
Research Article
J Dis Markers. 2024; 9(1): 1059.
Obesity Biomarkers and Leptin, IGF1, VEGFA Levels are Associated with Breast Cancer Risk in Mexican Women
González-Zamora A¹; Astorga AM²; González-Delgado MF³; Pérez-Morales R³*
1Laboratory of Evolutionary Biology, Faculty of Biological Sciences, University Juarez of the State of Durango, Mexico
2Ambulatory Care Medical Unit No. 53, Mexican Institute of Social Security, Mexico
3Laboratory of Cellular and Molecular Biology, Faculty of Chemical Sciences, University Juarez of the State of Durango, Mexico
*Corresponding author: Pérez-Morales RLaboratory of Cellular and Molecular Biology, Faculty ofChemical Sciences, University Juarez of the State ofDurango, Av. Artículo 123 s/n. Fracc Filadelfia, CP 35010,Gómez Palacio, Durango, México.Tel: 52 871 7158810Email: rebecapms@ujed.mx
Received: February 05, 2024 Accepted: March 14, 2024 Published: March 21, 2024
Abstract
Background: Breast cancer is the leading cause of death by can-cer in women. Obesity is a recognized risk factor for developing cancer, and the obesogenic microenvironment with high levels of glucose, lipids, hormonal, growth, and angiogenesis factors repre-sents an ideal microenvironment for carcinogenic promotion and progression.
Method: In this cross-sectional case‒control study, anthropo-metric measurements and biochemical parameters were analyzed. Leptin, IGF1 and VEGFA levels were analyzed by immunoassay, and genetic polymorphisms in LEP rs7799039, LEPR rs1137101, and VEGF rs699947 were determined by real-time PCR. Descrip-tive statistics, regression, correlation and multivariate discriminant analysis were performed.
Results: The average age of women was 54.70 and 50.35 years in cases and controls, respectively. We found an OR=1.71 (p=0.015) for women with obesity, OR=2.62 (p=0.001) for those with abdominal obesity, and OR=2.63 (p=0.0001) for those with diabetes. Significant differences in glucose (p=0.0001), leptin (p=0.002), IGF1 (p=0.02) and VEGFA (p=0.013) serum levels were found between cases and controls. The VEGF rs699947 polymorphism had an OR=1.85 (p=0.07) for the AA genotype with marginal significance. The correlation of the variables was different between the control and breast cancer groups, while the discriminant analysis managed to correctly classify more than 50% of women in each group; however, 28% of the controls and 46% of the cases should receive a more detailed follow-up in the risk of cancer and therapeutic treatment, respectively.
Conclusion: The obesogenic environment represents an impor-tant risk factor for the development of breast cancer in Mexican women.
Keywords: Breast cancer; Metabolic syndrome; Biochemical biomarkers; Leptin; Growth factors.
Introduction
Breast cancer is considered an epidemic of global dimensions, so it has been established as a priority health problem in several countries because it is currently the leading cause of mortality and disability among women [1]. Although it has been suggested that breast cancer mainly affects women with high socioeconomic status, there is currently a high incidence of breast cancer in women of all socioeconomic levels, which is exacerbated by the lack of an early diagnosis; therefore, patients are usually diagnosed in advanced stages, mainly in low-income countries [2]. According to data reported for all cancers in the Global Cancer Observatory, in 2020, breast cancer had the highest incidence in women among all other cancers, with 2,261,414 cases (11.7%), and the mortality was 15.5% (684, 996 cases), while in Mexico, it has the highest incidence in women among all cancers, with 105,963 cases (14.1%) and 46,082 deaths (13.2%) [3].
There are recognized risk factors for breast cancer, includ-ing family history, absence of pregnancy, menarche before age 12, first-term pregnancy after age 30, menopause after age 50, hormonal contraceptive use, and alcohol and tobacco use. Re-cently, obesity has been established as an important risk factor for developing breast cancer, mainly in postmenopausal wom-en [4].
Obesity is a public health problem worldwide. In Mexico, the prevalence of overweight was 39.1%, obesity was 36.1%, abdominal adiposity was 81.6%, and women had the highest prevalence [5].
Obesity is a chronic disease of complex etiology in which genetic and environmental factors play a role. In multifactorial obesity, several genes are involved, and environmental factors, such as nutritional status, microbiota, physical activity or exposure to chemical agents capable of modifying signaling pathways, particularly leptin/adiponectin, insulin/glucose, fatty acid metabolism, and the hypothalamic – pituitary – thyroid axis interact with these genes; all these alterations promote an increase in body mass and induce metabolic and immunological disturbances [6]. It has been proposed that cytokines derived from adipocytes, inflammatory factors, leptin resistance and insulin, as well as the hormonal load associated with obesity, play central roles in the initiation, promotion, and progression of cancer [7].
Recent studies have shown that adipose tissue exerts important endocrine control by secreting several hormones that can influence the pathophysiology of cancer, including leptin, which is one of the most abundant secreted adipocytokines. Leptin is a key mediator in energy balance and appetite control, and individuals with normal Body Mass Index (BMI) leptin reduce appetite by signaling brain receptors; however, in obesity, there is an overproduction of leptin, causing resistance in the system [8]. On the other hand, leptin may induce the overexpression of proliferation and angiogenesis factors, which may be directly related to the risk of developing cancer and the poor prognosis observed in obese patients with breast cancer [9]. Additionally, adipose tissue increases levels of the aromatase enzyme, resulting in high estrogen production, so obesity has also been associated with the imbalance of hormones such as estrogens, progesterone, androgens, and adrenal steroids, which may contribute to the progression of hormone-dependent cancer, such as some types of breast cancer [10].
Obesity promotes the development of coronary heart disease, liver cirrhosis, hypertension, stroke, dyslipidemia, metabolic syndrome, arthritis, insulin resistance and cancer through the dysfunction of signaling pathways that promote growth, differentiation, and angiogenesis. Insulin-like Growth Factor 1 (IGF1) is a polypeptide that is structurally similar to human proinsulin and is mainly produced by the liver by Growth Hormone (GH) stimulus. IGF1 levels are associated with the growth, regeneration and metabolism of lipids and carbohydrates and have also been associated with insulin sensitivity; in contrast, low serum IGF1 levels have been associated with insulin resistance, dyslipidemia, obesity, and metabolic syndrome [11]. In vitro studies indicate that IGF1 can induce proliferation in breast cancer cells via the receptor IGF1R and therefore have been implicated in the initiation and progression of this cancer [12].
Vascular Endothelial Growth Factor (VEGF) is a homodimeric glycoprotein expressed by different cell types, such as fibroblasts, keratinocytes, epithelial and endothelial cells, osteoblasts and inflammatory cells; that is closely related to the regulation of the growth and function of the vascular and lymphatic endothelium in both physiological and pathological processes [13]. VEGF plays a fundamental role in the regulation of the different stages of angiogenesis, studies have found that in carcinogenic progression, cells are capable of secreting a large amount of VEGFA to vascularize the tumor and metastasize [14].
Obesity, diabetes, and hypertension generate a microenvironment that can promote carcinogenic development, and an increase in the number of cases of breast cancer in obese women has been observed. This condition represents a worse prognosis of treatment with respect to nonobese patients, mainly in postmenopausal women who were diagnosed in stages III and IV; therefore, obesity has been associated with an increase in cancer mortality, a decrease in the quality of life of patients and a low response to treatment [15].
An obesogenic environment is the sum of conditions that favors the development of overweight and obesity. In recent decades, it has been observed that the Mexican population is highly sedentary, consumes more ultra-processed products and low nutritional quality. As result, Mexico has a high rate of obesity, and there is an increase in the number of cases of breast cancer because most Mexican women are diagnosed in advanced stages. In this regard, some factors are specific to each population, such as body composition, diet, distribution of risk factors, differential expression of genes that influence the biology of the tumor, and other environmental factors that may be related to the response to treatment; therefore, it is necessary to conduct specific epidemiological studies that analyze risk and poor prognosis biomarkers. In this study, some biomarkers were determined in healthy women and patients with breast cancer in a population of Mexican women to characterize the alterations associated with obesity and cancer risk in both groups.
Materials and Methods
Subjects
A cross-sectional case‒control study was conducted. Wom-en recently diagnosed with primary breast cancer and women without a cancer diagnosis were included. The breast cancer diagnosis was performed by oncologists and pathologists in Ambulatory Care Medical Unit No. 53 of Mexican Institute of Social Security in Gómez Palacio, Durango, México. All participants included in this study received general information about the study, signed an informed consent letter, and answered a questionnaire about their lifestyle, as well as epidemiological data. The study adhered to the Declaration of Helsinki and was approved by the Bioethics Committee of the Faculty of Chemical Sciences, University Juarez of the State of Durango (R-2017-123301538X0201-028).
Biological Samples
Body measurements were performed in all participants to obtain BMI and Waist/Hip Index (WHI) following the standard criteria. Blood samples of 168 women with primary breast can-cer and 168 samples of women without cancer diagnosis were collected under conditions of 8–12 hours of fasting, 6 ml with-out anticoagulant to obtain serum and 4 ml with EDTA to extract the DNA. The serum was obtained within two hours after ob-taining the sample and immediately processed to glucose, cho-lesterol, and triglyceride tests, and to immunoassay. Aliquots were kept at –70 °C until analysis.
Determination of Glucose, Cholesterol, and Triglyceride Levels
The biochemical parameters were determined in an auto-matic analyzer that includes colorimetric, potentiometric, and kinetic tests (System Vitros Chemistry 250 of Ortho-Clinical Diagnostics Johnson & Johnson). All determinations included their respective standards and calibrators. The concentrations of each parameter were expressed as milligrams, nanograms or picograms per deciliter.
Leptin, IGF1 and VEGFA Quantification by ELISA
Leptin, growth and angiogenesis factors in serum were determined by enzyme-linked immunosorbent assay (ELISA) (Sigma‒Aldrich, Germany) following the manufacturer’s rec-ommendations of the corresponding kits: RAB0333 for leptin, RAB0228 for IGF1 and RAB00507 for VEGFA. The plate was read on a Multiskan FC microplate reader (Thermo Fisher Scientific, Waltham, Massachusetts, U.S.) properly calibrated with a stan-dard curve for each case.
Genotyping of Polymorphisms in LEP rs7799039, LEPR rs1137101 and VEGF rs699947
Genomic DNA was purified from blood samples by the stan-dard salting out method, and DNA concentration and purity were established using a NanoDrop 2000 (Thermo Fisher Scien-tific Inc., Germering, Germany). The single nucleotide polymor-phisms were genotyped by real time PCR in a Step One Real-Time PCR (Applied Biosystems, Foster City, CA, USA) and hybridiza-tion probes (Integrated DNA Technologies rhAmp®), Hs.GT. rs7799039.A.1, HsGT.rs.1137101.G.1, and Hs.GT.rs699947.C.1 in accordance with the manufacturer’s instructions.
Statistical Analysis
Descriptive statistics such as chi squared, Student’s T test, logistic regression, correlation analysis between the different parameters and a discriminant analysis of grouping with variables of interest were performed. Statistical significance was considered if p<0.05. All statistical were performed with R software version 4.1.0.
Results
General Characteristics of the Study Population
The study population consisted of 336 women (168 cases and 168 controls), with a mean age of 54.70 years for the case group and 50.35 years for the control group (p=0.003). In the anthropometric evaluation, a higher frequency of obesity was found in the cases (48%), compared to the control group (36%) (p=0.01); a high frequency of diabetes was found in the cases (32%) compared to the controls (15%) (p=0.0003), while hypertension frequencies were 38% in the cases and 28% in the controls (p=0.06) with marginal significance (Table 1).
Parameter
Breast cancer cases
n=168
Mean (SD)Controls
n=168
Mean (SD)P – value
Age
54.70 (12.03)
50.35 (11.45)
0.003 a
Body Mass Index
30.51 (6.11)
28.67 (4.7)
0.006 a
Waist/hip ratio
0.92 (0.13)
0.88 (0.79)
0.001 a
Categorical variables
n (%)
n (%)
Obesity
82 (48)
60 (36)
0.01 b
Abdominal obesity
148 (88)
124 (74)
0.001 b
Menopause
115 (68)
109 (65)
0.48
Diabetes
53 (32)
25 (15)
0.0003 b
Hypertension
63 (38)
47 (28)
0.06 b
Family history of cancer
96 (57)
83 (49)
0.15 b
Breast cancer characteristics
n (%)
Histological type
Invasive Ductal Carcinoma
118 (83.1)
Invasive Lobular Carcinoma
24 (16.9)
Stage
II
4 (1.9)
IIIA
40 (28.1)
IIIB
84 (59.5)
IV
14 (10.5)
Molecular type
Luminal A
65 (65.6)
Luminal B
6 (6.0)
Her2
15 (15.1)
Triple negative
13 (13.3)
aT student's test.
bFisher's exact test.
P – value was considered statistically significant if p<0.05.
Table 1: General characteristics of the women included in the study.
In the pathological characteristics of the cases of breast cancer, the most frequent were invasive ductal carcinoma, stage IIIB and molecular type luminal A. These data are shown in Table 1. The logistic regression analysis showed that the variables of obesity (OR=1.71, CI=1.10–2.65), abdominal obesity (OR=2.62, CI=1.47–4.68), and diabetes (OR=2.63, CI=1.54–4.50) increased the probability of suffering from breast cancer, while the significance of hypertension was marginal (OR=1.54, CI=0.97–2.49) (Table 2).
Parameter
OR (CI 95%)
P – value
Obesity
1.71 (1.10 – 2.65)
0.015*
Abdominal obesity
2.62 (1.47 – 4.68)
0.001*
Diabetes
2.63 (1.54 – 4.50)
0.0001*
Hypertension
1.54 (0.97 – 2.49)
0.06*
Age
1.03 (1.01 – 1.05)
0.001*
Menopause
1.17 (0.75 – 1.84)
0.48
*Statistically significant difference p<0.05.
Table 2: Comorbidities and risk factors present in the study groups.
Biochemical and Hormonal Parameters and Growth Factors
In the biochemical parameters, a median glucose of 99.5 mg/dL was found for the cases and 85 mg/dL in the controls (p=0.0001), while cholesterol and triglycerides showed no significant difference. Regarding leptin levels, 7.4 ng/dL was found in the cases and 8.94 ng/dL in the controls (p=0.002), IGF1 levels were 2.55 ng/dL in the cases and 0.33 ng/dL in the controls (p=0.02), while VEGFA levels were 47.8 pg/dL in cases and 24.1 pg/dL in controls (p=0.013). The fasting Triglyceride/Glucose index (TyG) was determined in both groups, and a median of 9.01 was found for the cases and 8.79 for the controls (p=0.001) (Table 3).
Serum level
Breast cancer cases
Median (Min – Max)Controls
Median (Min – Max)P – value
99.5 (59 – 423)
85 (60 – 495)
0.0001*
Cholesterol mg/dL
182 (70 – 326)
185 (93 – 301)
0.43
Triglycerides mg/dL
160 (40 – 1033)
149 (39 – 965)
0.64
Leptin ng/dL
7.4 (0.06 – 15.6)
8.94 (0.14 – 38.6)
0.002*
IGF1 ng/dL
2.55 (0.03 – 290)
0.33 (0.11 – 32.51)
0.02*
VEGFA pg/dL
47.8 (1.02 – 704.18)
24.1 (8.2 – 352.2)
0.013*
TyG index
9.01 (7.47 – 11.03)
8.79 (6.02 – 12.38)
0.001*
*U Mann – Whitney test was considered statistically significant if p<0.05.
TyG index: Triglycerides/Glucose index = Ln[triglyceride (mg/dl) * glucose (mg/dl)/2].
Table 3: Biomarker levels of the women included in the study.
LEP rs7799039, LEPR rs1137101 and VEGF rs699947 Gene Polymorphisms
To determine the influence of the genetic polymorphisms on the serum levels of leptin and VEGFA, the functional polymorphisms LEP rs7799039, LEPR rs1137101 and VEGF rs699947 were analyzed. All polymorphisms were in Hardy-Weinberg equilibrium, and the genotypic and allelic frequencies of the polymorphisms are shown in Table 4.
Polymorphism
Cases
n (%)Controls
n (%)OR (CI 95%)
P-value
LEP rs7799039
GG
63 (37)
55 (33)
1
GA
76 (45)
84 (50)
0.78 (0.49 – 1.27)
0.33
AA
29 (18)
29 (17)
0.87 (0.46 – 1.63)
0.67
A allele frequency
0.40
0.42
0.90 (0.66 – 1.23)
0.53
X2
0.60
LEPR rs1137101
AA
42 (25)
41 (24)
1
AG
94 (56)
91 (54)
1.00 (0.6 – 1.69)
0.97
GG
32 (19)
36 (22)
0.86 (0.45 – 1.64)
0.66
G allele frequency
0.47
0.49
0.93 (0.67 – 1.28)
0.68
X2
0.89
VEGF rs699947
AA
19 (11)
29 (17)
1
AC
82 (49)
82 (49)
1.52 (0.79 – 2.93)
0.20
CC
67 (40)
56 (34)
1.85 (0.94 – 3.66)
0.07
C allele frequency
0.64
0.58
1.32 (0.96 – 1.82)
0.08
X2
0.20
The chi-square test corresponds to the distribution of genotypes between cases and controls.
*Statistically significant difference p<0.05.
Table 4: Frequency of polymorphisms and their association with the risk for breast cancer.
To analyze the association of the polymorphisms as risk factors for breast cancer, codominant and additive logistic regression models were performed; however, no significant associations were found (Table 4).
Protein Levels in Serum and Genetic Polymorphisms
When comparing leptin levels for the different genotypes of LEP rs7799030 and LEPR rs1137101 (Figure 1A and 1B), no statistically significant difference was found in neither the cases nor the controls. In the comparison of the protein levels according to VEGF rs699947 by genotype, no statistically significant difference was found; however, in the cases group, the significance was marginal (Figure 1C).
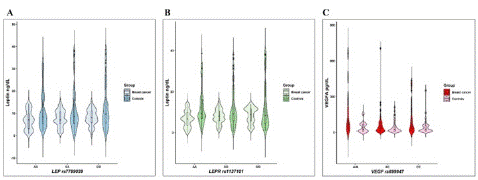
Figure 1: Leptin and VEGF levels in cases of breast cancer (BC) and the control group (CG). A) Leptin levels according to LEP rs7799039 genotypes p=0.6, B) Leptin levels according to LEPR rs1137101 genotypes p=0.7, C)VEGFA levels according to VEGF rs699947 genotypes p=0.07 to BC. A Kruskal‒Wallis test was performed between the genotypes of each group.
Correlation Analysis
The correlation between all the numerical variables for each group was analyzed (Table 5). In the control group, a significant positive correlation (p<0.05) was found between age and WHR, age and cholesterol level, age and glucose level, BMI and leptin level, BMI and triglyceride level, WHR and glucose level, WHR and triglyceride level, cholesterol level and triglyceride level, and leptin level and IGF1 level. After Bonferroni correction, all correlations remained. In the group of cases, a significant positive correlation was found (p<0.05) between age and WHR, age and glucose level, BMI and leptin level, BMI and triglyceride level, WHR and glucose level, cholesterol level and triglyceride level, triglyceride level and leptin level, and triglyceride level and VEGFA level, and a significant negative correlation was found between IGF1 and VEGFA level. All correlations remained after the Bonferroni correction.
Variables
Breast cancer cases
Controls
Rho
P-value
Rho
P-value
Age – WHR
0.28
0.0003*
0.27
0.0004*
Age – cholesterol
0.06
0.39
0.17
0.02*
Age – glucose
0.17
0.02*
0.26
0.0004*
BMI – leptin
0.48
0.0001*
0.32
0.0001*
BMI – triglycerides
0.22
0.003*
0.19
0.01*
WHR – glucose
0.21
0.004*
0.17
0.02*
WHR – cholesterol
0.07
0.32
0.11
0.13
WHR – triglycerides
0.13
0.07
0.17
0.02*
Cholesterol – triglycerides
0.42
0.0001*
0.30
0.0001*
Triglycerides – leptin
0.16
0.04*
0.08
0.26
Triglycerides – VEGFA
0.19
0.02*
-0.07
0.53
Leptin – IGF1
-0.05
0.55
0.34
0.0002*
IGF1 – VEGFA
-0.23
0.005*
-0.09
0.42
*Spearman correlations with statistical significance p<0.05.
The correlations that are significant in both groups are highlighted in bold.
BMI: Body Mass Index; WHR: Waist-Hip Ratio; IGF1: Insulin-like Growth Factor 1; VEGFA: Vascular Endothelial Growth Factor Isoform A.
Table 5: Correlations in the variables of interest in controls and breast cancer patients.
Discriminant Analysis
Discriminant analysis is a multivariate method used for finding the relationship between variables and classifying individuals into groups, which can represent a prediction resource for methodical monitoring of individuals. It was found that the model correctly classified 73% of the controls and 54% of the cases, while it classified 27% of the controls as cases and 46% of the cases as controls.
Discussion
The ages of both groups included in this study were in a similar age range that was between 22 and 94 years, and the highest age was between 51 and 55 years. These data are consistent with the findings that breast cancer can occur in women of all ages, so this variable is no longer considered one of the main risk factors. Regarding obesity, a very high percentage of women with obesity and abdominal obesity was found in both groups; however, in the cases with breast cancer, these values were significantly higher than in the control group. In a study reported by Namazi et al., 2019 [16], an association between abdominal obesity and breast cancer (RR=2.29, CI 95% 1.12–4.68) was found, in our study, these values were slightly higher, with OR=2.62 for abdominal obesity and OR=1.71 for obesity categorized by BMI. These data are important due to their biological relevance, since the abdominal circumference is an estimate of visceral adipose tissue, which is closely related to metabolic syndrome and cancer. In this context, Chen et al., 2016 [17] and Iyengar et al., 2019 [18], in independent studies, reported an increased risk of developing breast cancer by presenting a higher percentage of visceral fatty tissue. Obesity and metabolic syndrome frequently lead to other metabolic diseases, such as diabetes and hypertension, which are diseases related to increased blood glucose levels, insulin resistance, and dyslipidemia; in this study, we found that diabetes and hypertension increase the risk of developing breast cancer, as reported by other authors [19,20]. Metabolic syndrome is characterized by the coexistence of risk factors such as abdominal obesity, atherogenic dyslipidemia, elevated blood pressure, a prothrombotic and proinflammatory state, insulin resistance, and higher glucose levels. These factors are associated with type 2 diabetes. The presence of abdominal obesity and systemic metabolic abnormalities is an important factor for the initiation, promotion, and progression of cancer because it provides an inflammatory microenvironment rich in lipids, carbohydrates, growth factors, and angiogenic factors that tumor cells require to sustain their growth [21]. Regarding the biochemical parameters in this work, higher glucose levels were found in the group of women with breast cancer. Similar data were reported by Kebede et al. [22] and Gioseffi et al. [23], who reported hyperglycemia in patients with breast cancer, while in women in the control group, the serum glucose levels were lower. In our study, the levels of cholesterol and triglycerides did not show significant differences; however, another study reported that hypercholesterolemia causes cellular stress due to the accumulation of lipids, inducing the expression of factors that increase tumor development and metastasis [24]. In addition, other authors suggest that increased serum triglycerides may compromise muscle glucose metabolism and lead to decreased insulin sensitivity. The fasting TyG index has been analyzed, and the results indicate that it is a good surrogate marker of insulin resistance, with the advantage that this TyG index is economically feasible, so it can be analyzed in epidemiological studies [25]. In this study, TyG was higher in the cases than in the controls, as has also been reported in other studies [26].
Leptin is a hormone related to obesity because it is secreted by adipose tissue and has also been associated with breast cancer risk due to its ability to stimulate signaling pathways that induce proliferation, survival, and metastasis. Studies have reported higher leptin levels in healthy subjects with high BMI [27] and in patients with breast cancer [28]; however, in other comparative studies, higher leptin levels are observed in controls than in cases, although in both groups, the BMI – leptin correlation is preserved [29]. In this study, we also found higher leptin levels in the controls than in the cases. In this regard, leptin levels reported in various studies show great variability, and the differences found for this parameter should be interpreted based on the population analyzed and other factors, such as the circadian cycle and levels of hormones such as thyroid and sex hormones, since it has been observed that all these factors influence leptin levels, making it difficult to compare the results obtained for each population [30]. In addition to these limitations, it has been suggested that serum leptin levels can be modified in women with breast cancer because the hormone binds to its receptor in the target tissue, so it does not circulate in the serum. A study carried out in breast tissue showed higher expression of leptin and its receptor in tumor tissue, so this explanation is plausible [31].
The presence of other factors, such as IGF1 and VEGFA, increases the risk of developing breast cancer by stimulating the growth and angiogenesis pathways that a tumor requires to grow and migrate. In this study, we found higher levels of IGF1 and VEGFA in breast cancer cases, and similar data were reported by other studies [32,33]. These results are important because IGF1 promotes growth, which is overexpressed in cancer cells to maintain proliferation, while VEGFA promotes vascularization of tumoral tissue, providing nutrients and oxygen to sustain the high metabolic demand; this high energy demand is sustained by the hyperglycemia and dyslipidemia observed in patients, so the obesogenic microenvironment is a potent initiating and carcinogenic promoter agent, and it is also capable of directing the progression of cancer by promoting metastasis. Regarding the LEP rs7799039, LEPR rs1137101 and VEGF rs699947 polymorphisms, we did not find any significant association with breast cancer, as has been reported in other studies [34-36]. Likewise, we did not find a relation between polymorphism and protein levels in either the case or control group; it was observed that for VEGFA, in the group with breast cancer, there was a marginal decrease in individuals carrying the CC genotype, and a study reported that there were higher levels of VEGF in subjects carrying the AA genotype [37].
Bivariate correlations were performed between the different variables of interest to determine if there were differences between the correlations of the case and control groups. The results were interesting since in both groups, a correlation was observed between age and WHR, age and glucose level, BMI and leptin level, BMI and triglyceride level, WHR and glucose level, and cholesterol level and triglyceride level. However, for some variables, the r2 value was higher in the cases than in controls, while the correlations between triglyceride level and leptin level, and triglyceride level and VEGFA level, and the negative correlation between IGF1 level and VEGFA level were found only in breast cancer cases. It was important that the correlations remained significant after Bonferroni correction, indicating that the relation between these pair of variables is very important in the group with breast cancer. The correlation between BMI and leptin level has been widely documented in different populations, so BMI is a good indicator of circulating leptin levels in serum. Likewise, triglyceride levels correlate with BMI, leptin levels and VEGFA levels in cases, so the analysis of low–cost markers such as BMI and blood chemistry parameters (glucose, cholesterol and triglycerides) are routine markers that should be included in cancer treatment. To reduce hyperglycemia and dyslipidemia, which are essential for cancer progression, these markers, such as leptin, IGF and VEGF, are an indirect indicator of other pathways that could be functioning, which when overexpressed, decrease the response to chemotherapy treatment [38].
The interaction between multifactorial pathologies such as cancer and obesity represent a challenge for its analysis and interpretation; therefore, multivariate statistical methods have been implemented to understand these relations. In this study, we incorporated a discriminant analysis with the numerical categorization of obesity, diabetes, hypertension, glucose levels, cholesterol levels, triglyceride levels, leptin levels, IGF1 levels and VEGFA levels. The resulting model adequately classified 73% of the controls and 54% of the cases; however, 27% of the controls and 46% of the cases were classified in a different group. These results are interesting because there are women who do not yet have cancer but that the model classifies as potential cases, so the patients in this group should have a detailed follow–up, improve their lifestyle, and reduce the factors that feed the obesogenic microenvironment, which ultimately affects the initiation, promotion and progression of cancer [39]. Regarding the population of cases, it is likely that the 46% that were classified as controls may have a better prognosis for treatment. It is important to highlight that most of the cases were luminal A molecular type, which has expressed ER markers, so the therapy of choice for these cases is chemotherapy and hormone therapy, and good results have been observed with longer survival periods [40]. Therefore, information on the molecular type of breast cancer and the concomitant treatment of metabolic alterations related to obesity, diabetes and hypertension could have a positive impact on the prognosis of cancer.
The analysis of the microenvironment associated with obesity and its role in the development of breast cancer generates a complex model for its study due to the multiple factors involved; however, analyzing the characteristics associated with obesity and cancer allows decisions to be made in clinical practice and improves the prognosis of patients, as well as reducing risk factors in the population that already present some alterations that may favor carcinogenic development. Moreover, the different forms of interaction between the obesogenic microenvironment and its participation in the development of breast cancer are represented in Figure 2.
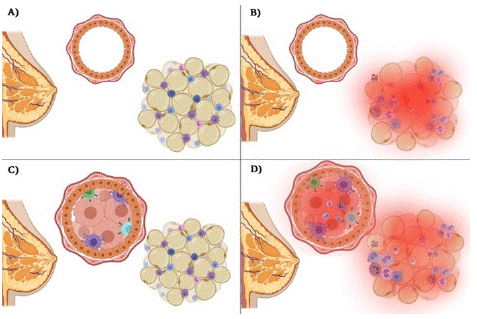
Figure 2: Normal and obese microenvironment in women with breast cancer and controls without a diagnosis of breast cancer. A) A functional microenvironment in adipose tissue is presented, and a normal phenotype of mammary gland epithelial cells is shown. B) Dysfunctional adipose tissue, characterized by an inflammatory microenvironment, and a normal phenotype of breast epithelial cells. In this condition, the risk of developing breast cancer is increased due to proinflammatory factors and growth factors secreted by adipose tissue. C) A malignant tumor phenotype is presented; however, there is a functional microenvironment of adipose tissue, so the treatment will have the appropriate effect as it does not present other poor prognostic factors. D) A malignant tumor phenotype and an obesogenic microenvironment with hyperglycemia, dyslipidemia, growth factors, and angiogenesis that represent a favorable niche for growth, proliferation, and invasion of other tissues; therefore, it can also be associated with a poor prognosis of the disease.
Conclusions
The results obtained in this study indicate that the presence of obesity, abdominal obesity, diabetes and hypertension increase the risk of developing breast cancer in Mexican women and that low–cost biomarkers such as BMI and blood chemistry are useful and easy access tools for monitoring and screening patients. To improve this work, the analysis should incorporate the measurement of HDL and LDL cholesterol, the measurement of insulin, increase the sample size and monitor the subjects with risk characteristics.
Author Statements
Disclosure Statement
We confirm that all authors listed on this manuscript have read and approved the final version of the manuscript, and they agree with the content and conclusions presented. Additionally,
all authors have made substantial contributions to the research and the preparation of the manuscript.
Acknowledgements
The authors thank María del Lourdes Froto Madariaga and Alicia Sophie Pulido González for their support in sample collection and DNA purification.
Author Contributions
RPM designed the study, carried out the experimental work and wrote the manuscript, AMAR provided the data of the patients, obtained the informed consent and carried out the sampling of the patients, GAB obtained the informed consent and carried out the sampling of the women included in the group control, performed the ELISA assays and wrote the manuscript, MFGD performed the genotyping analyses, AGZ designed the study, performed the experimental work, performed the statistical analyses, figures, and wrote the manuscript. All authors approved the final version of the manuscript.
References
- Arnold M, Morgan E, Rumgay H, Mafra A, Singh D, Laversanne M. Current and future burden of breast cancer: Global statistics for 2020 and 2040. Breast. 2022; 66: 15-23.
- Grabinski VF, Brawley OW. Disparities in breast cancer. Obstet Gynecol Clin North Am. 2022; 49: 149-165.
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A. Global cancer statistics 2020: GLOBOCAN Estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021; 71: 209-249.
- Zuo Q, Band S, Kesavadas M, Madak Z. Obesity and postmenopausal hormone receptor-positive breast cancer: Epidemiology and mechanism. Endocrinol. 2021; 162: 1-10.
- Barquera S, Hernández-Barrera L, Trejo-Valdivia B, Shamah T, Campos-Nonato I, Rivera-Dommarco J. Obesity in Mexico, prevalence and trends in adults. Ensanut 2018-19. Salud Publica Mex. 2020; 62: 682-692.
- Di Domenico M, Pinto F, Quagliuolo L, Contaldo M, Settembre G, Romano A. The role of oxidative stress and hormones in controlling obesity. Front Endocrinol (Lausanne). 2019; 10: 540.
- Wu Q, Li B, Li Z, Li J, Sun S, Sun S. Cancer-associated adipocytes: key players in breast cancer progression. J Hematol Oncol. 2019; 12: 95.
- Herrera-Vargas AK, García-Rodríguez E, Olea-Flores M, Mendoza-Catalán MA, Flores-Alfaro E, Navarro-Tito N. Pro-angiogenic activity and vasculogenic mimicry in the tumor microenvironment by leptin in cancer. Cytokine Growth Factor Rev. 2021; 62: 23-41.
- Ackerman SE, Blackburn OA, Marchildon F, Cohen P. Insights into the link between obesity and cancer. Curr Obes Rep. 2017; 6: 195-203.
- Kharb R, Haider K, Neha K, Yar MS. Aromatase inhibitors: Role in postmenopausal breast cancer. Arch Pharm (Weinheim). 2020; 353: e2000081.
- Takahashi Y. The role of growth hormone and insulin-like growth factor-I in the liver. Int J Mol Sci. 2017; 18: 1447.
- Sarfstein R, Nagaraj K, Le Roith D, Werner H. Differential effects of insulin and IGF1 receptors on ERK and AKT subcellular distribution in breast cancer cells. Cell. 2019; 8: 1499.
- Apte RS, Chen DS, Ferrara N. VEGF in signaling and disease: Beyond discovery and development. Cell. 2019; 176: 1248-1264.
- Al Kawas H, Saaid I, Jank P, Westhoff CC, Denkert C, Pross T. How VEGF-A and its splice variants affect breast cancer development - clinical implications. Cell Oncol (Dordr). 2022; 45: 227-239.
- Lee K, Kruper L, Dieli-Conwright CM, Mortimer JE. The impact of obesity on breast cancer diagnosis and treatment. Curr Oncol Rep. 2019; 21: 41.
- Namazi N, Irandoost P, Heshmati J, Larijani B, Azadbakht L. The association between fat mass and the risk of breast cancer: A systematic review and meta-analysis. Clin Nutr. 2019; 38: 1496-1503.
- Chen GC, Chen SJ, Zhang R, Hidayat K, Qin JB, Zhang YS. Central obesity and risks of pre- and postmenopausal breast cancer: A dose–response meta-analysis of prospective studies. Obesity Rev. 2016; 17: 1167–1177.
- Iyengar NM, Arthur R, Manson JE, Chlebowski RT, Kroenke CH, Peterson L. Association of body fat and risk of breast cancer in postmenopausal women with normal body mass index: a secondary analysis of a randomized clinical trial and observational study. JAMA Oncol. 2019; 5: 155–163.
- Jafari N, Kolla M, Meshulam T, Shafran JS, Qiu Y, Casey AN. Adipocyte-derived exosomes may promote breast cancer progression in type 2 diabetes. Sci Signal. 2021; 14: e2807.
- Islam D, Islam MS, Jesmin. Association of hypertension, hyperlipidemia, obesity, and demographic risk factors with breast cancer in Bangladeshi women. Medicine (Baltimore). 2022; 101: e31698.
- Dong S, Wang Z, Shen K, Chen X. Metabolic syndrome and breast cancer: prevalence, treatment response, and prognosis. Front Oncol. 2021; 11: e629666.
- Kebede T, Melak T, Sina AA, Fasil A. Assessment of serum uric acid, urea, and glucose levels and associated factors among breast cancer patients attending a tertiary hospital in Bahirdar, Ethiopia: A comparative cross-sectional study. Ethiop J Health Sci. 2022; 32: 1183-1192.
- Gioseffi C, Padilha PC, Chaves GV, Oliveira LC, Peres WAF. Body weight, central adiposity, and fasting hyperglycemia are associated with tumor characteristics in a brazilian cohort of women with breast cancer. Nutrients. 2022; 14: 4926.
- Liu W, Chakraborty B, Safi R, Kazmin D, Chang CY, McDonnell DP. Dysregulated cholesterol homeostasis results in resistance to ferroptosis increasing tumorigenicity and metastasis in cancer. Nat Commun. 2021; 12: 5103.
- Ramdas Nayak VK, Satheesh P, Shenoy MT, Kalra S. Triglyceride glucose (TyG) index: A surrogate biomarker of insulin resistance. J Pak Med Assoc. 2022; 72: 986-988.
- Alkurt EG, Özkan MB, Turhan VB. Predictive value of triglyceride/glucose index (TyG) in predicting breast cancer in patients with breast mass. Eur Rev Med Pharmacol Sci. 2022; 26: 4671-4676.
- Singh P, Sharma P, Sahakyan KR, Davison DE, Sert-Kuniyoshi FH, Romero-Corral A. Differential effects of leptin on adiponectin expression with weight gain versus obesity. Int J Obes (Lond). 2016; 40: 266-74.
- Pan H, Deng LL, Cui JQ, Shi L, Yang YC, Luo JH. Association between serum leptin levels and breast cancer risk: An updated systematic review and meta-analysis. Medicine (Baltimore). 2018; 97: e11345.
- Fontvieille E, His M, Biessy C, Navionis AS, Torres-Mejía G, Ángeles-Llerenas A. Inflammatory biomarkers and risk of breast cancer among young women in Latin America: a case-control study. BMC Cancer. 2022; 22: 877.
- Anusha K, Hettiaratchi UP, Athiththan LV, Perera PP. Inter-relationship of serum leptin levels with selected anthropometric parameters among a non-diabetic population: a cross-sectional study. Eat Weight Disord. 2019; 24: 551-556.
- Cárdenas Cárdenas E, Tenorio-Torres A, Méndez JP, Orozco-Arguelles L, Leal-García M, Coral-Vázquez RM. Leptin and its receptor are overexpressed in breast cancer tissue of postmenopausal Mexican-Mestizo women with obesity. Ann Diagn Pathol. 2022; 60: 151705.
- Tan VY, Bull CJ, Biernacka KM, Teumer A, Richardson TG, Sanderson E. Investigation of the interplay between circulating lipids and IGF-1 and relevance to breast cancer risk: An observational and mendelian randomization study. Cancer Epidemiol Biomarkers Prev. 2021; 30: 2207-2216.
- Liu G, Chen XT, Zhang H, Chen X. Expression analysis of cytokines IL-5, IL-6, IL-8, IL-17 and VEGF in breast cancer patients. Front Oncol. 2022; 12: 1019247.
- Geriki S, Bitla AR, Srinivasarao PV, Hulikal N, Yootla M. Association of single nucleotide polymorphisms of adiponectin and leptin genes with breast cancer. Mol Biol Rep. 2019; 46: 6287-6297.
- Atoum MF, Hamaid Alparrey AA. Association of leptin receptor Q223R gene polymorphism and breast cancer patients: A case control study. Asian Pac J Cancer Prev. 2022; 23: 177-182.
- Madrid-Paredes A, Casado-Combreras MÁ, Pérez-Ramírez C, Segura-Pérez AM, Chamorro-Santos C, Vergara-Alcalde E. Association of ABCB1 and VEGFA gene polymorphisms with breast cancer susceptibility and prognosis. Pathol Res Pract. 2020; 216: 152860.
- Nunes FD, Ferezin LP, Pereira SC, Figaro-Drumond FV, Pinheiro LC, Menezes IC. The association of biochemical and genetic biomarkers in VEGF pathway with depression. Pharmaceutics. 2022; 14: 2757.
- Dieli-Conwright CM, Courneya KS, Demark-Wahnefried W, Sami N, Lee K, Buchanan TA. Effects of aerobic and resistance exercise on metabolic syndrome, sarcopenic obesity, and circulating biomarkers in overweight or obese survivors of breast cancer: A randomized controlled trial. J Clin Oncol. 2018; 36: 875-883.
- Zhou X, Zhang J, Lv W, Zhao C, Xia Y, Wu Y. The pleiotropic roles of adipocyte secretome in remodeling breast cancer. J Exp Clin Cancer Res. 2022; 41: 203.
- Gao JJ, Swain SM. Luminal A breast cancer and molecular assays: A review. Oncologist. 2018; 23: 556-565.