
Special Article - Nicotine Addiction and Withdrawal
Austin J Drug Abuse and Addict. 2016; 3(1): 1007.
Maternal Smoking and Perinatal Outcomes
Watanabe H¹*and Fukuoka H²
¹Department of Children and Women’s Health, Osaka University of Graduate School of Medicine, Japan
²Waseda University Comprehensive Research Foundation, Japan
*Corresponding author: Hiroko Watanabe, Department of Children and Women’s Health, Osaka University Graduate School of Medicine, Osaka 565-0871, Japan
Received: January 15, 2016; Accepted: February 01, 2016; Published: February 02, 2016
Abstract
Nicotine is one of the major components in tobacco smoke, and it can cross the placenta. It enters the fetal circulation and accumulates in the fetal compartments from as early as seven weeks of gestation, even with passive smoking. Elevated fetal carbon monoxide levels may result in hypoxia due to reduced availability of hemoglobin for oxygen transport. Exposure to hazardous substances in cigarette smoke could lead to adverse pregnancy and birth outcomes. In particular, maternal nicotine exposure is associated with many adverse fetal, placental, and postnatal health outcomes, including both shortterm and long-term complications. Despite increased awareness of the harmful effects of smoking during pregnancy, approximately 20% of women continue to smoke throughout pregnancy in the world. Reducing smoking in pregnancy is a global public-health priority. We recommend clinical and public-health strategies aimed at the primary and secondary prevention of tobacco exposure for fetuses and children.
Keywords: Maternal Smoking; Perinatal Outcomes
Introduction
Cigarette smoke is estimated to contain 4,000 chemicals, including nicotine, tar, arsenic, lead, and hydrogen cyanide [1,2]. Exposure to hazardous substances in cigarette smoke could lead to adverse pregnancy and birth outcomes. In particular, maternal nicotine exposure is associated with many adverse fetal, placental and postnatal health outcomes, including both short-term and long-term complications. Epidemiological studies have provided indirect but compelling evidence that maternal cigarette smoking has serious consequences on all aspects of human reproduction. Many reports have linked maternal smoking with increased risk of fertility problems, spontaneous abortion, placenta abruption, fetal growth restriction, stillbirth, preterm birth, low birth weight, obesity, and type 2 diabetes mellitus in human and animal studies [3-5]. Despite increased awareness of the harmful effects of smoking during pregnancy, 10-23% of women continue to smoke throughout pregnancy in the US and Europe [6,7] and 5.1% of women in Japan [8]. Smoking is strongly associated with socioeconomic conditions, being more prevalent among less-educated women and those in the lowest income group [9,10] .Reducing smoking in pregnancy is a global public-health priority. This review focused on the effects of maternal nicotine exposure during pregnancy on birth outcomes.
Prevalence of Maternal Smoking Rates
The World Health Organization (WHO) estimates that the prevalence of smoking is approximately 22% of women in developed countries and 9% of women in underdeveloped countries [11]. The 2011 Pregnancy Risk Assessment Monitoring System (PRAMS) polled women from 24 states in the US. The self-reported data showed that about 23% of reproductive-aged women smoked during the three months before pregnancy, and about 10% of women smoked during the last three months of pregnancy. About 55% of women who smoked before pregnancy reported they had quit smoking by the last three months of pregnancy. The highest prevalence of those smoking during the last three months of pregnancy (15-16%) was in the age groups of 24 years and younger (Figure 1) [12]. A strong correlation has been seen between maternal smoking during pregnancy and young age, unmarried status, and being from a low socioeconomic status [13].
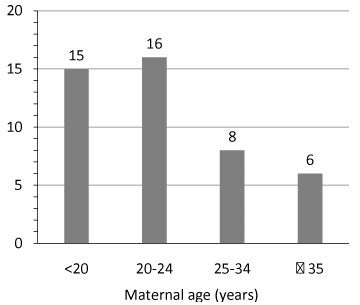
Figure 1: Prevalence of smoking during the last three months of pregnancy
from 24 PRAMS states, 2011. Source: [12].
The Centers for Disease Control and Prevention’s (CDC) PRAMS showed the trends of smoking three months before pregnancy, during, and after pregnancy data from 40 states in the US from 2000 to 2010. During this decade, the prevalence of smoking in the three months before pregnancy remained unchanged at about one in four women. However, the prevalence of smoking declined in the last three months of pregnancy from 13.2% to 11.6% and after delivery from 17.8% to 16.6%. The data showed that 40% of women who quit smoking during pregnancy relapsed within six months after delivery (Figure 2) [14].
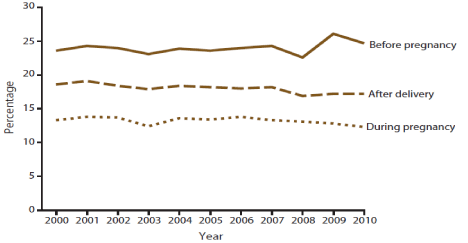
Figure 2: Trends of smoking before, during, and after pregnancy from nine
states in the US, 2000-2010. Source: [14].
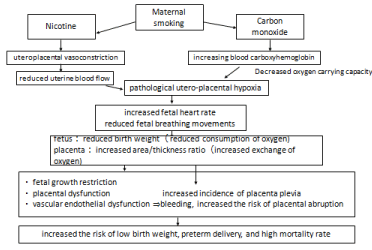
Figure 3: Maternal smoking exposure and perinatal outcomes. Source:
Smoking and pregnancy by Ministry of Health, Labour, and Welfare [25].
Other parts of the world had a greater decrease in smoking for women. Dias-Dame et al., conducted population-based survey in Brazil, data of 7,572 women showed that the prevalence of smoking before pregnancy decreased from 28% in 2007 to 22% in 2013, and the prevalence of smoking during pregnancy decreased from 22% in 2007 to 18% in 2013 [15]. In Australia, the prevalence of smoking in pregnancy in New South Wales declined from 22.1% in 1994 to 13.5% in 2007. The largest decrease in smoking in pregnancy rates was among the highest socioeconomic group, and smaller declines were observed among teenage and remote rural mothers [16].
Maternal and Newborn Cord Plasma Cotinine Concentration
Nicotine is one of the major components in tobacco smoke, and it can cross the placenta. From the placenta, it enters the fetal circulation and accumulates in the fetal compartments from as early as seven weeks of gestation, even with passive smoking [17]. Cotinine, nicotine’s metabolite measured in plasma, saliva, or urine, is a reliable marker of recent smoking status [18]. Maternal and newborn plasma cotinine levels are strongly associated, as the placenta does not bar plasma cotinine from moving between pregnant mother and her fetus.
Umbilical cord plasma cotinine correlates with the selfreported daily number of cigarettes at the end of pregnancy [19]. Berlin et al., [20] collected the data from 11 mothers who smoked cigarettes (average 95.1, SD±96, range 10-420) the week preceding delivery. Maternal blood was drawn on average 6h earlier than the umbilical cord blood. The mothers’ and newborns’ plasma cotinine concentrations were highly correlated (r=0.97, p<0.001). Similar results were reported in Hukkanen et al., study [21].
Nicotine also accumulates in breast milk. Studies have indicated that the amount of nicotine found in breast milk is 2.9 times greater than that found in maternal blood plasma [17] and that the amount of cotinine, the major metabolite of nicotine, present in the urine of infants breastfed by smoking mothers is, on average, 10 times higher than that found in bottle-fed children whose mothers smoke [22]. Urinary cotinine levels in infants breastfed by smoking mothers are similar to those found in adult smokers [23].
Maternal Smoking Exposure and Perinatal Outcomes
Placentation is an explosive process that occurs before embryonic development, which ultimately depends on placental function. The progenitor population of embryonic Cytotrophoblasts (CTBs) forms a polarized epithelium attached to a basement membrane that forms around the stromal cores containing the intrinsic vasculature of the placenta. Chronic exposure to tobacco constituents in early pregnancy likely affects placental development directly or indirectly by reducing blood flow, which creates a pathologically hypoxic environment. Nicotine depresses active amino-acid uptake by human placental villi and trophoblast invasion, and cadmium decreases the expression and activity of 11 beta-hydroxysteroid dehydrogenase type 2, which is causally linked to fetal growth restriction [24]. Smoking decreases the flow of uterine blood to the placenta through mechanisms that include vasoconstriction. Evidence has shown that smoking is a causal factor in intrauterine growth restriction and may precipitate a preterm delivery.
Figure 3 shows the effects of maternal smoking exposure and the perinatal outcomes [25]. Maternal smoking results in high levels of carbon monoxide in the circulation, which approximates the levels found in cord blood samples. Elevated fetal carbon monoxide levels may result in hypoxia due to reduced availability of hemoglobin for oxygen transport. This fetal hypoxia may be the cause of growth retardation, although it has also been suggested that nicotine-induced placental vasoconstriction may impede the transfer of oxygen and nutrients. Smoking may increase the risk of intrauterine infection, and it may also stimulate the production of prostaglandin E2, which causes contractions in myometrial tissue, and leading to premature labor. In addition, smoking reduces the level of type III collagen, which may increase the risk of preterm rupture of the membranes [26].
Smoking mothers have newborns with dramatically lower birth weight, on average 200 g less than gestation-matched controls, than nonsmoking mothers [27]; a more recent study reported a 377-g decrease in average birth weight for infants of mothers who smoked during pregnancy [28]. Reductions in birth weight have serious consequences, as they are strongly associated with infant morbidity and mortality [29].Varner et al., analyzed 137,297 singleton births and their linked birth certificate data from the 2004 to 2010 PRAMS. The study’s multiple logistic regression analysis revealed that women who had a previous poor birth outcome were 22% more likely to smoke during a subsequent pregnancy. Women who had a previous low birth weight and preterm birth were 30% and 13%, respectively, more likely to smoke during pregnancy than were mothers with routine previous births [30].
Ion et al., [31] reviewed 13,359 deliveries from 2012 to 2014 in UK, with complete data for 5,066 and 4,793 women in the self-reported and measured, respectively, groups for exhaled carbon monoxide. Self-reported exposure was associated with earlier delivery (-0.19 weeks, 95% confidence interval (CI): -0.32 to -0.05) and reduced birth weight (-56 g, 95% CI: -97 to -16 g) but not associated with an increase in the risk of preterm birth or small-for-gestational age (SGA).Similarly, in a study of 3,661Japanese women who gave birth to single full-term infants (37-42 weeks), Watanabe et al., reported woman who smoked 10 cigarettes a day during pregnancy were associated with a 110.5-g decrease in birth weight. Women who smoked more than 10 cigarettes per day had a 2.5 times (CI: 1.8-3.5) higher chance of having an SGA infant than did nonsmokers, but no such effect was observed among women who smoked fewer than 10 cigarettes per day [32].
Environmental Tobacco Exposure
Environmental Tobacco Smoke (ETS)consists of almost all the constituents of inhaled cigarette smoke, including tar, nicotine, carbon monoxide, and carbon dioxide.ETS exposure can also cause harmful effects to the fetus, such as low birth weight and preterm delivery [33,34].The World Health Organization has estimated that40% of children and 35% of female nonsmokers were exposed to secondhand smoke in 2004 [35], and tobacco kills nearly 6 million people each year and, among them, 10 % were due to second hand smoke [36]. According to the 2010 Global Adult Tobacco Survey in China, 65.1% of nonsmoking women from 15-49 years of age were exposed to ETS at home and 52.6% were exposed in the workplace [37].
Some studies indicated that ETS exposure of nonsmoking, pregnant women decreased mean birth weight. Infants born to women exposed to passive smoking during pregnancy were 40-50 g lighter than were those born to unexposed mothers, primarily due to growth restriction [38]. Leonardi-Bee et al., meta-analysis indicated that nonsmoking, pregnant women exposed to secondhand smoke are estimated to be 23% more likely to experience stillbirth and 13% more likely to give birth to a child with a congenital malformation, but no significant change was seen for perinatal or neonatal death [39]. However, Pineles et al., meta-analysis showed that ETS exposure was not significantly associated with miscarriage [40].
Health Problems for Offspring
ETS harms children’s respiratory health, and it has been linked to a higher risk of middle ear infections, bronchitis and pneumonia, coughing and wheezing, decreased lung function, and asthma development [41]. Smaller size at birth is generally associated with a reduced risk for being overweight later in life; however, recent research suggests that mothers who smoke during pregnancy have children at increased risk for later obesity. The combination of small size at birth and becoming overweight in later life is not only characteristic of the epidemiologic transition from acute to chronic disease, but it also confers a high risk of cardiovascular outcomes in adulthood. In a meta-analysis by Oken et al., children whose mothers smoked during pregnancy were at an elevated risk for becoming overweight (pooled adjusted odds ratio (OR) = 1.50, 95% CI: 1.36, 1.65) compared with children whose mothers did not smoke during pregnancy [42].
Child obesity is a major public-health problem in the world. Behl. et al., [6] reviewed 42 human studies investigating the relationship between childhood overweight/obesity and exposure to maternal smoking during pregnancy. The studies included cross-sectional, prospective, or retrospective designs. Ethnic groups were also different, including Asian, Hispanic, American, and European subjects. In 14 of the observational studies, maternal smoking during pregnancy was associated with being overweight at 3-33 years of age (adjusted OR = 1.50, 95% CI: 1.36, 1.65).
However, a large retrospective study by Sharma et al., [43] reported a significant association between maternal smoking and child obesity only in white and black children, but not in Hispanic, American Indian, or Asian/Pacific Islander children. In addition, a meta-analysis conducted by Weng. et al., [44] suggests that children with mothers who smoke regularly during pregnancy are 47% more likely to be overweight at 3-8 years of age compared with same age children of mothers who do not smoke during pregnancy (adjusted OR= 1.47, 95% CI: 1.26 to 1.73). Based on these human epidemiological studies, current evidence supports an association between maternal smoking and childhood obesity.
Nicotine Replacement Therapy
Nicotine Replacement Therapy (NRT) is considered a good smoking-cession tool in the nonpregnant population. It was introduced to help with smoking cession, because the administration of nicotine in the form of NRT was thought to be less harmful than exposure to nicotine through smoking. Kepaya et al., analyzed the data of 2009-2010PRAMS, and one in five pregnant smokers was offered NRT for smoking cession [45]. The FDA has classified nicotine as a “pregnancy category D drug,” meaning there is evidence of risk to the human fetus. Therefore, NRT should be reserved for women unable to quit using nonpharmacological methods.
Leung et al., [46] reviewed the efficacy and safety of smokingcessation strategies in pregnancy. NRT administered as gum may be better than using transdermal forms to avoid high levels of nicotine in the fetal circulation. A small trial demonstrated that bupropion is an effective aid for smoking cessation and it does not appear to be associated with an increased risk of major congenital malformations.
However, in aprospective cohort study of 1,217 women in India, Gupta et al., reported that smokeless-tobacco use was associated with an average reduction of 105 g in birth weight and a reduction in gestational age of 6.2 days. The adjusted ORwas 1.4 for preterm delivery (< 37 weeks) and 1.6 for low birth weight [33]. In addition, a meta-analysis of NRT clinical trials for prenatal smoking cession by Coleman et al., reported that NRT had no significant effect on quit rates [47]. Currently, insufficient evidence has been found on the safety of NRT during pregnancy and on adverse NRT effects on perinatal outcomes and both short and long-term consequences for the child. Cochrane’s review on pharmacological interventions for smoking cession during pregnancy concluded that insufficient evidence was available to demonstrate NRT improved smoking cession in pregnant women [47].
Conclusion
Tobacco smoke toxins readily cross the placental membrane, and even nonsmoking mothers have a risk of exposure to ETS, either from the home or workplace, during pregnancy. Quitting smoking as soon as pregnancy is known positively affects fetal growth and development, in both short-term and long-term consequences. Reducing smoking in pregnancy is a global health priority. Educational anti-tobacco campaigns and quit-smoking initiatives should target both mothers and fathers to ensure smoke-free living conditions and a healthy environment for all family members. We recommend clinical and public-health strategies aimed at the primary and secondary prevention of tobacco exposure for fetuses and children.
References
- Rustemeier K, Stabbert R, Haussmann HJ, Roemer E, Carmines EL. Evaluation of the potential effects of ingredients added to cigarettes. Part 2: chemical composition of mainstream smoke. 2002; 40: 93-104.
- Swauger JE, Steichen TJ, Murphy PA, Kinsler S. An analysis of the mainstream smoke chemistry of samples of the U.S. cigarette market acquired between 1995 and 2000. 2002; 35: 142-156.
- Warren GW, Alberg AJ, Kraft AS, Cummings KM. The 2014 Surgeon General’s report: “The health consequences of smoking--50 years of progress”: a paradigm shift in cancer care. 2014; 120: 1914-1916.
- Jangir RN, Jain GC. Diabetes mellitus induced impairment of male reproductive functions: a review. 2014; 10: 147-157.
- Petrik JJ, Gerstein HC, Cesta CE, Kellenberger LD, Alfaidy N, Holloway AC. Effects of rosiglitazone on ovarian function and fertility in animals with reduced fertility following fetal and neonatal exposure to nicotine. 2009; 36: 281-290.
- Behl M, Rao D, Aagaard K, Davidson TL, Levin ED, Slotkin TA, et al. Evaluation of the association between maternal smoking, childhood obesity, and metabolic disorders: a national toxicology program workshop review. Environ Health Perspect. 2013; 121: 170-180.
- Tong VT, Dietz PM, Morrow B, D’Angelo DV, Farr SL, Rockhill KM, et al. Centers for Disease Control and Prevention (CDC). Trends in smoking before, during, and after pregnancy--Pregnancy Risk Assessment Monitoring System, United States, 40 sites. 2000-2010. 2013; 62: 1-19.
- Yasuda T, Ojima T, Nakamura M, Nagai A, Tanaka T, Kondo N, et al. Postpartum smoking relapse among women who quit during pregnancy: Cross-sectional study in Japan, J Obstet Gynaecol Res. 2013; 39: 1505- 1512.
- Schneider S, Maul H, Freerksen N, Potschke-Langer M. Who smokes during pregnancy? An analysis of the German Perinatal Quality Survey 2005. 2008; 122: 1210-1216.
- Bombard JM, Dietz PM, Galavotti C, England LJ, Tong VT, Hayes DK, et al. Chronic diseases and related risk factors among low-income mothers. 2012; 16: 60-71.
- Mackay J, Eriksen M. The world tobacco atlas. Geneva, Switzerland: World Health Organization Publications. 2006.
- Information for health care providers and public health professionals: preventing tobacco use during pregnancy.CDC.
- Kvale K, Glysch RL, Gothard M, Aakko E, Remington P. Trends in smoking during pregnancy, Wisconsin 1990 to 1996. 2000; 99: 63-67.
- Tong VT, Dietz PM, Morrow B, D’Angelo DV, Farr SL, Rockhill KM, et al; Centers for Disease Control and Prevention (CDC). Trends in smoking before, during, and after pregnancy--Pregnancy Risk Assessment Monitoring System, United States, 40 sites, 2000-2010. 2013; 62: 1-19.
- Dias-Dame JL, Cesar JA. Disparities in prevalence of smoking and smoking cessation during pregnancy: a population-based study. 2015; 2015: 345430.
- Mohsin M, Bauman AE, Forero R. Socioeconomic correlates and trends in smoking in pregnancy in New South Wales, Australia. See comment in PubMed Commons below J Epidemiol Community Health. 2011; 65: 727- 732.
- Jauniaux E, Gulbis B, Acharya G, Thiry P, Rodeck C. Maternal tobacco exposure and cotinine levels in fetal fluids in the first half of pregnancy. 1999; 93: 25-29.
- SRNT Subcommittee on Biochemical Verification. Biochemical verification of tobacco use and cessation. 2002; 4: 149-159.
- Nafstad P, Kongerud J, Botten G, Urdal P, Silsand T, Pedersen BS, et al. Fetal exposure to tobacco smoke products: a comparison between selfreported maternal smoking and concentrations of cotinine and thiocyanate in cord serum. 1996; 75: 902-907.
- Berlin I, Heilbronner C, Georgieu S, Meier C, Spreux-Varoquaux O. Newborns’ born blood plasma cotinine concentrations are similar to that of their delivering smoking mothers. Drug Alcohol Depend. 2010; 107: 250-252.
- Hukkanen J, Jacob P 3rd, Benowitz NL. Metabolism and disposition kinetics of nicotine. 2005; 57: 79-115.
- Amir LH. Maternal smoking and reduced duration of breastfeeding: a review of possible mechanisms. 2001; 64: 45-67.
- Mascola MA, Van Vunakis H, Tager IB, Speizer FE, Hanrahan JP. Exposure of young infants to environmental tobacco smoke: breast-feeding among smoking mothers. 1998; 88: 893-896.
- Zdravkovic T, Genbacev O, McMaster MT, Fisher SJ. The adverse effects of maternal smoking on the human placenta: a review. 2005; 26: 81-86.
- The prevalence of smoking in Japan. Ministry of Health, Labour and Welfare. The National Health and Nutrition Survey.
- Hadley CB, Main DM, Gabbe SG. Risk factors for preterm premature rupture of the fetal membranes. 1990; 7: 374-379.
- Services US. DoHaH. The health benefits of smoking cessation: a report of the Surgeon General. U.S. Public Health Service. Office on Smoking and Health. 1990.
- Wang X, Zuckerman B, Pearson C, Kaufman G, Chen C, Wang G, et al. Maternal cigarette smoking, metabolic gene polymorphism, and infant birth weight. 2002; 287: 195-202.
- Mathews TJ, Menacker F, MacDorman MF. Infant mortality statistics from the 2000 period linked birth/infant death data set. Natl Vital Stat Rep. 2002; 50: 1-28.
- Varner SB, Ihongbe T, Masho SW. The Impact of Prior Poor Birth Outcomes on Smoking Behavior on Subsequent Pregnancies: Analysis of the National PRAMS Data. 2015.
- Ion RC, Wills AK, Bernal AL. Environmental Tobacco Smoke Exposure in Pregnancy is Associated With Earlier Delivery and Reduced Birth Weight. 2015; 22: 1603-1611.
- Watanabe H, Inoue K, Doi M, Matsumoto M, Ogasawara K, Fukuoka H, Nagai Y. Risk factors for term small for gestational age infants in women with low prepregnancy body mass index. 2010; 36: 506-512.
- Gupta PC, Subramoney S. Smokeless tobacco use, birth weight, and gestational age: population based, prospective cohort study of 1217 women in Mumbai, India. 2004; 328: 1538.
- Shah NR, Bracken MB. A systematic review and meta-analysis of prospective studies on the association between maternal cigarette smoking and preterm delivery. 2000; 182: 465-472.
- World Health Organization (WHO): WHO Report on the Global Tobacco Epidemic. Exposure to second-hand smoke. 2008.
- World Health Organization. WHO report on the global tobacco epidemic, 2013: enforcing bans on tobacco advertising, promotion and sponsorship. Luxembourg: World Health Organization; 2013.
- Centers for Disease Control and Prevention. Current tobacco use and secondhand smoke exposure among women of reproductive age--14 countries, 2008-2010. 2012; 61: 877-882.
- US Department of Health and Human Services: Women and Smoking. A report of the Surgeon General. United States Department of Health and Human Services. Washington, DC, USA. 2001.
- Leonardi-Bee J, Britton J, Venn A. Secondhand smoke and adverse fetal outcomes in nonsmoking pregnant women: a meta-analysis. 2011; 127: 734- 741.
- Pineles BL, Park E, Samet JM. Systematic review and meta-analysis of miscarriage and maternal exposure to tobacco smoke during pregnancy. 2014; 179: 807-823.
- Department of Health and Human Services. The Health Consequences of Involuntary Exposure to Tobacco Smoke: A Report of the Surgeon General. Atlanta (GA): Centers for Disease Control and Prevention (US); 2006.
- Oken E, Levitan EB, Gillman MW. Maternal smoking during pregnancy and child overweight: systematic review and meta-analysis. Int J Obes (Lond). 2008; 32: 201-210.
- Sharma AJ, Cogswell ME, Li R. Dose-response associations between maternal smoking during pregnancy and subsequent childhood obesity: effect modification by maternal race/ethnicity in a low-income US cohort. Am J Epidemiol. 2008; 168: 995-1007.
- Weng SF, Redsell SA, Swift JA, Yang M, Glazebrook CP. Systematic review and meta-analysis of risk factors for childhood overweight identifiable during infancy. Arch Dis Child. 2012; 97: 1019-1026.
- Kapaya M, Tong V, Ding H. Nicotine replacement therapy and other interventions for pregnant smokers: Pregnancy risk assessment monitoring system. 2009-2010. 2015; 78: 92-100.
- Leung LW, Davies GA. Smoking Cessation Strategies in Pregnancy. J Obstet Gynaecol Can. 2015; 37: 791-797.
- Coleman T, Chamberlain C, Davey MA, Cooper SE, Leonardi-Bee J. Pharmacological interventions for promoting smoking cessation during pregnancy. Cochrane Database Syst Rev 2012; 12: 9.