
Research Article
J Drug Discov Develop and Deliv. 2014;1(1): 7.
Effect of Oil, Surfactant and Co-Surfactant Concentrations on the Phase Behavior, Physicochemical Properties and Drug Release from Self-Emulsifying Drug Delivery Systems
Chukwuma O Agubata*1, Ifeanyi T Nzekwe2, Nicholas C Obitte1, Calister E Ugwu1, Anthony A Attama3 and Godswill C Onunkwo1
1Department of Pharmaceutical Technology and Industrial Pharmacy, University of Nigeria, Nigeria
2Department of Pharmaceutics and Pharmaceutical Technology, Nnamdi Azikiwe University, Nigeria
3Department of Pharmaceutics, University of Nigeria, Nigeria
*Corresponding author: Chukwuma O Agubata, Department of Pharmaceutical Technology and Industrial Pharmacy, University of Nigeria, Nsukka, Enugu State, Nigeria
Received: July 17, 2014; Accepted: Aug 07, 2014; Published: Aug 11, 2014
Abstract
Microemulsions are isotropic, thermodynamically stable systems. The aim of this study was to evaluate the effect of oil, surfactant and co-surfactant concentrations on the phase behavior, physico-chemical properties and drug release of Self-Emulsifying Drug Delivery Systems (SEDDS). Solubility of artemether in Peceol® (oil), Labrasol® (surfactant), Transcutol® (co-surfactant) and their mixtures was studied while pseudoternary phase diagrams were constructed using water titration method as surfactant efficiency and water solubilization capacity were examined. Artemether SEDDS were prepared by dissolving artemether in the oil prior to further mixtures with surfactant, cosurfactants and characterized by evaluation of phase stability, self-emulsification, pH, viscosity, drug precipitation, refrigeration thaw cycle, centrifugation, drug release and dispersion. SEDDS prepared with surfactant-co-surfactant mixture (Smix) at 3:1 ratio had the largest zone of microemulsion in the pseudoternary phase diagrams and highest surfactant efficiency. Formulations with higher Labrasol® content showed faster self-emulsification, while artemether release and dispersion from capsule-filled SEDDS was optimum and fastest using Peceol®/ Smix ratio of 1:2 and a Labrasol®/Transcutol® ratio of 3:1. Combinations of oil, surfactant and co-surfactants at varied ratios produced self-emulsifying systems with different emulsification, drug release and dispersion qualities.
Keywords: Microemulsion; Pseudoternary phase diagram; Selfemulsification; Artemether
Introduction
Self-emulsifying formulations are isotropic mixtures of oil, surfactant, co-solvent and solubilized drug [1]. These formulations can rapidly form oil in water (o/w) fine emulsions when dispersed in aqueous phase under mild agitation and are commonly called Self-Emulsifying Drug Delivery Systems (SEDDS). The rapid emulsification of these formulations in the gastrointestinal tract can provide both improved oral bioavailability and a reproducible plasma concentration of drug. Furthermore, the droplet size of the emulsion would influence the extent of absorption of the orally administered drug. SEDDS would require a relatively high intrinsic lipophilicity of the drug substance since the active ingredient should be dissolved in a limited amount of oil. Self-emulsification occurs when the entropy change that favors dispersion is greater than the energy required to increase the surface area of the dispersion [2]. The free energy of the conventional emulsion is a direct function of the energy required to create a new surface between the oil and water phases. The two phases of emulsion tend to separate with time to reduce the interfacial area and subsequently the emulsion is stabilized by emulsifying agents, which form a monolayer over emulsion droplets, which reduces the interfacial energy and provides a barrier to prevent coalescence [3]. Emulsification process may be associated with the ease with which water penetrates the oil-water interface with formation of liquid crystalline phase resulting in swelling at the interface, thereby causing greater ease of emulsification [4]. Large interfacial surface area provided by fine droplet size of the formulation promotes rapid release of the drug substance and/or formation of mixed micelles containing the drug [5]. Lipids (e.g. triglycerides) affect the oral bioavailability of drugs by changing biopharmaceutical properties such as increasing dissolution rate and solubility in the intestinal fluid, protecting the drug from chemical as well as enzymatic degradation in the oil droplets and the formation of lipoproteins promoting lymphatic transport of highly lipophilic drugs [6]. Many drugs degrade in the physiological system through enzymatic or hydrolytic cleavages under acidic pH of stomach [7]. Such drugs when presented in form of SEDDS can be well protected against these degradation processes as liquid crystalline phase in SEDDS act as barrier between degrading environment and the drug. The most widely recommended surfactants for SEDDS are non-ionic surfactants with relatively high Hydrophile-Lipophile- Balance (HLB) values. The hydrophilicity of the surfactants assists the immediate formation of oil-in-water droplets and rapid spreading of the formulation in aqueous media. SEDDS are often referred to as Self-Microemulsifying Drug Delivery Systems (SMEDDS) if they form transparent microemulsions. The flexibility of the surfactant film is important and enables the existence of several different structures including droplet-like shapes, aggregates and bicontinuous structures [8]. The interface of microemulsions is stabilized by an appropriate combination of surfactant and/or co-surfactant. The lipid mixtures with higher surfactant and co-surfactant/oil ratios lead to the formation of self-microemulsifying formulation [9].
It is important to note that compositional variables (oil, presence of other amphiphiles, hydrophilic molecules or electrolytes) as well as temperature may have an influence on hydrophilic and hydrophobic properties, the geometry of the surfactant molecule and the efficiency of a surfactant to generate microemulsion [10]. In most cases, single chain surfactants alone are unable to reduce the oil/water interfacial tension sufficiently to enable a microemulsion to form [11]. The efficiency of a surfactant usually represents the amount of an amphiphile required to completely homogenize equal quantities of oil and water [12]. Oils, surfactants and co-surfactants have different physico-chemical properties and their interactions modify the characteristics of the resultant self-emulsifying drug delivery systems.
Artemether is an antimalarial drug used for the treatment of multidrug resistant strains of Plasmodium falciparum malaria. Artemether is a relatively lipophilic and unstable drug [13]. Studies indicate that the bioavailability of artemether increases with the administration of fatty meals [14]. Hence, the objective of this study was to investigate the effect of oil, surfactant and co-surfactant concentrations on the phase behavior, physicochemical properties and drug release from self-emulsifying drug delivery systems containing artemether.
Materials and Methods
Materials
The following materials were used as procured without further purification: artemether (Hangzhou Dayang Chemical, China), Peceol®- glycerol monooleate, Labrasol® - caprylocaproyl macrogol-8- glyceride, Transcutol® - diethylene glycol monoethyl ether (Gattefosse, St. Priest, France). All other reagents and solvents were analytical grade.
Solubility of drug in oil, surfactant and co-surfactant for SEDDS
Solubility studies of artemether in Peceol® (oil), Labrasol® (surfactant), Transcutol® (co-surfactant) and different oil-surfactant/ co-surfactant mixtures were performed visually and confirmed with shake flask method. The solubility was observed visually by first saturating the vehicle with a known weight of the drug and then adding an increasing drop-wise amount of the vehicle and allowing for equilibration for 24 h before further addition until the drug completely dissolved. The solubility study was then performed using the shake flask method. An excess of each drug was separately added to 5 ml of oil, surfactants and oil/surfactant mix in a screwcapped tube and mixed. The tubes were then kept at 37 ± 1°C in an isothermal water bath shaker for 24 h after which each sample was centrifuged. The resulting supernatant was filtered, diluted appropriately with 1 M methanolic HCl, heated at 60 ± 2°C for 3 h for artemether derivatization and analysed using UV spectrophotometer (Spectrumlab 752s, UK) at wavelength of 254 nm.
Construction of pseudoternary phase diagrams
The pseudoternary phase diagrams were constructed using the water titration method. A series of SEDDS was prepared by varying mass ratio of oil to surfactant (or surfactant mixture, Smix) from 9:1 to 1:9. The ratio of surfactant to co-surfactant was optimized by varying their mass ratio from 1:0, 1:1, 2:1, 3:1, to 4:1 (Labrasol®/Transcutol®). Each pre-concentrate mixture was titrated drop-wise with distilled water at room temperature and agitated after each drop. For the purpose of conversions, 35 drops of water was equivalent to 0.5 ml. The end point of the titration was taken as the point when the solution became cloudy and turbid, and the quantity of water required was recorded. The pseudoternary phase diagram was established to delineate the area of microemulsion and boundary of phases. The pseudoternary phase diagrams were plotted using SigmaPlot® 12.3 software.
Surfactant efficiency (Smin) and water solubilization capacity (Wmax)
The efficiency of a surfactant usually represents the amount of an amphiphile required to completely homogenize equal quantities of oil and water. It was determined at equal oil to water weight fractions in order to avoid effects of domain curvature on the surfactant efficiency measurement [15]. The surfactant efficiency of the surfactants or Smix was determined at experimental temperature of 25 ± 1°C and was expressed as the minimum concentration of the surfactant required to obtain a single phase microemulsion (Smin, %w/w). The result was compared with values extrapolated from a graph.
The water solubilization capacity (Wmax) of the surfactant-oil pre-concentrate at constant surfactant to oil mass ratio 1:1, was determined by titrating the mixtures with distilled water (drop wise) to the water solubilization limit which was detected visually as the transition from transparent to turbid/cloudy system upon addition of excess water. The transparent samples containing Smin and Wmax were allowed to equilibrate for a minimum of 72 h and then examined visually for transparency. Clear isotropic one phase systems were designated as microemulsions.
Formulation of unloaded self-microemulsifying drug delivery systems
Based on microemulsion area in the pseudoternary phase diagram and safety requirement, appropriate quantities of Peceol®, Labrasol® and Transcutol® were mixed together in different selected ratios to obtain homogenous self- microemulsifying systems as presented in Table 1. A 3 x 2 factorial design was adopted for the SEDDS formulation using 2 independent variables (oil/surfactant ratio, and surfactant/ co-surfactant ratio (Kmin)) with 3 and 2 use levels respectively. A formulation without co-surfactant was used as control.
Formulation
Ratio of
Peceol® oil: surfactant mix
Ratio of Labrasol® (surfactant): Transcutol® (co-surfactant)
Km
Peceol®
(g)
Labrasol®
(g)
Transcutol®
(g)
A
4:6
4:1
0.40
0.48
0.12
B
4:6
3:1
0.40
0.45
0.15
C
1:2
4:1
0.25
0.60
0.15
D
1:2
3:1
0.25
0.56
0.19
E
1:3
4:1
0.33
0.54
0.13
F
1:3
3:1
0.33
0.50
0.17
G
4:6
1:0
0.40
0.60
-
Table 1: Optimized mass ratios and weights of oil, surfactant and co-surfactant for 1 g Unloaded SEDDS.
Test for phase separation and self-emulsification time of unloaded SEDDS
Phase separation
A 2 ml quantity of each formulation was stored for 48 h at ambient temperature and observed thereafter for phase separation. Also 1 ml samples of each SMEDDS batch was diluted to 10 ml and 100 ml with distilled water at 25°C, stored for a period of 24 h and observed afterwards for phase separation.
Self-emulsification time
Self-emulsification of the formulations was studied using a magnetic stirrer – beaker assembly. A 1 ml portion of each unloaded SMEDDS was introduced into a beaker containing 250 ml of distilled water, maintained at 37 ± 1°C under continuous stirring at 50 rpm. The self emulsification time was taken as the time for a preconcentrate to form a homogenous mixture upon dilution.
Formulation of artemether SEDDS
Artemether loaded self-microemulsifying drug delivery systems were prepared using the formulae in Tables 2 and 3. The required amounts of artemether were individually dissolved in appropriate quantities of Peceol® oil in a beaker. The required weights of the liquid excipients were converted to volumes using their densities for easy measurement. Subsequently, calculated amounts of Labrasol® and Transcutol® were added based on the formula and thereafter mixed thoroughly.
Formulation
code
Artemether
(g)
Oil:surfactant
Smix
(Km)
Peceol®
(g)
Labrasol®
(g)
Transcutol®
(g)
A
0.067
4:6
4:1
0.40
0.48
0.12
B
0.067
4:6
3:1
0.40
0.45
0.15
C
0.067
1:3
4:1
0.25
0.60
0.15
D
0.067
1:3
3:1
0.25
0.56
0.19
E
0.067
1:2
4:1
0.33
0.54
0.13
F
0.067
1:2
3:1
0.33
0.50
0.17
G
0.067
4:6
1:0
0.40
0.60
---
Table 2: Formula for the formulation of 1 g liquid SEDDS loaded with artemether.
Formulation
code
Artemether
(g)
Oil:Smix
Smix
(Km)
Peceol®
(g)
Labrasol®
(g)
Transcutol®
(g)
A
0.02
4:6
4:1
0.120
0.144
0.036
B
0.02
4:6
3:1
0.120
0.135
0.045
C
0.02
1:3
4:1
0.075
0.180
0.045
D
0.02
1:3
3:1
0.075
0.168
0.057
E
0.02
1:2
4:1
0.099
0.162
0.039
F
0.02
1:2
3:1
0.099
0.150
0.051
G
0.02
4:6
1:0
0.120
0.180
--
Table 3: Formula for the formulation of artemether loaded SEDDS capsule (1 capsule).
pH of SEDDS samples
The pH of the SEDDS samples was evaluated using a validated pH meter (HANNA Instruments, Padova, Italy). In each case, the electrode was immersed into 50 ml quantities of each liquid SMEDDS pre-concentrate and the reading recorded. Each measurement was performed in triplicate and the average and standard deviation calculated.
Viscosity measurement of SEDDS
The viscosity of the SEDDS samples was measured using an Ostwald u-tube viscometer. The lower larger bulb of the viscometer was filled with each liquid SEDDS sample and suspended in a thermostat water bath maintained at room temperature (25°C). The samples were drawn into the upper bulb by suction through the top of the second tube arm. The meniscus of the liquid was adjusted to be just above the upper etched mark of the upper bulb and the time for the meniscus to fall from the upper to lower mark of the upper bulb was recorded. The average of three determinations was then calculated and recorded. The result was then appropriately related to flow times of water with viscosity of 1 cSt.
Stability studies of artemether SEDDS
Phase separation and drug precipitation
Test for phase separation was performed as earlier described for unloaded SEDDS. Drug precipitation in 2 ml SMEDDS samples was visually examined after storage at room temperature (25oC) for 48 h and after diluting 1 part of the sample with 10 parts and 100 parts of distilled water respectively and subsequent storage at room temperature.
Refrigeration thaw cycle
Different 2 ml samples of each labeled test formulation were separately transferred to a transparent screw capped bottle and stored in a refrigerator at 2 °C for 24 h after which they were removed and stored at 25°C and 40°C. A single refrigeration thaw cycle test was performed. The samples were then observed for phase separation and drug precipitation.
Centrifugation
A 5 ml sample of each SEDDS formulation was transferred into a glass test tube and inserted into a laboratory centrifuge (Uniscope SM800B, England) and centrifuged at 4,000 rpm for 5 min. Thereafter, the samples were observed for phase separation and drug precipitation.
Test for self-emulsification time of artemether loaded SEDDS
Self-emulsification of the loaded formulations was studied using a magnetic stirrer–beaker assembly as described for unloaded formulations. A 1 ml volume of each loaded SEDDS was introduced into a beaker containing 250 ml of distilled water, maintained at 37 ± 1°C under continuous stirring at 50 rpm. The self-emulsification time was taken as the time for a pre-concentrate to form a homogenous mixture upon dilution.
Drug partition between the oil and water
The partitioning of artemether between Peceol® oil and water was evaluated by mixing 20 mg of artemether with 5 ml of Peceol® oil and 5 ml of distilled water in a separating funnel and shaking vigorously. The mixtures were allowed to equilibrate for 48 h and the aqueous compartment was assayed after appropriate dilutions at 254 nm using spectrophotometer (Spectrumlab 752s UV-VIS, UK). Artemether containing test samples were derivatized with 1 N HCl at 80 ± 2 oC and diluted (200 fold) prior to assay.
Drug release and dispersion of encapsulated SEDDS
Drug release and dispersion studies were performed using a magnetic stirrer-beaker assembly. Test SEDDS capsules were submerged in 250 ml SGF (pH 1.2) in a beaker maintained at 37 ± 1°C and rotated at 50 rpm. The study was performed on artemether capsules samples C, D, E, F and G (Table 3) only with the exemption of samples A and B since the later showed phase separation during self-emulsification studies. Test solutions (5 ml) were withdrawn at 2 min interval and replaced with 5 ml of fresh SGF. The withdrawn test solutions were heated with 5 ml of 1N HCl at 80 ± 2 °C for 30 min, cooled to room temperature and diluted with distilled water to 20 ml. The treated test solutions were filtered and assayed at 254 nm using UV-VIS spectrophotometer (Spectrumlab 752s, UK).
For the statistical analysis, ANOVA was used to evaluate the relationship between several variables.
Results and Discussion
Solubility profile of artemether in different vehicles and mixtures
The result obtained (Figure 1) showed that artemether had solubility of 667.4 mg/ml in irvingia fat, 285.7 mg/ml in Transcutol®, 133.3 mg/ml in Labrasol® and 64.5 mg/ml in Peceol®. These relatively high solubilities of artemether made it easier to encapsulate its SEDDS since a single dose of 20 mg artemether could easily be dissolved in a 0.3 ml liquid SEDDS which was the target fill volume for a no. 1 sized hard gelatin capsule (0.48 ml total fill volume).
Key: P-S4:6 and P-S1:3 represents mixtures containing combinations of Peceol® and surfactant mixtures at 4:6 and 1:3 ratios respectively. Smix 3:1 and 4:1 represents mixtures containing combinations of Labrasol® and Transcutol® at 3:1 and 4:1 ratios respectively. Error bars represent± SD.
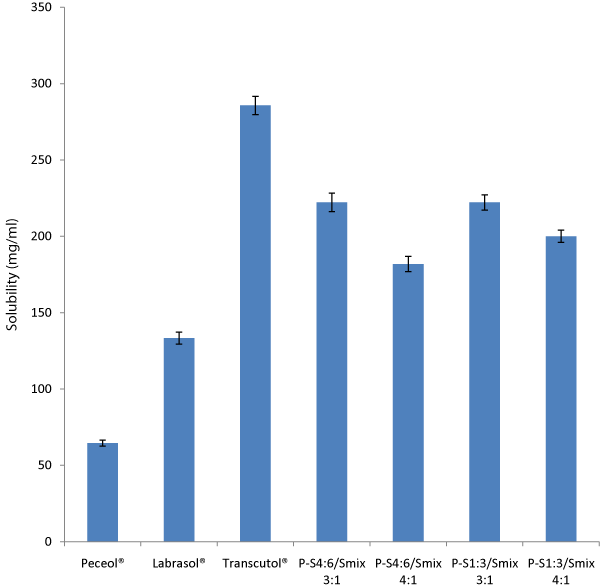
Figure 1: Solubility of artemether in Peceol®, Labrasol®, Transcutol® and their mixtures.
Key: P-S4:6 and P-S1:3 represents mixtures containing combinations
of Peceol® and surfactant mixtures at 4:6 and 1:3 ratios respectively. Smix
3:1 and 4:1 represents mixtures containing combinations of Labrasol® and
Transcutol® at 3:1 and 4:1 ratios respectively. Error bars represent± SD.
Pseudoternary phase diagrams, surfactant efficiency (Smin) and water solubilization capacity (Wmax)
The constructed pseudoternary phase diagrams are presented in Figure 2 – 4. The pseudoternary phase diagrams showed that the zone of microemulsion (upper zone) was largest in SEDDS prepared with Labrasol®-Transcutol® surfactant mixture (Smix) at 3:1 ratio. SEDDS prepared with surfactant – cosurfactant mixtures of 3:1 and 4:1 ratios remained as microemulsions even upon infinite water titration or dilution of 2:8 and 1:9 oil/Smix pre-concentrates. Although formulations containing Smix of 4:1 had a slightly smaller zone at areas of higher Peceol® oil content compared to those with 1:1 and 2:1 Smix, however at areas of higher surfactant concentration and lower oil content, the former had a larger microemulsion zone. Furthermore, preparations containing 1:1 and 2:1 Smix remained as microemulsion after infinite water titration only at 1:9 oil/ Smix mixture.
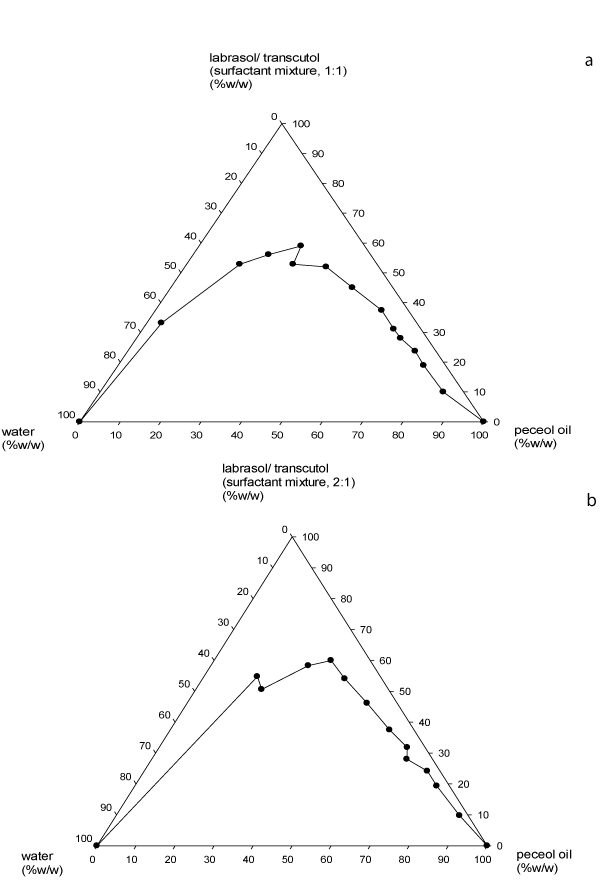
Figure 2: Pseudoternary phase diagrams of Peceol«, Labrasol«/Transcutol«
(1:1) and watera; Peceol«, Labrasol«/Transcutol« (2:1) and waterb
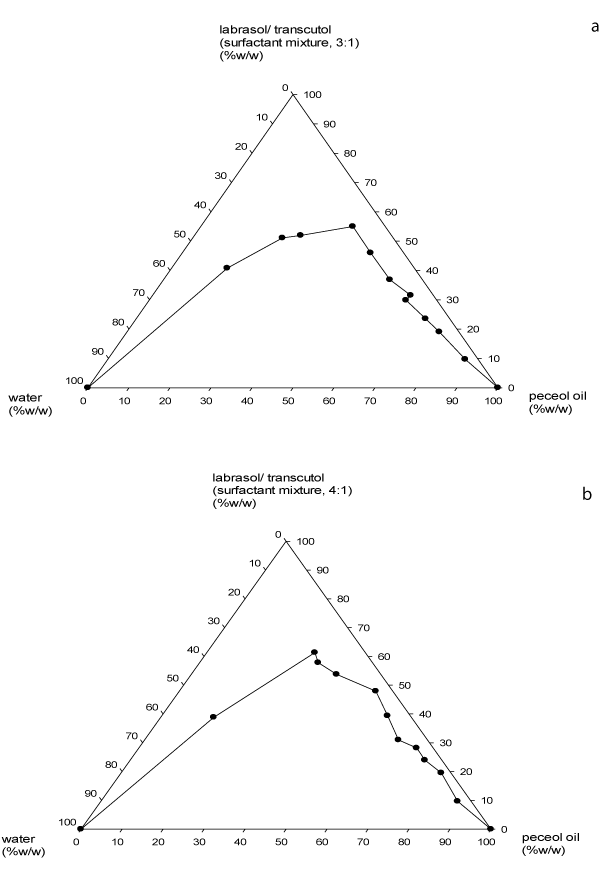
Figure 3: Pseudoternary phase diagrams of Peceol«, Labrasol«/ Transcutol«
(3:1) and watera; Peceol«, Labrasol«/Transcutol« (4:1) and waterb
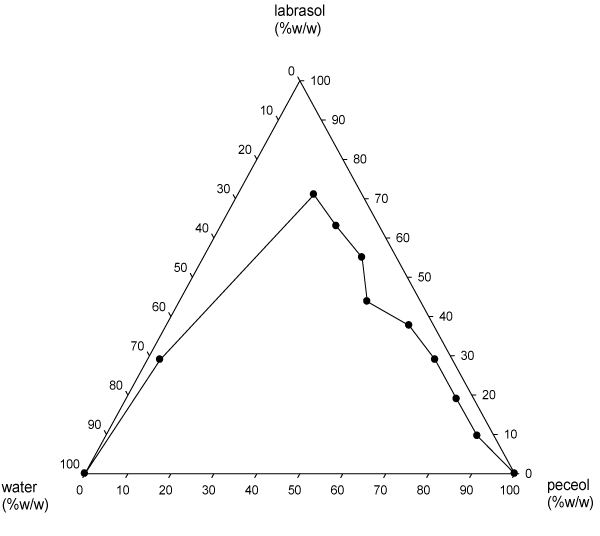
Figure 4: Pseudoternary phase diagram of Peceol«, Labrasol« and water.
Different microemulsions can be prepared by selecting appropriate oil, surfactant, co-surfactant (or co-solvent) and water concentrations within the microemulsion area. However this present study involved the use of pre-concentrates consisting oil and surfactants and the pseudoternary diagram was only used to select the appropriate oil, surfactant and co-surfactant mixtures.
These systems often require high surfactant concentrations in order to provide very low interfacial tension (≤ 10-3 mN/m) and sufficient interfacial coverage to microemulsify entire oil and water phases [16]. The ease and degree of surface tension lowering was increased at high Smix content. In order to reduce the interfacial tension to significantly low levels, a co-surfactant was combined with the surfactant. The importance of addition of co-surfactant was shown in improved microemulsification capacity of Labrasol® (surfactant) upon addition of Transcutol® (co-surfactant/co-solvent). The role of co-surfactants in lowering the surface tension can be explained using Gibbs adsorption isotherm for multicomponent systems. The Gibbs adsorption isotherm for multi component system relates the changes in concentration of a component in contact with a surface with changes in the surface tension, which results in a corresponding change in surface energy. For a binary system containing two components, the Gibbs adsorption equation in terms of surface excess can be expressed using Equation 1:
-d γow = Γ1 dμ1 + Γ2 dμ2 ---------------(1)
Where γow is the surface tension, Γ1 and Γ2 are the surface excesses of components 1 and 2, and μ1 and μ2 are the chemical potentials of components 1 and 2. The equation relates interfacial tension to interfacial composition (surface /interfacial excess) and chemical potential of mixture components. In the SEDDS formulated, increase in concentration of the Labrasol® (surfactant) and Transcutol® (cosurfactant) may have increased the surface excess and the chemical potential of these components. Consequently, a reduction in surface tension might have occurred. Surfactant/co-surfactant adsorption layer at the oÏl/water interface may affect the interactions with the dispersed and continuous phases. Therefore, concentrations of Labrasol® and Transcutol® affect the ease of self-emulsification of SEDDS.
For effective additive performance, it is vital that the surfactant and co-surfactant adsorb at the interface without significant interaction. Microemulsion properties such as phase behavior and stability depend very much on the properties of the interfacial films such as interfacial tension, spontaneous curvature (Ho) and film rigidity. Since the oil-water interfacial tension (γow) is the work required to increase the area of an interface by unit amount, then the formation of microemulsions requires γow to be low. Lowering γow to the optimum value might have been achieved better by using surfactant–co-surfactant mixture. Moreover, the co-surfactant/cosolvent can also improve the solubility of loaded drugs. Labrasol® has an HLB value of 14 which implied it is hydrophilic resulting in improved microemulsification.
In addition, the oil used also affected the Smin and Wmax. The minimum concentration of the surfactant required to obtain a single phase microemulsion (Smin, %w/w) was observed to be relatively lowest for Labrasol®/ Transcutol® surfactant mixture of 3:1 ratio (Table 4). The surfactant efficiency of the system improved with the addition of Transcutol® (lower Smin). However lower Transcutol® content seem to provide optimum result (3:1) and the microemulsion zone of the pseudoternary phase diagram provided a more complete discriminatory platform.
Surfactant (Smix)
Oil
Smin %w/w
Wmax %w/w
Labrasol®
Peceol®
66.25
12.40
Labrasol®/ Transcutol® (1:1)
Peceol®
58.00
10.00
Labrasol®/ Transcutol® (2:1)
Peceol®
55.00
7.69
Labrasol®/ Transcutol® (3:1)
Peceol®
52.00
8.05
Labrasol®/ Transcutol® (4:1)
Peceol®
55.00
4.12
Table 4: Pseudoternary phase diagram of Peceol®, Labrasol® and water.
The Wmax of the formulations (Table 4) seem to decrease with the addition of and increase in Transcutol® content. A close examination of this behavior in relation with other results (pseudoternary phase diagram and Smin) might imply that the inclusion of Transcutol® had a slightly negative influence at higher oil concentration but produced positive effects on mixtures containing lower oil content.
At higher oil: surfactant ratios (high oil content), the amount of Labrasol® present was too small to microemulsify the larger oil since some part of the Labrasol® was further replaced by Transcutol®. However as the surfactant concentration increased, the amount of Labrasol® and Transcutol® present became enough for each to perform its functions effectively. At this point, the solubilization capacity increased and the zone of microemulsion expanded.
Earlier reports have also shown that in some cases smaller molecular volume triglycerides could be solubilized by nonionic surfactants of polyoxyethylene n-alkyl ethers, to a great extent than the larger molecular volume triglycerides [17]. Low molecular volume triglycerides may penetrate the interfacial monolayer and have a better interaction with the surfactant (Labrasol®).
Therefore based on the results obtained and the requirement for safety, 4:6, 1:2 and 1:3 combinations of Peceol® and surfactant mixtures were used for the formulation of self- microemulsifying drug delivery systems. The surfactant mixtures (Smix) of Labrasol® (surfactant) and Transcutol® (co-surfactant / cosolvent) were combined at two different mass ratios (Km) of 3:1 and 4:1. Furthermore, these pre-concentrates can be mixed with small volumes of water before administration, to form self-emulsifying microemulsions.
Phase separation and drug precipitation studies
No phase separation or drug precipitation occurred in all the formulations after storage for 48 h and after appropriate dilutions, single refrigeration thaw cycle and centrifugation. However all the formulations turned cloudy after dilution of 1 part of SEDDS with 100 parts of distilled water while phase separation was observed in formulations prepared with oil/Smix ratio of 4:6 after this dilution.
Physicochemical properties of SEDDS
The pH of artemether SEDDS were within 5.0 – 5.2 (Table 5). All the preparations were slightly acidic because of the usually slightly acidic nature of the oils. The high solubility of artemether in these oils might be because artemether is a base, which made it easier for it to dissolve in the slightly acidic oils through pH based ionization although artemether also has intrinsic lipid solubility. Generally, the viscosity of the SEDDS showed that there was reduced resistance to flow therefore self-emulsification was not impeded.
Drug
oil
Smix/ surf
Oil/Smix
Km
pH
SD
Viscosity
SD (cSt)
Self-emulsification
time of loaded
SEDDS (sec)
ARM
pec
lab/trans
4:6
3:1
5.1
39.82
7
ARM
pec
lab/trans
4:6
4:1
5.0
41.84
5
ARM
pec
lab/trans
1:2
3:1
5.2
37.46
7
ARM
pec
lab/trans
1:2
4:1
5.1
41.44
6
ARM
pec
lab/trans
1:3
3:1
5.2
37.12
8
ARM
pec
lab/trans
1:3
4:1
5.1
40.04
6
ARM
pec
lab
4:6
-
5.0
43.10
5
Key: ARM Artemether ; pec áPeceol® ; lab áLabrasol® ; trans áTranscutol®
Table 5: pH, viscosity and self-emulsification time of drug loaded SEDDS.
Self-emulsification of SEDDS
Formulations with higher Labrasol® surfactant content showed lower self- emulsification time (faster self-emulsification) while formulations with higher Transcutol® co-surfactant/ co-solvent had higher self-emulsification time (slower self-emulsification). This could be observed in Tables 5-7. Labrasol® has high intrinsic self-emulsification ability and the presence of high amounts of Transcutol® (acting more as a co-solvent) in some batches reduced the quantity of Labrasol® present in these batches, hence reducing the self-emulsification of the said batch. Therefore a balance was required since Transcutol® was necessary for effective solubilization of the drugs and also to facilitate drug release and dispersion. The difference in self-emulsification times of the different batches in the bulk liquid SEDDS was actually very small and since the observation times were fast (in seconds), it was actually difficult atimes to concretely differentiate between batches. However, significant differences were observed in artemether SEDDS capsules (Table 7) where formulations prepared with 1:2 and 1:3 oil/ Smix and also containing Km 4:1 surfactant/ co-surfactant mixture had significantly lower (p < 0.05) self-emulsification times.
Oil
Smix/ surf
Oil/Smix
Km
Self-emulsification
time of unloaded
SEDDS (sec)
Peceol®
Labrasol®/Transcutol®
4:6
3:1
7
Peceol®
Labrasol®/Transcutol®
4:6
4:1
5
Peceol®
Labrasol®/Transcutol®
1:2
3:1
7
Peceol®
Labrasol®/Transcutol®
1:2
4:1
5
Peceol®
Labrasol®/Transcutol®
1:3
3:1
8
Peceol®
Labrasol®/Transcutol®
1:3
4:1
5
Peceol®
Labrasol®
4:6
-
5
Table 6: Self-emulsification time of unloaded SEDDS.
Formulation
Oil/Smix
Km
Capsule leakage
Point (min)
Self- emulsification
Point (min)
Self- emulsification time
(sec)
Remark
A
4:6
3:1
4.37
4.57
20
Phase
Separation
B
4:6
4:1
4.13
4.28
15
Phase separation
C
1:2
3:1
3.31
3.44
13
Emulsified & cloudy
D
1:2
4:1
4.42
4.48
6
Emulsified & cloudy
E
1:3
3:1
5.02
5.12
10
Emulsified & cloudy
F
1:3
4:1
2.49
2.56
7
Emulsified & cloudy
G
4:6
1:0
5.40
5.58
18
Emulsified & cloudy
Table 7: Self-emulsification time of artemether SEDDS capsules.
Drug release and dispersion profile of encapsulated SEDDS
Artemether release and dispersion from SEDDS capsules (Figure 5) revealed that formulations C with a Peceol®/ Smix ratio of 1:2 and a Labrasol®/Transcutol® ratio of 3:1 (Km) had the highest and fastest artemether release (significant difference at p < 0.05). The result showed that the batches can be ranked in terms of the fastest and highest drug release as follows; C > D > E > F > G. This showed that a threshold of surfactant and co-surfactant concentration was required for optimum drug release. A balance between self emulsification and effective diffusion of drug was required.
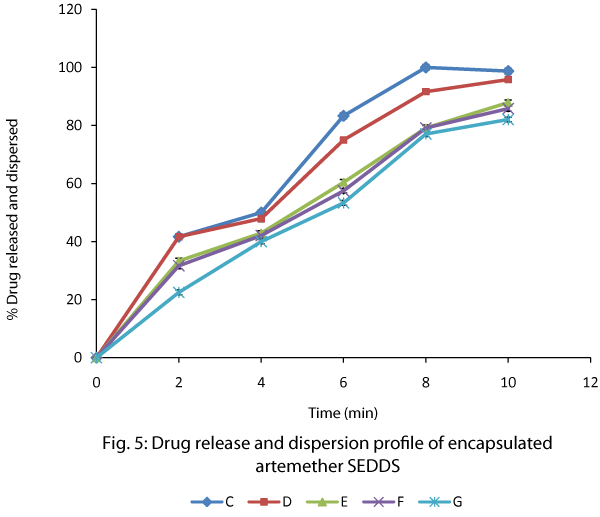
Figure 5: Drug release and dispersion profile of encapsulated artemether SEDDS.
The SEDDS capsules generally showed very fast drug release and dispersion with the fastest capsule showing 100% drug release in 8 min. Microemulsions are dynamic systems in which the interface is continuously and spontaneously fluctuating [18]. The presence of co-surfactants allowed the interfacial film sufficient flexibility to take up different curvatures required to form microemulsion over a wide range of composition [19]. The droplets have very high surface to volume ratio which were able to efficiently solubilize the drug. The cosurfactants must have reduced the interfacial tension and increased the fluidity of the interface. However, the appearance of the drug in the medium and in vivo distribution would depend on the Peceol® oil-water partition coefficient of artemether which was determined to be in the ratio of 4.12:1. Artemether partitioned more into the Peceol® oil, thereby indicating its lipophilicity. Lipid soluble drugs with favorable partition coefficient are usually effectively absorbed after oral administration.
Conclusion
Optimum concentration of oil, surfactant and co-surfactant is required to prepare self-emulsifying drug delivery systems that are effective in self-emulsification, drug release and dispersion. Combinations of surfactants and co-surfactants are selected based on functionality of the mixture. Mixtures with higher surfactant content were easily self-emulsified while higher co-surfactants improved artemether release and dispersion. Variations in oil, surfactant and co-surfactant/co-solvent ratios created systems with varied thermodynamics and entropy as observed from self- emulsification process. The choice of excipient type and combination would then depend on desired outcome and application.
Acknowledgement
The authors wish to acknowledge Gattefosse, St. Priest France for the gift of Peceol®, Labrasol® and Transcutol® used in this work.
References
- Gursoy RN, Benita S. Self-emulsifying drug delivery systems (SEDDS) for improved oral delivery of lipophilic drugs. Biomed Pharmacother. 2004; 58: 173-182.
- Mistry RB, Sheth NS. A Review: Self emulsifying drug delivery system. Int J Pharm Sci. 2011; 3: 23-28.
- Constantinides PP. Lipid microemulsions for improving drug dissolution and oral absorption: physical and biopharmaceutical aspects. Pharm Res. 1995; 12: 1561-1572.
- Groves MT, Mustafa RM. Measurement of the 'spontaneity' of self-emulsifiable oils. J Pharm Pharmacol. 1974; 26: 671-681.
- El-Laithy HM. Self-nanoemulsifying drug delivery system for enhanced bioavailability and improved hepatoprotective activity of biphenyl dimethyl dicarboxylate. Curr Drug Deliv. 2008; 5: 170-176.
- Hauss DJ, Fogal SE, Ficorilli JV, Price CA, Roy T, Jayaraj AA, Keirns JJ. Lipid-based delivery systems for improving the bioavailability and lymphatic transport of a poorly water-soluble LTB4 inhibitor. J Pharm Sci. 1998; 87: 164-169.
- Usui F, Maeda K, Kusai A, Nishimura k, Yamamoto K. Inhibitory effects of water soluble polymers on precipitation of RS-8359. Int J Pharm. 1997; 154: 59-66.
- Talegaonkar S, Azeem A, Ahmad FJ, Khar RK, Pathan SA, Khan ZI. Microemulsions: a novel approach to enhanced drug delivery. Recent Pat Drug Deliv Formul. 2008; 2: 238-257.
- Meinzer A, Mueller E, Vonderscher J. Microemulsion- A suitable galenical approach for the absorption enhancement of a low soluble compound? BT Gattefosse. 1995; 88: 21-26.
- Kahlweit M. Microemulsions. Annual Reports Section "C" (Physical Chemistry). 1999; 95: 89-115.
- Bhargava HN, Narurkar A, Lieb LM. Using micro emulsions for drug delivery. Pharm Tech. 1987; 11: 46-52.
- Djekic L, Primorac M. The influence of cosurfactants and oils on the formation of pharmaceutical microemulsions based on PEG-8 caprylic/capric glycerides. Int J Pharm. 2008; 352: 231-239.
- De Spiegeleer BM, D'Hondt M, Vangheluwe E, Vandercruyssen K, De Spiegeleer BV, Jansen H, et al. Relative response factor determination of ╬▓-artemether degradants by a dry heat stress approach. J Pharm Biomed Anal. 2012; 70: 111-116.
- Lefèvre G, Thomsen MS. Clinical pharmacokinetics of artemether and lumefantrine (Riamet®). Clin Drug Invest. 1999; 18: 467-480.
- Sjoblom J, Lindbergh R, Friberg SE. Microemulsions–phase equilibria characterization, structures, applications and chemical reactions. Adv Colloid Interf Sci. 1996; 65: 125-287.
- Wennerstrom H, Balogh J, Olsson U. Interfacial tensions in microemulsions. Colloids surf A: Physicochem Eng Aspects. 2006; 291: 69-77.
- Warisnoicharoen W, Lansley AB, Lawrence MJ. Nonionic oil-in-water microemulsions: the effect of oil type on phase behaviour. Int J Pharm. 2000; 198: 7-27.
- Lam AC, Schechter RS. The theory of diffusion in microemulsions. J Colloid Interface Sci 1987; 120: 56-63.
- Ghosh PK, Murthy RS. Microemulsions: a potential drug delivery system. Curr Drug Deliv. 2006; 3: 167-180.