
Research Article
J Dis Markers. 2023; 8(2): 1054.
Repurposing the Incidental Detection of Internal Hemorrhoids as an Independent Predictor of Coronary Artery Disease Risk
Eroglu E¹*; Turkmen I²; Algul S³; Reddy V4; Altinli E¹
1Department of General Surgery, Memorial Hospital, Bahcelievler, Istanbul, Turkey
2Department of Radiology, Memorial Hospital, Bahcelievler, Istanbul, Turkey
3Department of Physiology, Yuzuncu Yil University, School of Medicine, Van, Turkey
4Heersink School of Medicine, University of Alabama, Birmingham, AL, USA
*Corresponding author: Eroglu E Department of General Surgery, Memorial Hospital, Bahcelievler, Istanbul, Turkey Tel: +90 212 408 45 45; Fax: +90 212 654 76 28 Email: erogluersanmd@gmail.com
Received: April 05, 2023 Accepted: May 12, 2023 Published: May 19, 2023
Abstract
The relationship between anal canal hemorrhoids and cardiovascular diseases has been demonstrated in retrospective population-based studies. Despite the etiological similarities, the real-time correlation between hemorrhoids and cardiovascular risk is not known yet. We aimed to investigate the importance of incidentally detected internal hemorrhoids in predicting cardiovascular risk as determined by Carotid Intima-Media Thickness (CIMT) and Epicardial Fat Thickness (EFT) measurements. Totally, 269 patients who underwent colonoscopy for benign reasons were enrolled in this single-center cross-sectional study. The groups with and without hemorrhoids were compared for CIMT, EFT, and other risk factors. Independent predictors for increased CIMT and EFT values were evaluated by regression analysis. The mean age of the patients was 43.5±13.9 years and 34.6% were female. The prevalence of internal hemorrhoids was 30.5% (n=82). The presence of hemorrhoids was one of the independent predictors for increased CIMT along with hypertension and smoking (OR: 4.29, 2.63, and 2.36, respectively). The independent predictors of increased EFT were female gender, high body mass indexes, and high platelet levels (OR: 2.27, 1.47, and 1.01 respectively). Incidentally diagnosed internal anal canal hemorrhoids, albeit for other reasons, can be a useful predictor for the risk of atherosclerosis and cardiovascular diseases.
Keywords: Hemorrhoid; Carotid intima-media thickness; Epicardial fat; Atherosclerosis
Abbreviations: BMI: Body Mass Indexes; CI: Confidence Interval; CIMT: Carotid Intima Media Thickness; CT: Computed Tomography; EFT: Epicardial Fat Thickness; IBD: Inflammatory Bowel Disease; MRI: Magnetic Resonance Imaging; NS: Non-Significant; OR: Odds Ratio; SD: Standard Deviation; VEGF: Vascular Endothelial Growth Factor
Introduction
Although anal canal hemorrhoids are among the most common clinical conditions in the general population, most patients are asymptomatic. While patients who have thrombosed external hemorrhoids and advanced internal hemorrhoids are admitted with bleeding, pain, or difficulty in defecation, patients who have early-stage internal hemorrhoids are incidentally diagnosed during proctoscopy or colonoscopy. The most common etiologies are dietary habits, sliding anal canal lining due to constipation, abdominal obesity, alcohol intake, and/or increased pressure on the lower rectum [1-3]. The most prominent demonstrated physiological abnormality is an increased resting anal canal pressure along with elevated matrix metalloproteinase activity and a degenerated collagen structure [4,5]. In addition, a study has shown that increased Vascular Endothelial Growth Factor (VEGF) and Nitric Oxide Synthase (NOS), which indicate increased inflammatory activity, was observed in internal hemorrhoid tissue samples [6,7].
Presently, cardiovascular diseases are also seen as frequently as hemorrhoids. However, cardiovascular disease remains one of the leading causes of death despite developing technology and increasing treatment options as it still cannot be completely prevented due to the increasing incidence of obesity-related sedentary lifestyles [8]. Many asymptomatic patients aged between 40 and 70 are admitted to the hospital with sudden cardiac death or an extensive myocardial infarction. The diagnosis, often determined after the cardiovascular event has occurred, is the main reason for increased mortality and morbidity [9]. In recent years, studies have focused on practical and reliable tests that can be used to predict high-risk populations, apart from the techniques used routinely in diagnosis and screening [10].
Carotid Intima-Media Thickness (CIMT) was investigated in many studies as the earliest predictor of morphologic or functional arterial wall deterioration and atherosclerosis [11]. According to some studies, CIMT measurements reflect the systemic pro-inflammatory state and it can be used as a surrogate marker for atherosclerotic plaque development. Even though routine CIMT measurement is not recommended by the main guidelines, this method is a well-established non-invasive technique with reliable age-sex-adjusted cut-offs to define cardiovascular risk [12].
Epicardial Fat (EF) is the deposit of visceral fat located between the myocardium and pericardium and has been shown to predict the risk of coronary artery disease [13]. EF embryologically develops from the same origin as omental and mesenteric adipose tissue [14]. Adipokines and proinflammatory cytokines released from adipose tissue play an important role in the development of atherosclerosis [15]. EF levels are evaluated as EF Thickness (EFT) with two-dimensional echocardiography or EF volume with cross-sectional methods (MRI or CT). These measurements are useful in predicting the risk of coronary artery disease [13].
However, these methods have not yet been widely used in clinical practice. In a population-based retrospective study on the relationship between hemorrhoids and cardiovascular diseases, it was shown that the risk of cardiovascular events is increased in patients with hemorrhoids [16]. From this point, we hypothesize that, regardless of other proven etiologies, the simultaneous development of these two conditions caused by increased vascular inflammatory stress can be used as a clinical predictor. Our aims in this study are to evaluate the relationship between internal anal canal hemorrhoids and cardiovascular risk as well as to increase awareness among clinicians who are performing colonoscopy about this small finding incidentally revealed during the procedure.
Materials and Methods
Study Design, Patient Enrollment, and Sample Collection
This cross-sectional study included 400 consecutive patients who underwent a colonoscopy for any reason at our hospital from July 2022 to October 2022. Demographic characteristics, chronic diseases, body mass indexes, habits, and drugs used by the patients were recorded. Patients who have chronic inflammatory conditions which may affect CIMT or EF measurements were excluded (Figure 1). In addition, according to the results of colonoscopy, patients with findings such as cancer and inflammatory bowel disease were also excluded as well as patients who had inadequate bowel preparation. Biochemistry and hemogram parameters were evaluated in fasting venous blood samples taken from the remaining 269 patients. The study was conducted in compliance with the principles outlined in the Declaration of Helsinki and was approved by the Institutional Ethics Committee (permit number: 2022/07-11). All patients provided written informed consent.
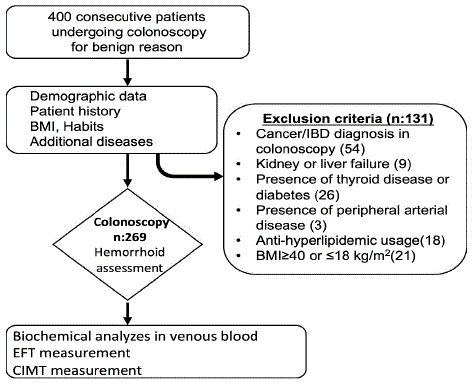
Figure 1: Flowchart of the cross-sectional study design.
Colonoscopy Assessment
After 8 hours of fasting and proper bowel preparation, the patient underwent a colonoscopy. All colonoscopy procedures were performed under conscious sedation by the same endoscopist with an Olympus tc35 XC700 ST (Tokyo, Japan) colonoscope. Before the procedure was terminated, a retroflection maneuver was performed and the anal canal and dentate line were evaluated at 360 degrees. Hemorrhoids that originated above the dentate line were considered internal hemorrhoids. Cases of external hemorrhoids were not included in the study.
Carotid Intima-Media Thickness (CIMT) and Epicardial Fat thickness (EFT) Measurements
CIMT and EFT measurements were performed as a single-blind assessment by a radiologist with a Siemens ACUSON S2000 Ultrasound system (Siemens Medical Solutions, Malvern, PA). Measurements were made from 4 different points in a segment of at least 10mm on the anterolateral and posterolateral longitudinal axis of the common carotid artery and the average of these values was obtained. Previously defined age and gender-adjusted cutoffs were used for significant CIMT elevation [17] (Figure S1). EFT measurements were performed by the same radiologist. A hypoechoic area between the epicardial surface and the parietal pericardium from at least two locations on the right ventricle-free wall was measured during diastole. The average of the three measurements taken from both locations was analyzed. The values equal to or above 5mm were used as a cutoff as determined by previous studies for the definition of increased EF thickness [18] (Figure S2).
Statistical Analysis
All statistical analyses were carried out using SPSS software (V21.0, IBM Inc, Chicago, Illinois, USA). The compliance of variables to the normal distribution was investigated using Kolmogorov-Smirnov and Shapiro-Wilk tests. While the normally distributed data were presented as mean and standard deviation, skewed data were shown as the median and interquartile range (Q25-Q75). Two group comparisons were done by using the Mann-Whitney U test or Student t-test according to data distribution patterns. Pearson or Spearman analyses were used for the correlations among variables. The differences between the categorical variables were assessed using the Chi-square test or Fischer’s exact test. For logistic regression analysis, the possible risk factors identified by univariate analysis or recent studies were included in the regression models to determine independent predictors of increased CIMT or EF thickness. To determine the optimal final model, some variables were excluded using the backward likelihood ratio stepwise method. Hosmer-Leme show goodness-of-fit statistics were used for the post hoc test. A 5% type 1 error was used to infer statistical significance.
Results
The mean age of the patients was 43.5±13.9 years, 93(34.6%) were female, and 176(65.4%) were male. The prevalence of internal hemorrhoids was 30.5%. According to internal hemorrhoid classification, 32(39%) patients were grade 1, 26(31.7%) patients were grade 2, 16(19.5%) patients were grade 3, and 8 (9.8%) patients were grade 4. The mean Body Mass Index (BMI) was 25.1±2.9kg/m2. EF thickness was increased in 41(15.2%) of the patients, while CIMT was higher than the cut-off value in 55(20.4%) patients. Other characteristics of the study population are shown in Table 1. The mean age in the hemorrhoids group was significantly higher than in the non-hemorrhoid group, 53.5 years and 39.1 years respectively (p<0.001). While the platelet levels were significantly lower in the hemorrhoids group, serum creatinine and AST values were significantly higher in the hemorrhoids group than in the non-hemorrhoids group. The comparison of other parameters between groups is shown in Table 2. EFT was ≥5mm in 19.5% of patients in the group with hemorrhoids and 13.4% in the non-hemorrhoid group. However, this difference was not statistically significant (p: 0.19). In the hemorrhoids group, CIMT was above the cutoff value in 22% of the patients. In the non-hemorrhoid group, CIMT was above the limit in only 9.6% of the patients, and this difference between the two groups was statistically significant (p<0.001) (Figure 2). In the correlation analysis, there was a weak but statistically significant positive correlation between CIMT and EFT (r:0.26, p:0.01). There was no correlation between hemorrhoid grade and CIMT or EFT values. In the multivariate logistic regression analysis, independent predictors for increased EFT were found to be female gender, high BMI, and high platelet levels. Factors that independently predicted increased CIMT were the presence of internal hemorrhoids, hypertension, and smoking (Tables 3 and 4).
Age (years)
43.5±13.9
Gender
Male n, (%)
176(65.4)
Female n,(%)
93(34.6)
Smoker n,(%)
42(15.6)
Alcohol users n, (%)
28(10.4)
Hypertension n,(%)
36(13.4)
BMI ≥25 kg/m2 n, (%)
130(48.3)
WBC, (103/µL)
7.3±1.7
Hemoglobin, (g/dL)
14.1 [12.6-15.1]
Platelet, (103/µL)
275[212-355]
Glucose, (mg/dL)
94[90-97]
AST, (U/L)
22[17-32]
Creatinine, (mg/dL)
0.8[0.6-1]
Trigliceride, (mg/dL)
148.2±29.5
HDL, (mg/dL)
47 [45-50]
LDL, (mg/dL)
134.5±18.2
VLDL, (mg/dL)
32[30-35]
Hemorrhoid n, (%)
82(30.5)
Hemorrhoid stage, median (min-max)
2(1-4)
Epicardial Fat thickness (mm.)
3.4[2.9-4.1]
Carotid intima-media thickness (mm.)
0.61±0.1
Continuous variables were shown as mean ± Standard Deviation (SD) or median and interquartile range- [Q25-Q75] according to their distribution pattern. BMI: Body Mass Indexes; HDL: High-Density Lipoprotein, LDL: Low-Density Lipoprotein; VLDL: Very Low-Density Lipoprotein; WBC: White Blood Cell Count, AST: Aspartate Transaminase.
Table 1: General characteristics of the study population (n=269).
Hemorrhoid (n=82)
Non-hemorrhoid (n= 187)
P values
Age, mean SD (years)
53.5±13.7
39.1±11.4
<0.001
Gender
Male n, (%)
52(29.5)
124(70.5)
0.64
Female n,(%)
30(32.3)
63(67.7)
Smoker n,(%)
19(23.2)
23(12.3)
0.02
Alcohol users n, (%)
11(13.4)
17(9.1)
0.28
Hypertension n,(%)
18(22)
18(9.6)
0.006
BMI ≥25 kg/m2 n, (%)
44(53.7)
86(46)
0.24
WBC, (103/µL)
7.2±1.4
7.4±1.8
0.33
Hemoglobin, (g/dL)
14.1[12.6-14.9]
14.1[12.5-15.2]
0.6
Platelet, (103/µL)
255[224-298]
288[207-383]
0.02
Glucose, (mg/dL)
94[88-100]
94[91-97]
0.06
AST, (U/L)
24[19-33]
18.5[16-23]
<0.01
Creatinine, (mg/dL)
0.9[0.7-1.1]
0.7[0.6-0.9]
0.02
Trigliceride, (mg/dL)
145.9±48.1
149.2±15.6
0.2
HDL, (mg/dL)
48[43-52]
47[45-50]
0.83
LDL, (mg/dL)
131.7±29.3
135.7±9.8
0.23
VLDL, (mg/dL)
32.5[28-36]
32[30-34]
0.27
EFT (mm.)
3.4[2.6-4.7]
3.4[3-4]
0.36
CIMT (mm.)
0.66±0.1
0.59±0.08
<0.001
Normally distributed variables were shown as mean±standard deviation, non-normally distributed variables were shown as median and interquartile range Q25-Q75, SD: Standard Deviation; BMI: Body Mass Indexes; HDL: High-Density Lipoprotein; LDL: Low-Density Lipoprotein; VLDL: Very Low-Density Lipoprotein; WBC: White Blood Cell Count; AST: Aspartate Transaminase; EFT: Epicardial Fat Thickness; CIMT: Carotid Intima-Media Thickness.
Table 2: Comparison of hemorrhoid versus non-hemorrhoid groups in terms of demographic and clinical features.
Univariate Analysis
Multivariate Analysis*
%95 Cl
%95 Cl
OR
Lower bound
Upper bound
p values
OR
Lower bound
Upper bound
p values
Age (years)
1.01
0.98
1.03
0.38
Gender (Female)
1.10
0.55
2.21.
0.76
2.37
1.00
5.57
0.048
Hemorrhoid status **
1.57
0.78
3.13
0.19
BMI (kg/m2)
1.35
1.18
1.54
<0.01
1.47
1.26
1.72
<0.001
Triglyceride (mg/dL)
1.01
1.00
1.02
0.02
Platelets (103/µL)
1.00
1.00
1.01
0.05
1.00
1.00
1.01
0.017
*Most reliable model which is found at step 4 by the stepwise backward likelihood ratio method was presented in the table. ** Presence of internal hemorrhoids was considered as a reference value for the regression analysis. CI: Confidence Interval; OR: Odds Ratio; BMI: Body Mass Indexes.
Table 3: Logistic regression analysis demonstrates the independent predictors for increased Epicardial Fat thickness (n=269).
Univariate Analysis
Multivariate Analysis*
%95 Cl
%95 Cl
OR
Lower bound
Upper bound
p values
OR
Lower bound
Upper bound
p values
Age (years)
1.03
1.01
1.05
0.001
Gender (Female)
1.00
0.53
1.86
0.99
Hemorrhoid status **
5.05
2.69
9.45
<0.001
4.29
2.24
8.22
<0.001
BMI (kg/m2)
1.04
0.94
1.15
0.39
Hypertension**
3.97
1.89
8.35
<0.001
2.63
1.14
6.09
0.023
Smoking **
3.38
1.66
6.86
0.001
2.36
1.06
5.26
0.035
*Most reliable model which is found at step 4 by the stepwise backward likelihood ratio method was presented in the table ** Presence of the condition was considered as a reference value for the regression analysis. CI: Confidence Interval; OR: Odds Ratio; BMI: Body Mass Indexes.
Table 4: Logistic regression analysis demonstrates the independent predictors for increased Carotid intima-media thickness (n=269).
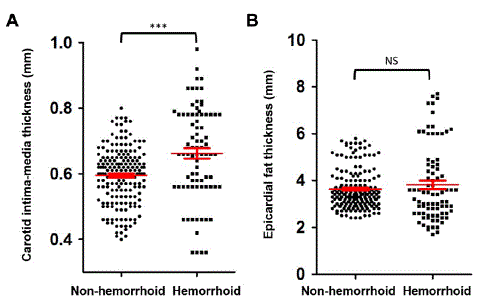
Figure 2: (A) Comparison of hemorrhoid and non-hemorrhoid groups in terms of carotid intima thickness and (B) Epicardial fat thickness.
Discussion
Individuals with a high risk of coronary events who are genetically at risk or with comorbid conditions such as hyperlipidemia, hypertension, diabetes, or renal failure can be followed closely in clinical practice. However, there is a need for additional screening methods that also cover patients in the low-risk group [19]. Anal canal hemorrhoids are quite common in the general population and many patients do not apply to the hospital since they are asymptomatic [20]. In this study, we evaluated the relationship between anal canal internal hemorrhoids incidentally detected and the risk of coronary artery disease. According to our results, the presence of internal hemorrhoids independently predicts increased CIMT, which is used as a well-established marker of early atherosclerosis. A population-based cohort study by Chang et al. showed that patients with hemorrhoids had a 1.27-fold increased risk of coronary events after adjusting for confounding factors [16]. Based on the author's opinion, similar dietary habits and physical inactivity in the etiology of hemorrhoids and coronary artery disease, as well as the higher incidence of hemorrhoids and coronary artery disease in the obese population are possible explanations for this association. In our study, morbidly obese (BMI≥40kg/m2) patients were excluded. However, CIMT measurements were found to be significantly higher in patients with hemorrhoids compared to those without hemorrhoids, both in obese patients and in individuals with normal BMI. Therefore, we think that, apart from obesity, a chronic inflammatory process may play a role in the pathophysiology of both diseases, but the details of this association are not known yet [16,21,22]. In a nationwide study by Hu et al. presenting longitudinal data of approximately eighty thousand patients, the presence of hemorrhoids was found to be associated with peripheral arterial occlusive disease independent of other risk factors [23]. Because of the common details in the etiology of occlusive peripheral artery disease and coronary artery disease, we considered the results of these two large studies in line with our results.
The importance of EF in the pathogenesis of coronary artery disease has been vigorously investigated during the last decade [13,24-26]. Besides coronary artery disease, there are publications related to myocardial stress and vascular endothelial chronic inflammation [27,28]. However, there is no study in the literature investigating the relationship between EF and anal canal hemorrhoids. In our study, we did not find a significant relationship between EFT and anal canal internal hemorrhoids. The fact that the mean BMI in our study population was above the overweight limit may have caused a decrease in the sensitivity of the basal EFT measurement.
Although the single-centered and cross-sectional design of our study seems to be a limitation, we think that it is important to present the first real-time data in this field for encouraging future studies.
In conclusion, although different factors are involved in the pathophysiology of venous and arterial diseases, this relationship observed in large population-based studies was also confirmed in our cross-sectional study. However, prospective studies with more parameters are needed to explore the casualty relationship between these two diseases. Authors should discuss the results and how they can be interpreted from the perspective of previous studies and of the working hypotheses. The findings and their implications should be discussed in the broadest context possible. Future research directions may also be highlighted.
References
- Ho YH, Buettner PG. Open compared with closed haemorrhoidectomy: meta-analysis of randomized controlled trials. Tech Coloproctol. 2007; 11: 135-43.
- Sun Z, Migaly J. Review of Hemorrhoid Disease: Presentation and Management. Clin Colon Rectal Surg. 2016; 29: 22-9.
- Thomson WH. The nature and cause of haemorrhoids. Proc R Soc Med. 1975; 68: 574-5.
- Plackett TP, Kwon E, Gagliano RA, Oh RC. Ehlers-danlos syndrome-hypermobility type and hemorrhoids. Case Rep Surg. 2014; 2014: 171803.
- Serra R, Gallelli L, Grande R, Amato B, De Caridi G, et al. Hemorrhoids and matrix metalloproteinases: A multicenter study on the predictive role of biomarkers. Surgery. 2016; 159: 487-94.
- Han W, Wang ZJ, Zhao B, Yang XQ, Wang D, et al. Pathologic change of elastic fibers with difference of microvessel density and expression of angiogenesis-related proteins in internal hemorrhoid tissues. Zhonghua Wei Chang Wai Ke Za Zhi. 2005; 8: 56-9.
- Wijers CH, van Rooij IA, Marcelis CL, Brunner HG, de Blaauw I, et al. Genetic and nongenetic etiology of nonsyndromic anorectal malformations: a systematic review. Birth Defects Res C Embryo Today. 2014; 102: 382-400.
- Riquelme R, Rezende LFM, Marques A, Drenowatz C, Ferrari G. Association between 24-h movement guidelines and cardiometabolic health in Chilean adults. Sci Rep. 2022; 12: 5805.
- Wong CX, Brown A, Lau DH, Chugh SS, Albert CM, et al. Epidemiology of Sudden Cardiac Death: Global and Regional Perspectives. Heart Lung Circ. 2019; 28: 6-14.
- Poredoš P, Cífková R, Marie Maier JA, Nemcsik J, Šabovič M, et al. Preclinical atherosclerosis and cardiovascular events: Do we have a consensus about the role of preclinical atherosclerosis in the prediction of cardiovascular events? Atherosclerosis. 2022; 348: 25-35.
- Naqvi TZ, Lee MS. Carotid intima-media thickness and plaque in cardiovascular risk assessment. JACC Cardiovasc Imaging. 2014; 7: 1025-38.
- Tosetto A, Prati P, Baracchini C, Manara R, Rodeghiero F. Age-adjusted reference limits for carotid intima-media thickness as better indicator of vascular risk: population-based estimates from the VITA project. J Thromb Haemost. 2005; 3: 1224-30.
- Ansaldo AM, Montecucco F, Sahebkar A, Dallegri F, Carbone F. Epicardial adipose tissue and cardiovascular diseases. Int J Cardiol. 2019; 278: 254-60.
- Chau YY, Bandiera R, Serrels A, Martínez-Estrada OM, Qing W, et al. Visceral and subcutaneous fat have different origins and evidence supports a mesothelial source. Nat Cell Biol. 2014; 16: 367-75.
- Liu L, Shi Z, Ji X, Zhang W, Luan J, et al. Adipokines, adiposity, and atherosclerosis. Cell Mol Life Sci. 2022; 79: 272.
- Chang SS, Sung FC, Lin CL, Hu WS. Association between hemorrhoid and risk of coronary heart disease: A nationwide population-based cohort study. Medicine (Baltimore). 2017; 96: e7662.
- Randrianarisoa E, Rietig R, Jacob S, Blumenstock G, Haering HU, et al. Normal values for intima-media thickness of the common carotid artery--an update following a novel risk factor profiling. Vasa. 2015; 44: 444-50.
- Mookadam F, Goel R, Alharthi MS, Jiamsripong P, Cha S. Epicardial fat and its association with cardiovascular risk: a cross-sectional observational study. Heart Views. 2010; 11: 103-8.
- Lechner K, von Schacky C, McKenzie AL, Worm N, Nixdorff U, et al. Lifestyle factors and high-risk atherosclerosis: Pathways and mechanisms beyond traditional risk factors. Eur J Prev Cardiol. 2020; 27: 394-406.
- Reese GE, von Roon AC, Tekkis PP. Haemorrhoids. BMJ Clin Evid. 2009; 2009: 0415.
- Yetkin E, Ileri M. Dilating venous disease: Pathophysiology and a systematic aspect to different vascular territories. Med Hypotheses. 2016; 91: 73-6.
- Zhu Y, Xian X, Wang Z, Bi Y, Chen Q, et al. Research Progress on the Relationship between Atherosclerosis and Inflammation. Biomolecules. 2018; 8: 80.
- Hu WS, Lin CL. Hemorrhoid is associated with increased risk of peripheral artery occlusive disease: A nationwide cohort study. J Epidemiol. 2017; 27: 574-7.
- Cabrera-Rego JO, Escobar-Torres RA, Parra-Jiménez JD, Valiente-Mustelier J. Epicardial fat thickness correlates with coronary in-stent restenosis in patients with acute myocardial infarction. Clin Investig Arterioscler. 2019; 31: 49-55.
- Iacobellis G, Assael F, Ribaudo MC, Zappaterreno A, Alessi G, et al. Epicardial fat from echocardiography: a new method for visceral adipose tissue prediction. Obes Res. 2003; 11: 304-10.
- Villasante Fricke AC, Iacobellis G. Epicardial Adipose Tissue: Clinical Biomarker of Cardio-Metabolic Risk. Int J Mol Sci. 2019; 20: 5989.
- Parsaei MS, Nabati M, Yazdani J, Bagheri B, Ghaemian A, et al. Relationship between epicardial fat and coronary microvascular dysfunction. Kardiol Pol. 2014; 72: 417-24.
- Sorop O, van de Wouw J, Chandler S, Ohanyan V, Tune JD, et al. Experimental animal models of coronary microvascular dysfunction. Cardiovasc Res. 2020; 116: 756-70.