defined as any one of the following: (a) thoracic aortic aneurysm, in which dilation of the aneurysmal aorta is ≥4.0 cm (b) acute aortic dissection, including either a Stanford type A dissection of the ascending aorta, or a Stanford type B dissection of the descending aorta) [22].
The study group patients were classified into four different subgroups according to type and location:
• Ascending aortic aneurysm group: 788 patients (63.3%) with aneurysms of the root, ascending, or arch of the aorta. The mean age was 62.4±13.9. Of these, 549 patients were male (69.7%) and 239 were female (30.3%).
• Descending aortic aneurysm group: 123 patients (9.9%) with aneurysms of the descending and thoracoabdominal aorta. The mean age was 69.6±13.3. Of these, 70 patients were male (56.9%) and 53 were female (43.1%).
• Stanford type A dissection group: 137 patients (11.0%) with a type A dissection. The mean age was 61.3±14.9. Of these, 94 patients were male (68.6%) and 43 patients were female (31.3%).
• Stanford type B dissection group: 196 patients (15.8%) presented a type B dissection originating from a tear in the descending thoracic aorta. The mean age was 65.0±14.9. Of these, 105 patients were male (53.6%) and 91 patients were female (46.4%).
Evaluation of radiology imaging studies to determine the presence of Simple Hepatic Cysts (SHC)
Only patients with non-syndromic TAD were included in this study. Patients with TAD and with a comorbidity of Marfan Syndrome were also included in this study. The presence of simple hepatic cysts was evaluated via computed tomography, magnetic resonance imaging, and/or echography scan records. All patients undergo chest CT or MRI preoperatively. Liver ultrasound examinations had been performed for other reasons in only 15% (19 out of 1244) of the imaging examinations that form the basis of this study. Patients were determined to have simple hepatic cysts based on the radiologist’s citation of its presence in the radiology report. Radiologic definition of a simple hepatic cyst refers to a round, low attenuation lesion with a size ≥5mm that is present in either one or both lobes of the liver. The radiologist(s) were not aware of the current study.
In addition to imaging data, relevant demographic information collected for all patients with a TAD included gender, age (at which the hepatic cyst(s) was initially discovered), and date of surgical intervention. This data was collected through the imaging scan reports, the Aortic Institute patient records, and electronic medical records.
Control group
The control group for this study were patients admitted through the Yale-New Haven Hospital emergency department with a diagnosis of trauma or motor vehicle accident during the 2004-2013 period and who also had an imaging study (CT and/or MRI) performed at admission. This control group was selected to avoid selection bias, reasoning that trauma patients are not expected to favor having either the presence of hepatic cysts or TAD. Patients with either a known or identified aortic disease, or with known or identified liver disease such as Polycystic Liver Disease (PCLD) favoring the presence of hepatic cysts were excluded from the study. The control group included 809 patients (mean age, 39.7±20.7 years; range, 2-94), of which 459 patients (56.7%) were male and 350 patients (43.2%) were female. Due to the nature of the radiology database, clinical or demographic information was not able to be collected for the control subjects.
Definition of risk factors and comorbidities
Risk factors and comorbidities were evaluated among individuals in the study population and defined as the following:
• Hypertension: systolic blood pressure greater than 140 mmHg and diastolic greater than 100 mmHg or treatment with at least 1 antihypertensive medication;
• Hyperlipidemia: total cholesterol level over 200 mg/dL or treatment with lipid control medications;
• Diabetes: medically recorded diagnosis of diabetes either diet-controlled, controlled by oral medications, or requiring insulin;
• Obesity: extreme obesity, BMI>40;
• Smoking: current or previous history of smoking;
• Cerebral vascular accident: history of stroke;
• Chronic Obstructive Pulmonary Disease (COPD);
• Myocardial infarction: history of myocardial infarction;
• Steroid use: use of oral steroids (i.e., prednisolone);
• Neurological deficit: history of significant pathological conditions affecting the central or peripheral nervous systems;
• Marfan syndrome: clinical and/or genetic diagnosis of Marfan Syndrome;
• Positive family history: presence of family history of aneurysm disease;
• Pulmonary/respiratory disease: history of significant pathological conditions affecting the lung parenchyma, pleura, or tracheobronchial tree;
• Concurrent uncontrolled malignancy: history of concurrent uncontrolled malignancy;
• Renal failure: history of chronic kidney disease or elevated creatine (>2).
Statistical analysis
Pearson’s Chi-Square test was used to compare proportions between categorical variables. If the sample size was too small, Fisher’s exact test was used instead. The 2-tailed unpaired t-test was used to compare continuous variables. The level of significance was set at alpha=5% and the p-value was given to 3 decimal places. Multivariate logistic regression was performed using R software. Multivariate analysis was used to control for potential confounding variables such as age and gender, given the difference in mean age and gender proportions between the study and control groups. Multivariate logistic regression models included either age, or both age and gender along with the anatomical groups in the study population (ascending aortic aneurysm, descending aortic aneurysm, Stanford type A aortic dissection, and Stanford type B aortic dissection). Multivariate logistic regression also controlled for sex due to the majority of literature studies suggesting an increased prevalence of hepatic cysts among females. The control population was set as the reference group in multivariate logistic regression models.
Ethical considerations
The study is approved by the Yale Human Investigation Committee.
Results
Overall prevalence of SHC
Among the 1244 patients from the study population, 177 patients (14.2%) had at least 1 SHC. Among the 809 patients from the control group, prevalence of SHC was 31 patients (3.8%), which was significantly lower than the prevalence in the study population (p<0.001).
The prevalence of SHC was evaluated separately for all four anatomical groups of the study population. Overall prevalence of SHC was 14.8% (117 out of 788), 11.4% (14 out of 123), 12.4% (17 out of 137), and 14.8% (29 out of 196), in patients with ascending aortic aneurysm, descending aortic aneurysm, and type A and type B aortic dissection, respectively (Figure 1). In all four anatomical subgroups of the study population, the prevalence of SHC was statistically greater than the control population (p<0.001).
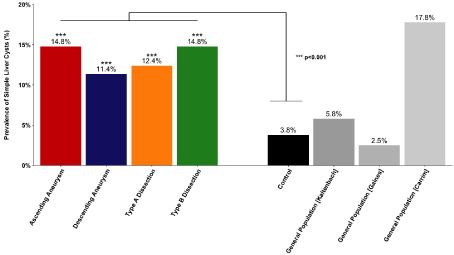
Figure 1: Overall prevalence of Simple Hepatic Cysts (SHC) in patients with Thoracic Aortic Disease (TAD) was greater in comparison to the control group and the
general population (historical controls). In all four anatomical subgroups of the study population, the prevalence of SHC was significantly different from the control
group (p<0.001).
Age-related prevalence of SHC
Prevalence of SHC was calculated for 10-year age groups among the TAD study population and control population. The prevalence of SHC was significantly greater in the study population compared to the control group for age groups 40-49 years (p=0.034) and 50-59 years (p=0.033) (Table 2, Figure 2). The prevalence of SHC was also greater but not significantly greater in the study population compared to the control group for age groups 30-39, 60-69, 70-79, and 80+ years. Test for trend analysis showed an increasing linear trend in the proportion of cases across age groups (p=0.036). Patients in the study group had a statistically higher rate of SHC than the following age groups from the historical controls from the literature: 40-49, 50-59, 60-69, and 70-79 (Gaines et al, Figure 2), and 80+ (Larssen et al, Figure 2).
Age Group
Odds Ratio (95 % Confidence Interval)
P value
0-19
-
-
20-29
-
-
30-39
1.34 (0.25, 5.91)
0.709
40-49
2.95 (1.06, 9.68)
0.034*
50-59
2.93 (1.12, 10.30)
0.033*
60-69
1.57 (0.71, 3.97)
0.272
70-79
1.34 (0.54, 4.10)
0.526
80+
1.82 (0.64, 6.70)
0.265
*Statistically significant result (p<0.05)
Table 2: Odds ratios with 95% confidence intervals of having Simple Hepatic Cysts (SHC) among patients with Thoracic Aortic Disease (TAD) compared to the control population by age group.
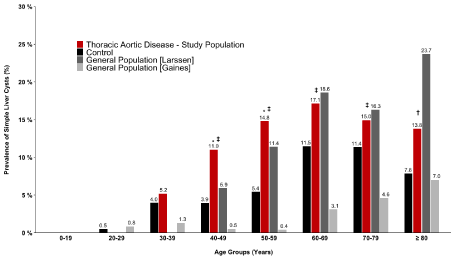
Figure 2: Prevalence of Simple Hepatic Cysts (SHC) among patients with Thoracic Aortic Disease (TAD) by age groups compared to the control group and
general population. Symbols denote statistically significant difference between the study population and *control population, ‡prevalence reported by Gaines et
al, †prevalence reported by Larssen et al. The prevalence of SHC was significantly greater in the study population compared to the control group for age groups
40-49 years (p=0.034) and 50-59 years (p=0.033).
Multivariate logistic regression was used to control for a potential confounding factor age given a statistically significant difference in mean age between the study population (63.4±14.3) and control group (39.7±20.1) (p<0.001). Controlling for age, the odds of having SHC among the study population was statistically 2.68 fold greater (95% CI: 1.77, 4.19) compared to the control group (Table 3). Controlling for age, the odds of having a SHC was statistically greater among all anatomical groups of the TAD study population with the exception of patients with descending aneurysms compared to the control group (Table 3). It is also worth noting that the odds of having a SHC among patients with a type A dissection was only borderline significant (p=0.043). The odds of having SHC was statistically strongest among patients with ascending aneurysm (p<0.001) and type B dissections (p=0.005) compared to the control group (Table 3).
Presence of Simple Hepatic Cysts (SHC)
Odds Ratio ± SE
95% Confidence Interval
P value
Age
1.03 ± 0.0044
(1.02, 1.04)
<0.001*
Male Sex
1.16 ± 0.18
(0.86, 1.58)
0.325
Thoracic Aortic Disease
2.68 ± 0.59
(1.77, 4.19)
<0.001*
Ascending Aneurysm
2.67 ± 0.60
(1.74, 4.23)
<0.001*
Descending Aneurysm
1.33 ± 0.49
(0.63, 2.71)
0.446
Type A Dissection
1.96 ± 0.65
(1.02, 3.76)
0.043*
Type B Dissection
2.32 ± 0.69
(1.29, 4.18)
0.005*
*Statistically significant result (p<0.05).
Table 3: Multivariate logistic regression results after controlling for patient age between patients with Thoracic Aortic Disease (TAD) and control group.
Sex controlled prevalence of SHC
Prevalence of SHC was calculated separately for males and females in both the TAD study population and control group (Figure 3). Among patients with ascending aortic aneurysm, prevalence of SHC was 15.1% (83 out of 549) for males and 14.2% (34 out of 239) for females (p=0.746). Among patients with descending aortic aneurysm, prevalence of SHC was 12.9% (9 out of 70) for males and 9.4% (5 out of 53) for females (p=0.760). Among patients with type A dissection, prevalence of SHC was 10.6% (10 out of 94) for males and 16.3% (7 out of 43) for females (p=0.516). Among patients with type B dissection, prevalence of SHC was 13.3% (14 out of 105) for males and 16.5% (15 out of 91) for females (p=0.536). So, there was no significant gender difference for all four categories of aortic disease. The prevalence of SHC was greater in males among patients with either ascending or descending aortic aneurysms and greater among females for patients with either type A or type B dissections; however, no statistically significant difference was observed between males and females within each anatomic subgroup (p>0.05, Figure 3). In the control group, prevalence of SHC was greater among females as expected from the literature [9,10,12,16,18,19]-however, this difference was not statistically significant (p=0.339). Among the historical controls, only one study (Kaltenbach et al.) showed a statistically greater prevalence of SHC among females (p<0.001, Figure 3).
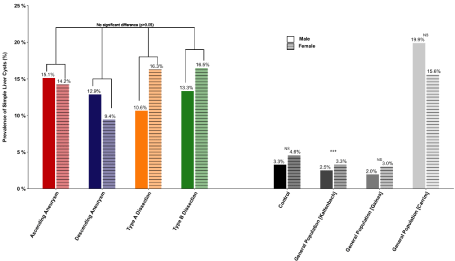
Figure 3: Prevalence of SHC in male and female patients with Thoracic Aortic Disease (TAD) compared to the control group and general population (historical
controls). No statistically significant difference in the prevalence of SHC was observed between males and females from both the study and control population.
Among the historical controls, only Kaltenbach et al. showed a statistically difference in the prevalence of SHC between males and females (p<0.001).
***statistically significant (p<0.001), NS = not statistically significant.
Incidence of SHC in ascending and descending aortic disease
According to the Stanford classification, type A dissections are defined to involve the ascending aorta, and type B to involve the descending aorta [23]. Furthermore, given that the pathogenesis, etiology, and even survival rates differ between ascending and descending thoracic aneurysms, we grouped the ascending aneurysm and type A dissections into one “ascending group” as well as the descending and type B dissections into one “descending group” in order to assess differences in the prevalence of SHC between these two aortic pathology groups [23]. Among the patients in the ascending aortic pathology group, overall prevalence of SHC was 14.5 % (134 out of 925) and in the descending aortic pathology group, overall prevalence of SHC was 13.5% (43 out of 319). However, the difference in the prevalence of SHC between these two pathology groups was not statistically significant (p=0.657). Even after controlling for potential confounding variables such as age and gender, the difference in the prevalence of SHC between these two groups was not statistically significant (p=0.482).
Clinical characteristics for patients with TAD
Detailed clinical baseline characteristics were collected and evaluated for patients in the TAD study population; however, these were not evaluated for patients in the control group due to the limitations in the radiology database. Complete clinical information was available for 98.3% of TAD study group patients with a SHC (174 out of 177) and 98.3% of TAD study group patients without a SHC (1049 out of 1067) (Table 4). After controlling for gender, the mean age of TAD patients with SHC (65.3±11.6) was statistically higher than the mean age of TAD patients without SHC (63.1±14.7) (p=0.049). Controlling for age and gender, TAD patients with SHC were more likely to be obese, have a stroke event, have COPD, have taken steroid medication, and to have neurological deficit, and pulmonary/respiratory disease; however, these differences were not statistically significant (Table 4).
Variable
TAD with Simple Hepatic Cysts
TAD without Simple Hepatic Cysts
P Value
No. of Subjects
Percentage
No. of Subjects
Percentage
Total No. of Patients
177
14.20%
1067
85.80%
-
Males
116
65.50%
702
65.80%
0.816
Mean Age
65.3 ± 11.6
-
63.1 ± 14.7
-
0.049*
Clinical information available
174
98.30%
1049
98.30%
-
Cancer
31
17.80%
207
19.70%
0.343
Obesity (BMI>40)
12
6.90%
68
6.50%
0.572
Diabetes
8
4.60%
145
13.80%
<0.001*
Hyperlipidemia
74
42.50%
485
46.20%
0.196
Hypertension
145
83.30%
874
83.30%
0.476
Positive Smoking History
90
51.70%
556
55.00%
0.666
Cerebrovascular Accident
20
11.50%
106
10.10%
0.643
Chronic Kidney Disease
17
9.80%
111
10.60%
0.637
COPDa
33
19.00%
178
17.00%
0.639
Myocardial Infarction
14
8.00%
107
10.20%
0.275
Steroid
24
13.80%
123
11.70%
0.648
Neurological deficit
9
5.20%
45
4.30%
0.652
Marfan Syndrome
9
5.20%
26
2.50%
0.003*
Positive Family History
48
27.60%
222
21.20%
0.049*
Respiratory Diseaseb
21
12.10%
88
8.40%
0.148
aChronic obstructive pulmonary disease.
bPulmonary or respiratory disease.
*Statistically significant result (p<0.05).
Table 4: Comparison of clinical characteristics between TAD study population patients with and without SHC.
Interestingly, after controlling for gender and age, TAD patients with SHC were significantly less likely to have diabetes (p<0.001), but more likely to have either a positive family history of TAD (p=0.049) or Marfan Syndrome (p=0.003). However, this may not reflect a real effect due to small sample size.
Relationship between aortic size and the presence of SHC
Among the patients with ascending and descending aortic aneurysms, mean sizes of the aorta were compared between those with and without SHC. Aortic sizes were available for 99.1% of patients with ascending aortic aneurysms (665 out of 671) and 98.2% for patients with descending aortic aneurysms (107 out of 109). For patients with ascending aortic aneurysms, the mean size of the aorta was 5.13±0.76 cm and 5.02±0.91 cm in those with and without SHC, respectively. For patients with descending aortic aneurysms, the mean size of the aorta was 6.18±2.04 cm and 5.84±1.61 cm in those with and without SHC, respectively.
The difference in mean aortic size between ascending aortic aneurysm patients with and without SHC was not statistically significant (p=0.151). Even after controlling for age and gender, the difference in mean aortic size between ascending aortic aneurysm patients with and without SHC was not statistically significant (p=0.262). Likewise, the difference in mean aortic size between descending aortic aneurysm patients with and without SHC was not statistically significant (p=0.597). After controlling for age and gender, the difference was also not found to be significant (p=0.547).
Discussion
Key findings in this study are as follows
Increased incidence of hepatic cysts in aneurysm patients: This investigation offers a very high suggestion that SHC and TAD aortic aneurysm (especially ascending aortic aneurysm) are strongly correlated. Our cardinal finding is that in all four categories of TAD (ascending aortic aneurysm, descending aortic aneurysm, type A dissection, and type B dissection), incidence of simple hepatic cysts was higher than in our control group (14.2% vs. 3.8%, p<0.001) and higher than expected for the general population from previously published literature. This preponderance of simple hepatic cysts was seen uniformly across all four thoracic aneurysm types.
Incidence of hepatic cysts as a function of age: Incidence of SHC generally increased with increasing age category among our TAD patients, although not uniformly for all categories.
Incidence of hepatic cysts as a function of aortic location: There was no consistent difference noted in SHC between ascending and descending aortic pathologies.
Relationship between aortic size and the presence of SHC: Precise aortic size did not appear to influence the likelihood of SHC in our TAD patients.
Possible pathophysiological link: While our study concerns simple hepatic cysts among patients without polycystic livers, hepatic cysts are also seen in Polycystic Liver Disease (PCLD), which is defined by the presence of 20 or more cysts and develops in association with Autosomal Dominant Polycystic Kidney Disease (ADPKD), the most commonly inherited kidney disorder that is characterized by the development of renal cysts and progressive increase of total kidney volume [8]. Studies have linked MMPs to playing a role in the hepatic cystogenesis of polycystic liver disease: for example, MMP-3 (stryomelysin), is found to be overexpressed in cystic cholangiocytes from patients with PCLD [24]. Furthermore, another study found that the overexpression of MMP-14 in fetal hepatic stem cells (hepatoblasts), to promote the formation of bile duct-like cysts [25].
Since these studies link MMP to its role in hepatic cystogenesis among patients affected by PCLD or ADPKD, the exact role of MMP in hepatic cystogenesis among patients with SHC is still unclear. However, these studies offer support to potentially link MMP expression in hepatic cystogenesis with thoracic aortic aneurysms, given that MMPs have already been implicated in the development of aortic aneurysms by multiple studies [26,27]. It may be possible that the TAD and SHC share a common genetic pathway or pathogenesis through elevated MMP levels.
Aortic aneurysm as two different diseases: In this study, we found a higher overall incidence of SHC in patients with ascending aortic pathology, compared to patients with descending aortic disease. After controlling for age, multivariable analysis showed that the odds of having SHC was statistically strongest among patients with ascending aortic aneurysms, (OR: 2.67, CI: [1.74, 4.23], p<0.001), compared to control patients. Therefore, the link between SHC and TAD seems to be stronger for the ascending aorta. This is supported by the fact that ascending and descending aortic aneurysm are two different diseases: ascending aortic pathology is largely non-atherosclerotic and noncalcified in nature, whereas descending aortic pathology has heavy arteriosclerosis and calcification [23]. Given that elevated MMP levels play a central role in ascending aortic aneurysms, in particular, and that MMPs have also been implicated in hepatic cystogenesis, it is possible that MMPs are a common factor responsible for SHC and ascending aortic aneurysms [28-30].
Strengths & Limitations
One strength of our study is that we have a screened a very large number of patients with thoracic aortic disease (N=1244) for the presence of simple hepatic cysts. However, this study is not without limitations.
This study is limited by its retrospective nature. As in most large clinical studies, there are some missing data points. Patients from the study population with missing documentation regarding the presence or absence of simple hepatic cysts from imaging scans were simply not included in this analysis. Missing-data in other data fields was handled using complete-case analysis, which can potentially produce biased results if systematic differences exist between patients with missing data and those with complete data. This seems unlikely to be the case here.
For our study, we adjusted for confounders using logistic regression models. Although this method is theoretically sound, in practice, there are other methods to reduce biases, including propensity scores (including inverse probability weighting), which would balance baseline covariates between patients in the study and the control population and mimic a key property of randomized control trials-thereby addressing a weakness of observational studies like ours. However, in order to choose a least biased propensity score model we would need to identify appropriate covariates that are correlated with at least one of the anatomical subgroups as well as SHC itself, and it is unclear what variables, beyond age and sex, would need to be included in the model. Another limitation in our study, attributable to the inherent nature of the radiology database, is unavailability of risk factors and comorbidities. As a result, we were only able to adjust for sex and age for patients in the control population, although we feel that these two are the most important potential confounders for our study.
Studies of this nature, of course, depend on diagnostic imaging sensitivity for detection of SHC. The overall prevalence of SHC among our control population was 3.8%, which is within the range of the prevalence rates for SHC in the general population as reported by our two historical controls, Kaltenbach et al. (5.8%) and Gaines et al. (2.5%), but is markedly lower than that reported by Carrim et al. (17.8%), whose high prevalence rate appears to be an outlier (Figure 1) [10,15,19]. Carrim et al. provides two potential explanations for this: one reason is that small cysts are misdiagnosed as hepatic hemangiomata (another common liver lesion) and another reason is due to the greater sensitivity of using CT scan (as opposed to ultrasound) for detection of hepatic cysts [15]. The latter reason is plausible since both Kaltenbach et al. and Gaines et al. use ultrasound as their modality for detection and have reported lower prevalence rates for SHC among the general population [10,19]. Although our study used only CT scans for detection of hepatic cysts among the control population, we reported a prevalence rate that was within the range of those reported by our two historical control studies (Kaltenbach et al. and Gaines et al.) that used ultrasounds for the detection of hepatic cysts among the general population [10,19].
Finally, our study merely conveys an association between hepatic cysts and TAD and does not necessarily represent a causal effect. We are only able to speculate on a physiological mechanism to explain the link between SHC and TAD and suggest that they might follow a common etiology. Further studies are warranted to explain a causal pathway between TAD and SHC.
Recommendations
Because Thoracic Aortic Aneurysms (TAA) is a lethal and asymptomatic disease, this study suggests that patients with documented simple hepatic cysts should be screened for TAA. Several clinical “guilty associates” of TAA have already been identified to aid in detection of silent TAA [7,21]. With our report, we wish to include SHC in our list of “guilty associates” that suggest evaluating for TAA. For Chest CT, there is not much value to be gained since the aorta is already imaged. However, for abdominal CT or MRI, extending the image just a few inches higher to include the thoracic segment may bring value via the potential to detect thoracic aortic aneurysms. Likewise, for liver ultrasounds, there is potential value to be gained by evaluating the thoracic segment by CT scan as well. We are aware that implementing these recommendations in clinical practice will pose a societal cost, but we believe that the yield will be high-at least 14% or more for the potentially lethal disease of TAD. We hope to heighten the awareness of clinicians of the association between SHC and TAD, and for them to recognize the potential life-saving value of diagnosing silent TAA in hepatic patients.
Conclusion
The key finding from our study is that the prevalence of SHC was statistically greater among TAD patients than control patients. After controlling for age, TAD patients were 2.68 times more likely to have a SHC compared to the control patients. The highest prevalence of SHC was observed in patients with ascending aortic aneurysm. Contrary to the pattern observed in the general population, females were not statistically more likely to have SHC in the TAD population. These results are suggestive that simple hepatic cysts can be used as a marker to detect patients at risk for developing thoracic aortic disease.
References
- WISQARS Leading Causes of Death Reports. 2015.
- Abdulameer H, Taii HA, Al-Kindi SG, Milner R. Epidemiology of fatal ruptured aortic aneurysms in the United States (1999-2016). J Vasc Surg. Elsevier. 2019; 69: 378-384.e2.
- Mathur A, Mohan V, Ameta D, Gaurav B, Haranahalli P. Aortic aneurysm. J Transl Intern Med. 2016; 4: 35-41.
- FastStats. 2021.
- Wundram M, Falk V, Eulert-Grehn J-J, Herbst H, Thurau J, Leidel BA, et al. Incidence of acute type A aortic dissection in emergency departments. Sci Rep. Nature Publishing Group. 2020; 10: 7434.
- Tanaka Y, Sakata K, Sakurai Y, Yoshimuta T, Morishita Y, Nara S, et al. Prevalence of Type A Acute Aortic Dissection in Patients with Out-Of-Hospital Cardiopulmonary Arrest. Am J Cardiol. 2016; 117: 1826-1830.
- Elefteriades JA, Sang A, Kuzmik G, Hornick M. Guilt by association: paradigm for detecting a silent killer (thoracic aortic aneurysm). Open Heart. 2015; 2: e000169.
- Rawla P, Sunkara T, Muralidharan P, Raj JP. An updated review of cystic hepatic lesions. Clin Exp Hepatol. 2019; 5: 22-29.
- Sanfelippo PM, Beahrs OH, Weiland LH. Cystic disease of the liver. Ann Surg. 1974; 179: 922-925.
- Gaines PA, Sampson MA. The prevalence and characterization of simple hepatic cysts by ultrasound examination. Br J Radiol. 1989; 62: 335-337.
- Jones EC, Chezmar JL, Nelson RC, Bernardino ME. The frequency and significance of small (less than or equal to 15 mm) hepatic lesions detected by CT. AJR Am J Roentgenol. 1992; 158: 535-539.
- Caremani M, Vincenti A, Benci A, Sassoli S, Tacconi D. Ecographic epidemiology of non-parasitic hepatic cysts. J Clin Ultrasound JCU. 1993; 21: 115-118.
- Huang JF, Chen SC, Lu SN, Lin ZY, Chuang WL, Hsieh MY, et al. Prevalence and size of simple hepatic cysts in Taiwan: community- and hospital-based sonographic surveys. Gaoxiong Yi Xue Ke Xue Za Zhi. 1995; 11: 564-567.
- Kreft B, Pauleit D, Bachmann R, Conrad R, Krämer A, Schild HH. [Incidence and significance of small focal liver lesions in MRI]. ROFO Fortschr Geb Rontgenstr Nuklearmed. 2001; 173: 424-429.
- Carrim ZI, Murchison JT. The prevalence of simple renal and hepatic cysts detected by spiral computed tomography. Clin Radiol. 2003; 58: 626-629.
- Larssen TB, Rørvik J, Hoff SR, Horn A, Rosendahl K. The occurrence of asymptomatic and symptomatic simple hepatic cysts. A prospective, hospitalbased study. Clin Radiol. 2005; 60: 1026-1029.
- Victor B, Galvão T, rios torres L, Cardia P, Nunes T, Salvadori P, et al. Prevalence of simple liver cysts and hemangiomas in cirrhotic and noncirrhotic patients submitted to magnetic resonance imaging * Prevalência de cistos simples e hemangiomas hepáticos em pacientes cirróticos e não cirróticos submetidos a exames de ressonância magnética. Radiol Bras. 2013.
- Horta G, López M, Dotte A, Cordero J, Chesta C, Castro A, et al. [Benign focal liver lesions detected by computed tomography: Review of 1,184 examinations]. Rev Med Chil. 2015; 143: 197-202.
- Kaltenbach TE-M, Engler P, Kratzer W, Oeztuerk S, Seufferlein T, Haenle MM, et al. Prevalence of benign focal liver lesions: ultrasound investigation of 45,319 hospital patients. Abdom Radiol NY. 2016; 41: 25-32.
- Chow K, Pyeritz RE, Litt HI. Abdominal visceral findings in patients with Marfan syndrome. Genet Med. Nature Publishing Group. 2007; 9: 208-212.
- Ziganshin BA, Theodoropoulos P, Salloum MN, Zaza KJ, Tranquilli M, Mojibian HR, et al. Simple Renal Cysts as Markers of Thoracic Aortic Disease. J Am Heart Assoc Cardiovasc Cerebrovasc Dis. 2016; 5.
- Ziganshin BA, Elefteriades JA. Thoracic Aortic Disease. In: Stergiopoulos K, Brown DL, editors. Evid-Based Cardiol Consult. London: Springer. 2014; 331-353.
- Elefteriades JA, Farkas EA. Thoracic aortic aneurysm clinically pertinent controversies and uncertainties. J Am Coll Cardiol. 2010; 55: 841-857.
- Urribarri AD, Munoz-Garrido P, Perugorria MJ, Erice O, Merino-Azpitarte M, Arbelaiz A, et al. Inhibition of metalloprotease hyperactivity in cystic cholangiocytes halts the development of polycystic liver diseases. Gut. 2014; 63: 1658-1667.
- Otani S, Kakinuma S, Kamiya A, Goto F, Kaneko S, Miyoshi M, et al. Matrix metalloproteinase-14 mediates formation of bile ducts and hepatic maturation of fetal hepatic progenitor cells. Biochem Biophys Res Commun. 2016; 469: 1062-1068.
- Barutcu I, Karakaya O, Esen AM, Dogan S, Saglam M, Kargin R, et al. Circulating stromelysin concentration is elevated in hypertensive aortic root dilatation. Heart Vessels. 2009; 24: 138-141.
- Rabkin SW. Chapter Seven - The Role Matrix Metalloproteinases in the Production of Aortic Aneurysm. In: Khalil RA, editor. Prog Mol Biol Transl Sci. 2017; 239-265.
- Huusko T, Salonurmi T, Taskinen P, Liinamaa J, Juvonen T, Pääkkö P, et al. Elevated messenger RNA expression and plasma protein levels of osteopontin and matrix metalloproteinase types 2 and 9 in patients with ascending aortic aneurysms. J Thorac Cardiovasc Surg. 2013; 145: 1117- 1123.
- Ikonomidis JS, Jones JA, Barbour JR, Stroud RE, Clark LL, Kaplan BS, et al. Expression of matrix metalloproteinases and endogenous inhibitors within ascending aortic aneurysms of patients with bicuspid or tricuspid aortic valves. J Thorac Cardiovasc Surg. 2007; 133: 1028-1036.
- Schmitt R, Tscheuschler A, Laschinski P, Uffelmann X, Discher P, Fuchs J, et al. A potential key mechanism in ascending aortic aneurysm development: Detection of a linear relationship between MMP-14/TIMP-2 ratio and active MMP-2. PLOS ONE. 2019; 14: e0212859.