
Research Article
J Drug Discov Develop and Deliv. 2014;1(2): 8.
Association between Polymorphic Variants of RAF1 Gene with Occurrence of Mammary Tumor and Aging in Canines
Bartosz Kempisty1,2*, Katarzyna Zaorska1, Dorota Bukowska3, Marcin Nowak4, Sylwia Ciesiólka1, Katarzyna Wojtanowicz-Markiewicz3, Karol Jopek1, Artur Bryja1, Pawel Antosik3, Klaus-Peter Brüssow5, Malgorzata Bruska2, Michal Nowicki1 and Maciej Zabel6
1Department of Histology and Embryology, Medicine Faculty I, Poznan University of Medical Sciences, Swiecickiego 6 St., 60-781, Poznan, Poland
2Department of Anatomy, Medicine Faculty I, Poznan University of Medical Sciences, Swiecickiego 6 St., 60-781 Poznan, Poland
3Institute of Veterinary Sciences, Faculty of Animal Breeding and Biology, Poznan University of Life Sciences, WojskaPolskiego 52 St.
4Department of Pathology, Faculty of Veterinary Medicine, Wroclaw University of Life Sciences, 50-375 Wroclaw, C.K. Norwida
5Institute of Reproductive Biology, Department of Experimental Reproductive Biology, Leibniz Institute for Farm Animal Biology, Dummerstorf, Germany
6Department of Histology and Embryology, Wroclaw Medical University, 6a Chalubinskiego St., 50-368, Wroclaw, Poland
*Corresponding author: Bartosz Kempisty, Department of Histology and Embryology, Medicine Faculty I, Poznan University of Medical Sciences, Swiecickiego 6 St., 60-781, Poznan, Poland
Received: Aug 12, 2014; Accepted: Sep 29, 2014; Published: Oct 02, 2014
Abstract
The induction of carcinogenesis as well as cancer growth and development are often associated with an altered expression of genes encoding protooncogenes and proteins responsible for regulation of cell division cycle. Although the role of RAF1 protein in development of apoptosis resistance mechanisms is well known in many types of human cancer, the association between changes in the structure of RAF1 gene and induction of canine carcinogenesis and/or aging remains still poorly recognized. Therefore, the goal of this study was focused on new mutations in the RAF1 gene as well as on association between frequency of RAF1 gene polymorphisms and the occurrence of mammary tumor in groups of domestic bitches of various ages.
In this study, blood samples were obtained from 22 female dogs diagnosed with mammary tumors. Moreover, blood samples were also collected from geriatric (>5 to 10 years old; n=15), mature adult (>2 to 5 years old; n=10) and young (from 1 to 2 years old; n=11) dogs. Thirty six bitches examined for other reasons served as controls.
After Sanger sequencing analysis, 13 single nucleotide variations were identified, of which two were localized in coding regions (exons 3 and 9) and the rest 11 - in introns (introns 3, 4, 5, 7, 8, 10 and 16). We observed differences in prevalence of heterozygotes and alternative alleles between study groups in 4 polymorphisms, two of which could indicate the putative protective variants (c.T237C, c.A918C) and the other two could indicate the putative risk variants (g.A4805C, g.A6902C) with reference to the cancer occurrence in dogs.
In conclusion, we demonstrated that RAF1 polymorphisms may serve as a protective and/or risk factor for the occurrence of mammary tumor in domestic bitches, manifesting a type- and localization of mutation-dependent manner. Although the frequency of occurrence of these polymorphisms was often not statistically significant, haplotype blocks revealed strong linkage between investigated polymorphisms.
Keywords: Mammary Tumor; RAF1 Polymorphisms; Aging; Canine
Introduction
The carcinogenesis is a complex process, involving many molecular, biochemical and morphological changes, which finally lead to growth and development of cancer [1,2,3]. It has been suggested that various types of cancer reflect type of affected tissue, tissue morphology as well as organ origin [4,5,6]. Moreover, several lines of experiments indicated that canine cancers display many similarities to human cancers, especially in respect to morphology, growth of cancer, clinical features and clinical prognosis. Therefore, some of the authors regarded canine cancers to represent a model of human carcinogenesis [7,8,9,10].
Induction of carcinogenesis was demonstrated to be strongly associated with molecular changes and disruptions. Furthermore, in several studies changes in gene sequences caused by spontaneous or heritable mutations/polymorphisms were found to lead in a simple way to disruptions in amino acid sequence and/orto formation of improper protein [7,11]. Induction of carcinogenesis is often caused by mutation in the genes encoding proteins responsible for cell cycle control, formation of free radical and oxygen species, apoptosis or uncontrolled prolongation of cell life as well as for formation of fibrous collagen and cell cytoskeleton [12,13,14].
The RAF1 is known as an oncogene that can be targeted to the mitochondria by BCL2 as well as it is known to belong to the main regulators of apoptotic cell death [15]. Active RAF1enhanced BCL2-mediated resistance to apoptosis and may be involved in phosphorylation of BAD. Alavi et al [16]. Demonstrated that FGFB and VEGF may differentially activate RAF1, which leads to protection through distinct pathways of apoptosis in human endothelial cells and chick embryo vasculature. The two studies showed that activation of RAF1 is strongly associated with resistance to apoptosis, which may provide the main reason for uncontrolled cell cycle divisions leading finally to induction of carcinogenesis.
The problem of mammary tumor of non-identified origin in aging bitches is increasing. Therefore, the aim of the study was searching for new molecular markers that would predict the occurrence of tumor in bitches as well as determining the correlation between frequency of RAF1 gene polymorphisms and the occurrence of mammary tumor in domestic bitchesin relation to aging.
Materials & Methods
Subjects and samples collection
Blood samples were obtained from 22 female dogs of mongrel dogs diagnosed with mammary tumors, histologically classified as malignant tumors, during surgery in the Small Animal Clinic, University of Life Sciences, Poznan, Poland. Blood was collected during standard surgery procedures. Thirty six bitches served as controls. The affected bitches were divided into 3 subgroups of different age, according to the classification by Jugdutt et al. [17]: geriatric (>5 to 10 years old; n=15), mature adult (>2 to 5 years old; n=10) and young ones (from 1 to 2 years old; n=11). Blood was collected from from the jugular vein into vials with EDTA and frozen at-80 °C until further analyses.
Molecular analyses
Genomic DNA was extracted from whole peripheral blood using QIAamp DNA Blood Mini Kit (Qiagen), according to the manufacturer’s instructions. DNA was re-suspended in 100 μl of Qiagen elution buffer and stored at -20 °C. Sixteens exons of the RAF1 gene (exon 2 - exon 17) were amplified in Polymerase Chain Reaction (PCR), including up to 70-bp flanking regions of every exon. The sequences of the primers used with thermal and time conditions are listed in Table 1. The reactions were carried out in a total volume of 12.5 μl containing: 10 x Taq DNA Polymerase buffer with MgCl2, 5 x GC-rich solution, 0.24 mMdNTPs, 0.5 μM of the primers, 1 unit of Taq Polymerase (Roche) and 40-60 ng of genomic DNA. The PCR cycle conditions were: an initial denaturation at 94 °C for 4 min, followed by 35 cycles of denaturation at 95 °C for 30 sec, annealing at temperatures shown in Table 1 for 1 min and elongation at 72 °C for 30/60 sec, with a final extension at 72 °C for 7 min. PCR products were purified using membrane plates (Millipore) and used as templates in PCR-sequencing reamplification. The latter reaction was performed in Veriti 96 well Thermal Cycler using BigDye Terminator v3.1 Cycle Sequencing Kit (Life Technologies) and one of the specific primers (Forward or Reverse). Reamplification products were purified with EDTA and ethanol precipitation and separated by electrophoresis using ABI 3130 sequencer (Applied Biosystems).
Exon
Sequences
Annealing temp./elongation
exon 2
F 5'-TGTCACTTAAGAGAAAGTCCACAT3'
59 °C / 30 sec
R 5'-TTTGCCCATCTACAAGGTGA-3'
exon 3
F 5'-CAAATAATGCTGTCATAAATCTGC-3'
58 °C / 30 sec
R 5'-GAGTTGTCTATGCACAAGGGAAT-3'
exon 4
F 5'-TGGGGATAATCGTACTTTGTAGG-3'
60 °C / 30 sec
R 5'-TGGCATAATGACAGCTTTAAACA-3'
exon 5
F 5'-CACATAAGACCTCTTGGAAACCA-3'
59 °C / 30 sec
R 5'-TCTAAAACCCACACTTGTCAGC-3'
exon 6
F 5'-AAATGGCTGAGGAAGATGAGA-3'
60 °C / 30 sec
R 5'-GGGATACAAAGGCACTCTGG-3'
exon 7
F 5'-GAATTTGCCCCTGAGTGTCT-3'
60 °C / 30 sec
R 5'-CCCAGGGTCCTGAGAAAGTA-3'
exon 8, exon 9
F 5'-CTTTGTTCCTGCAAGTTCTCC-3'
58 °C / 30 sec
R 5'-CCCCCAATTAAAAGTATTCTCG-3'
exon 10
F 5'-ATGGAGGAATGGGTGGATTT-3'
58 °C / 30 sec
R 5'-GCAATCAAGTGTCCCTGGAG-3'
exon 11
F 5'-TTCCATTTGTGTGGTACATGTTAT-3'
59 °C / 30 sec
R 5'-TGCAGCCCACTTCCTCTG-3'
exon 12
F 5'-GCCATCCACCCAGATCATAA-3'
58 °C / 30 sec
R 5'-ACCCCAGAGGCTTACTTTCC-3'
exon 13
F 5'-CTTTAGTTCCTCGCCAGGTG-3'
58 °C / 30 sec
R 5'-CCCAAACATGTATCTCAACCCTA-3'
exon 14
F 5'-GTGCAGAAAAGGCTGGAGAC-3'
58 °C / 30 sec
R 5'-GAAGGCCTCCCTGAGGTATC-3'
exon 15, exon 16, exon 17
F 5'-GAGGCTTCCTGAGCTTGTGT-3'
59 °C / 60 sec
R 5'-AAATGAGCTCTCTGCCTCCA-3'
Table 1: PCR primer pairs with thermal conditions used for the amplification of exons of RAF1 gene.
Statistical analyses
Genotypes obtained in the study were aligned with the reference sequences from Ensemble database. Single nucleotide variations were assessed and calculation of the chi-square test for deviation from Hardy-Weinberg Equilibrium (HWE) was performed. Genotype and allele frequencies were evaluated and compared between study and control groups using the Fisher’s exact test. Odds Ratio Values (OR) were also evaluated and p<0.05 was considered to indicate statistically significant differences. Additionally, we used Haplo view 3.2 software to obtainRAF1 gene structure. Linkage Disequilibrium Values (LD) was calculated as R2 value and Gabriel et al [18]. Algorithm was used.
Results
We performed Sanger sequencing of 16 exons (exon 2 to exon 17, exon 1 is a non-coding region of this gene) with approximately 70-bp non-coding splicing regions of RAF1 gene in 58 subjects, in total. Upon alignment of obtained and reference sequences 13 single nucleotide variations were identified. Two of them were localized in coding regions (exon 3 and 9) and the rest 11 - in introns (in tron 3, 4, 5, 7, 8, 10 and 16). Numbering of nucleotides was carried with reference to the first nucleotide in the first AUG coding triplet in exon2. All single nucleotide variations identified in the study are listed in Table 2.
Name of variation
Localization
ntchange
aa change
1
c.T237C
exon 3
T>C
syn: H79H (CAT>CAC)
2
g.A4805C
intron 3
A>C
-
3
g.A6902C
intron 4
A>C
-
4
g.G7478A
intron 5
G>A
-
5
g.G11494A
intron 7
G>A
-
6
g.C14523T
intron 8
C>T
-
7
c.A918C
exon 9
A>C
syn: S306S (TCA>TCC)
8
g.A23254T
intron 10
A>T
-
9
g.C28107T
intron 16
C>T
-
10
g.C28111T
intron 16
C>T
-
11
g.A28119G
intron 16
A>G
-
12
g.C28179T
intron 16
C>T
-
13
g.C28193T
intron 16
C>T
-
Table 2: Single nucleotide variations identified in RAF1 gene in studied subjects.
All of the nucleotide changes were substitutions and were biallelic. Both variations localized in exons were synonymous and did not change the amino acid sequence of the protein.
Genotype and allele frequencies
We determined frequencies of alleles and genotypes for all 13 variants. Distribution of all RAF1 genotypes was consistent with HWE. Frequencies and OR values for chosen variations are shown in Table 3. There were differences in genotype and allele frequencies between cancer and control group for heterozygote, alternative homozygote and for alternative alleles for several polymorphisms. Although the differences were not statistically significant or they remained at the boundary of statistical significance, nevertheless they could suggest an inclination to risk or protective variants with reference to a larger population of subjects.
Variation
Cancer
subjects
Controls
Pvalue
OR (95% CI)
c.T237C
Genotypefrequency
(n=22)
(n=36)
TT
21 (0,95)
31 (0,86)
CT
1 (0,05)
4 (0,11)
C vs. T
0,4025
2,6 (0,3-25,1)
CC
0
1 (0,03)
C vs. T
0,6978
1,9 (0,07-48,7)
subgroup Y (n=11)
TT 8 (0,73)
CT 2 (0,18)
CC 1 (0,09)
subgroup A (n=10)
TT 10 (1,0)
CT 0
Y vs. A
0,2891
5,5 (0,2-130,4)
CC 0
Y vs. A
0,5157
3 (0,1-82,4)
subgroup G (n=15)
TT 13 (0,87)
CT 2 (0,13)
Y vs. G
0,7358
1,5 (0,2-12,2)
CC 0
Y vs. G
0,4228
3,9 (0,1-104,7)
Allele frequency
T
43 (0,98)
66 (0,92)
C
1 (0,02)
6 (0,08)
C vs. T
0,2143
3,9 (0,5-33,6)
subgroup Y
T 18 (0,82)
C 4 (0,18)
subgroup A
T 20 (1,0)
C 0
Y vs. A
0,1315
10 (0,5-198,1)
subgroup G
T 28 (0,93)
C 2 (0,07)
Y vs. G
0,2159
3,1 (0,5-18,8)
g.A4805C
Genotypefrequency
(n=22)
(n=36)
AA
21 (0,95)
36 (1,0)
AC
1 (0,05)
0
T vs. C
0,3254
5,1 (0,2-130,7)
subgroup Y (n=11)
AA 11 (1,0)
subgroup A (n=10)
AA 10 (1,0)
subgroup G (n=15)
AA 15 (1,0)
Table 3 (1 of 3): Frequencies and odds ratio values for chosen single nucleotide variations for RAF1 gene. (Y=young; A=adult; G=geriatric)
Allele frequency
A
43 (0,98)
72 (1,0)
C
1 (0,02)
0
T vs. C
0,3277
5 (0,2-125,5)
subgroup Y
A 22 (1,0)
C 0
subgroup A
A 20 (1,0)
C 0
subgroup G
A 30 (1,0)
C 0
g.A6902C
Genotypefrequency
(n=22)
(n=36)
AA
19 (0,86)
34 (0,94)
AC
2 (0,09)
2 (0,06)
T vs. C
0,6095
1,7 (0,2-13)
CC
1 (0,05)
0
T vs. C
0,3254
5,1 (0,2-130,7)
subgroup Y (n=11)
AA 11 (1,0)
subgroup A (n=10)
AA 10 (1,0)
subgroup G (n=15)
AA 13 (0,87)
AC 2 (0,06)
Allele frequency
A
40 (0,91)
70 (0,97)
C
4 (0,09)
2 (0,03)
T vs. C
0,1585
3,5 (0,6-20)
subgroup Y
A 22 (1,0)
C 0
G vs. Y
0,3832
3,9 (0,2-86,4)
subgroup A
A 20 (1,0)
C 0
G vs. A
0,4167
3,6 (0,2-79)
subgroup G
A 28 (0,93)
C 2 (0,07)
Table 3 (2 of 3): Frequencies and odds ratio values for chosen single nucleotide variations for RAF1 gene. (Y=young; A=adult; G=geriatric)
c.A918C
Genotypefrequency
(n=22)
(n=36)
AA
21 (0,95)
31 (0,86)
AC
1 (0,05)
4 (0,11)
C vs. T
0,4025
2,6 (0,3-25,1)
CC
0
1 (0,03)
C vs. T
0,6978
1,9 (0,07-48,7)
subgroup Y (n=11)
AA 8 (0,73)
AC 2 (0,18)
CC 1 (0,09)
subgroup A (n=10)
AA 10 (1,0)
AC 0
Y vs. A
0,2891
5,5 (0,2-130,4)
CC 0
Y vs. A
0,5157
3 (0,1-82,4)
subgroup G (n=15)
AA 13 (0,87)
AC 2 (0,13)
Y vs. G
0,7358
1,5 (0,2-12,2)
CC 0
Y vs. G
0,4228
3,9 (0,1-104,7)
Allele frequency
A
43 (0,98)
66 (0,92)
C
1 (0,02)
6 (0,08)
C vs. T
0,2143
3,9 (0,5-33,6)
subgroup Y
A 18 (0,82)
C 4 (0,18)
subgroup A
A 20 (1,0)
C 0
Y vs. A
0,1315
10 (0,5-198,1)
subgroup G
A 28 (0,93)
C 2 (0,07)
Y vs. G
0,2159
3,1 (0,5-18,8)
Table 3 (3 of 3): Frequencies and odds ratio values for chosen single nucleotide variations for RAF1 gene. (Y=young; A=adult; G=geriatric)
We found higher OR values both for the CT heterozygote and for the alternative CC homozygote in control group in comparison with the cancer cases (OR=2.6 for the CT genotype, p=0.4025 and OR=1.9 for the CC genotype, p=0.6978) for c.T237C polymorphism. Also, there was a higher frequency of the alternative C allele in control group in comparison with the cancer group (OR=3.9, p=0.2143). Furthermore, we observed higher incidence of the same genotypes and the C allele in the youngest control subjects (Young group) in comparison with two older subgroups (Adults and Geriatrics) (OR=5.5 for Young vs. Adults and OR=1.5 for Young vs. Geriatrics for the CT genotype; OR=3 for Young vs. Adults and OR=3.9 for Young vs. Geriatrics for the CC genotype; OR=10 for Young vs. Adults and OR=3.1 for Young vs. Geriatrics for the C allele).
Alike, there was a higher prevalence of the AC heterozygotes and the alternative CC homozygotes in control subjects in comparison with cancer subjects (OR=2.6 for the AC genotype, p=0.4025 and OR=1.9 for the CC genotype, p=0.6978) in c.A918C polymorphism. Also, the frequency of the alternative C allele was almost 4-fold higher in control group vs. cancer group (OR=3.9, p=0.2143). Moreover, we observed a higher prevalence of heterozygotes, alternative homozygotes and alternative alleles among the youngest subjects (Young group) in comparison with the other two subgroups of more advanced age: Adults and Geriatrics (OR=5.5 for Young vs. Adults and OR=1.5 for Young vs. Geriatrics for the AC genotype; OR=3 for Young vs. Adults and OR=3.9 for Young vs. Geriatrics for the CC genotype; OR=10 for Young vs. Adults and OR=3.1 for Young vs. Geriatrics for the C allele).
On the contrary, we observed high OR values for AC heterozygote and the alternative C allele in tumor vs. control subjects (OR=5.1 for the AC genotype, p=0.3254 and OR=5 for the C allele, p=0.3277) in g.A4805C polymorphism. Those results could indicate a possible risk variant for cancer occurrence. However, the higher OR value for the alternative CC homozygote in cancer group was most likely due to a distinct number of subjects in each group, as the CC genotype was detected neither in control group, nor in the cancer group. Also, no differences between control subgroups were found and the OR results were all around the neutral ‘1’ value.
There were more consistent data for the other putative risk variant, g.A6902C. We observed much higher prevalence of the AC heterozygote, the alternative CC homozygote and the alternative allele C in cancer cases in comparison with control subjects (OR=1.7 for the AC genotype, p=0.6095; OR=5.1 for the CC genotype, p=0.3254; OR=3.5 for the C allele, p=0.1585). Moreover, there were also higher OR values for the heterozygote and the alternative allele in the most age advanced subgroup of controls (Geriatrics) in comparison with the younger subgroups (Adults and Young) (OR=3.9 for Geriatrics vs. Adults and OR=4.3 for Geriatrics vs. Young for the AC genotype; OR=3.6 for Geriatrics vs. Adults and OR=3.9 for Geriatrics vs. Young for the C allele). Although those results were not statistically significant, they could indicate a very possible risk variant for cancer occurrence, also with reference to age of a subject.
In reference to other identified variants it is worth mention that we also observed a slightly higher prevalence of the alternative AA homozygote (OR=2.1, p=0.3066) and for the alternative allele A (OR=1.8, p=0.1536) in control subjects vs. cancer subjects in the g.G7478A variant. There were also higher OR values in the Young subgroup in comparison with older subjects (OR=2.3 for Young vs. Adults and OR=2.3 for Young vs. Geriatrics for the CC genotype; OR=1.5 for Young vs. Adults and OR=1.9 for Young vs. Geriatrics for the C allele). Similarly, there was a higher incidence of the alternative AA homozygote (OR=3.9, p=0.1045) and alternative allele A (OR=1.9, p=0.1167) in control vs. cancer group in the g.G11494A variant. The genotype and allele frequencies in separate control subgroups were inconclusive and the OR values were mostly around the neutral ‘1’ value. Nevertheless, we assume that both variants could represent putative protective variants. Moreover, both variants were in 83% linkage in RAF1 gene structure and this strongly significant association was seen both in control and cancer subjects (83% and 80%, respectively). We did not observe differences in genotype and allele frequencies for the rest of the identified variants, the OR values were mostly around the neutral ‘1’ value or, if higher, were most likely due to a presence of a single genotype or allele in one of the groups.
Haplo view analysis
RAF1gene structure of case-control association study revealed 2 haplotype blocks, according to the Gabriel et al. [18] algorithm (Figure 1). The haplotype block 1 consisted of two variants: g.G7478A and g.G11494A and showed 83% of linkage between the polymorphisms. Both of them were also in strong linkage separately in control group (83%) and in tumor group (80%) (Data not shown). Interestingly, those two variants were observed to be the putative protective variants with reference to tumor occurrence. The haplotype block 2 consisted of two variants: g.C28107T and g.C28111T and showed 100% linkage. The same linkage was observed separately for the control and the tumor groups (data not shown).
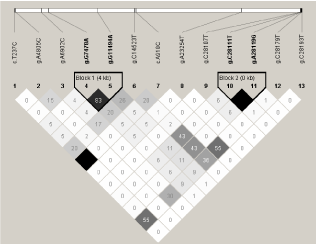
Figure 1: Structure of RAF1 gene shown as case-control association.
Similarly, a very strong linkage (100%) was observed for variants c.T237C and c.A918C in case-control association structure, but also in separate study groups. Both polymorphisms were suggested to be putative protective variants.
We also observed some differences in linkage disequilibrium values between control subjects and tumor patients with reference to several pair of variants. There were over 3-fold higher linkage values for the control group in comparison with the tumor group for variants: c.A918C (putative protective variant) and g.C28179T (72% vs. 23%) and for the variants: c.T237C (putative protective variant) and g. C28179T(72% vs. 23%). Also, we observed over 4-fold higher LD values for the control group in comparison with the tumor group for variants: g.C14523T and c.A918C (putative protective variant) (29% vs. 7%) and also for variants: c.T237C (putative protective variant) and g.C14523T (29% vs. 7%). On the contrary, we observed over 2-fold and over 3-fold higher LD values for the tumor group in comparison with the control group for variants: g.A6902C (putative risk variant) and g.C28179T (52% vs. 22%) and for variants: g.A6902C (putative risk variant) and g.C14523T (30% vs. 9%), respectively.
Discussion
Carcinogenesis is a process which may be associated with multiple molecular and biochemical changes finally leading to changes in cell and tissue morphology as well as induction of cancer growth and development. It was found that the main factors that lead to induction of carcinogenesis and cancer growth are associated with uncontrolled cells division and resistance to apoptosis [19,20,21]. Therefore, many new gene mutations and/or polymorphisms were found to be markers associated with frequency of cancer in several populations. Canine cancer, especially the mammary gland tumor, belong to the cancer types which display a lot of morphological and clinical prognosis similarities to human tumors [22,23]. Therefore, in many cases, canine cancer was found to provide a model of human carcinogenesis.
Aging is the process of multiple biochemical, molecular and metabolomics changes that involve many chemical reactions (e.g. free radical reactions) which finally lead to irreversible changes [24,25]. The aging is also characterized by molecular-genetic changes detected in telomerase activity and chromosome morphology [26,27].
This study was performed to identify new mutation and/ or polymorphisms in the RAF1 gene. Since the RAF1 proteins function is to protect cells from apoptosis pathway as well as to produce in the cells the mechanisms of apoptosis resistance, it was hypothesized that changes in the RAF1 gene structure may lead to induction of carcinogenesis and cancer growth in the case of uncontrolled cell division and protection against cell programmed death. After sequencing analysis we found 13 new single nucleotide polymorphisms, two of them localized in exons 3 and 9. Although all identified polymorphisms were synonymous, inducing no change of amino acid sequence in the protein structure, it is suggested that many of haplotype blocks revealed strong linkage of the polymorphisms. Even if the results were not statistically significant, it is worth mentioning them, as they were observed in study groups only separately, and could indicate slightly different pattern of genetic segregation in cancer and control subjects. Moreover, each pair of the discussed above polymorphic variants included one putative protective or risk variant, which could be of importance while considering genetic susceptibility to carcinogenesis in dogs. RAF1 gene structure and genotype and allele frequencies suggest it to be a candidate gene for cancer occurrence in female dogs.
In the case of association between aging process and RAF1 gene mutations, significant changes were observed in c.T237C polymorphisms and frequency of C allele between three age-related groups of females. The results were not statistically significant and the nucleotide substitution in this variant did not change the amino acid sequence of the protein. Nevertheless, the results suggest that variant c.T237C could be recognized as a possible protective variant in relation to cancer occurrence as well as it could be associated with aging process in bitches. Similarly, we found an increased prevalence of heterozygote, alternative homozygote and alternative allele in the youngest subjects (Young group) in comparison with the other two age-related groups. The results were not statistically significant and the nucleotide substitution did not change the amino acid sequence of the protein. Still, similarly to variant c.T237C, difference in genotype and allele frequencies in c.A918C variant could indicate not only the alternative allele C as a protective variant but also a lower incidence of the tumor presence as related to age of a subject. Interestingly, both putative protective variants (c.T237C and c.A918C) manifested a 100% linkage, which was seen both in control and tumor groups.
Although importance of proto-oncogenes and cell division cycle regulators in induction of carcinogenesis and/or growth and development of cancer was well recognized, the role of RAF1 expression remains still not entirely known. Moreover, few data are only available concerning association between mutations and polymorphisms of RAF1gene and the occurrence of mammary tumor in humans. There exists no evidence of the role of RAF1gene structure changes and occurrence of any types of cancers in domestic bitches. Therefore, the results of present study are of high significance for understanding of the role of this proto-oncogene in canine mammary tumor development as related to aging process. Kiefer et al. [28] used Non-Small-Cell Lung Cancer (NSCLC) cell lines as the model for investigation on variable pattern of cellular oncogene expression and focusing of Epidermal Growth Factor (EGF) binding sites in the NSCLC genome. They found that each investigated cell line showed its own oncogene expression pattern. Moreover, they concluded that the transcriptional activation of proto-oncogenes such as RAF1is involved in activation of EGF signaling pathway as well as it may serve as a marker of NSCLC development. In another study, Kang et al. [29] investigated mutation in several proto-oncogenes such as RAS, RAF and PIK3CA and AKT1 in the Extra Mammary Paget’s Disease (EMPD). They observed a distinct mutation prolife in EMPDs with 27 (19%) cases mutatedRAS and RAF oncogenes and 50 (35%) cases harboring oncogenic mutation in PIK3CA and AKT1. They demonstrated that mutation in these important proto-oncogene signaling pathways (RAS/RAF and PI3K/AKT) may be a reason for altered expression of related genes as well as are for their significant association with pathogenesis of EMPD. It may be concluded that these genes and encoded by them proteins may be used a target of pharmacogenetic therapy in skin cancer. In a similar study, Swanson et al. [30] searched for mutations in a member of RAS/ERK signaling pathway genes, such as RAF1, in relation to development of Noonan Syndrome (NS). They showed that RAS/ERK signaling pathway genes such as PTPN11, KRAS and RAF1were highly associated with development of various sporadic neoplasms, although this association was determined at variable levels of frequency.
The RAF1, as a main proto-oncogene, was previously used as a target gene and protein in analyzes of human cellular senescence, however the association between mutations/polymorphisms of RAF1 gene in relation to aging in canines was never investigated before. In the study of cellular senescence, the WI-38hTERT/GFP-RAF1-ER model of immortal cell line was previously used for studying RAF1- induced senescence of human fibroblasts [31]. The authors observed that, when the fibroblasts were cultivated in 5% oxygen, RAF1 activation generated negligible reactive oxygen species, whereas RAF1-induced senescence occurred efficiently in the culture even in the presence of anti-oxidants or inhibitors of DNA checkpoint pathways. They demonstrated that explicative and oxidative stresses are not the required factors, responsible for RAF1-induced cellular senescence. It was recently also demonstrated that accumulation of 8-oxo-7,8-dihydroguanine (8-oxoG) in the DNA structure leads to genetic instability and spontaneous mutagenesis, providing the reason for induction of carcinogenesis, induction of aging processes and development of various aging-related disease [32]. 8-oxoG is removed from the DNA via DNA Base Excision Repair Process (BER), which is initiated by 8-oxoguanine DNA glycosylase-1 (OGG1). The results obtained by German et al. [32] demonstrated that 8-oxoG was removed from DNA via activation of OGG1-BER. Moreover, it results in activation of RASGTPase, which leads to phosphorylation of the downstream RAStargets RAF1, MEK1,2 and ERK1,2. These results showed a novel mechanisms of OGG1-initiated DNA BER, which may serve as a marker of processes counteracting induction of aging.
In conclusion, RAF1 described as a proto-oncogene and/or apoptosis resistance protein is involved in induction of carcinogenesis, cellular senescence and processes of aging. The results obtained in this study revealed that RAF1 mutations may influence cancer development in a biphasic way, such as a risk or a protective factor.
Acknowledgements
Supported by the Polish National Centre of Science (Grant No. 5279/B/P01/2011/40).
References
- Yeramian A, Moreno-Bueno G, Dolcet X, Catasus L, Abal M, Colas E, et al. Endometrial carcinoma: molecular alterations involved in tumor development and progression. Oncogene. 2013; 32: 403-413.
- Castillo J, García P, Roa JC. [Genetic alterations in preneoplastic and neoplastic injuries of the gallbladder]. Rev Med Chil. 2010; 138: 595-604.
- Ke XS, Qu Y, Goldfinger N, Rostad K, Hovland R, Akslen LA, et al. Epithelial to mesenchymal transition of a primary prostate cell line with switches of cell adhesion modules but without malignant transformation. PLoS One. 2008; 3: e3368.
- Shi Z, Dragin N, Miller ML, Stringer KF, Johansson E, Chen J, et al. Oral benzo[a]pyrene-induced cancer: two distinct types in different target organs depend on the mouse Cyp1 genotype. Int J Cancer. 2010; 127: 2334-2350.
- Looyenga BD, Hammer GD. Origin and identity of adrenocortical tumors in inhibin knockout mice: implications for cellular plasticity in the adrenal cortex. Mol Endocrinol. 2006; 20: 2848-2863.
- Zhao H, Adler KB, Bai C, Tang F, Wang X. Epithelial proteomics in multiple organs and tissues: similarities and variations between cells, organs, and diseases. J Proteome Res. 2006; 5: 743-755.
- Kempisty B, Zaorska K, Bukowska D, Nowak M, Lange K, Brüssow KP, et al. Association between mitogen-activated protein kinase 1 (MAP2K1) gene polymorphisms and the risk of adenocarcinoma during aging in bitches. MedWeter. 2014; 70: 483-490.
- Rasotto R, Goldschmidt MH, Castagnaro M, Carnier P, Caliari D, Zappulli V. The dog as a natural animal model for study of the mammary myoepithelial Basal cell lineage and its role in mammary carcinogenesis. J Comp Pathol. 2014; 151: 166-180.
- Carvalho MI, Pires I, Prada J, Queiroga FL. A role for T-lymphocytes in human breast cancer and in canine mammary tumors. Biomed Res Int. 2014; 2014: 130894.
- Richards KL, Motsinger-Reif AA, Chen HW, Fedoriw Y, Fan C, Nielsen DM, et al. Gene profiling of canine B-cell lymphoma reveals germinal center and postgerminal center subtypes with different survival times, modeling human DLBCL. Cancer Res. 2013; 73: 5029-5039.
- Wozna M, Kempisty B, Piotrowska H, Dorszewska J, Bukowska D, Nowicki M. The immunological, biochemical and molecular bases of canine senescence and Carcinogenesis. Med Vet. (Praha). 2012; 57: 350-359.
- Hughes BT, Sidorova J, Swanger J, Monnat RJ Jr, Clurman BE. Essential role for Cdk2 inhibitory phosphorylation during replication stress revealed by a human Cdk2 knockin mutation. Proc Natl Acad Sci U S A. 2013; 110: 8954-8959.
- Tasaki M, Kuroiwa Y, Inoue T, Hibi D, Matsushita K, Ishii Y, et al. Oxidative DNA damage and in vivo mutagenicity caused by reactive oxygen species generated in the livers of p53-proficient or -deficient gpt delta mice treated with non-genotoxic hepatocarcinogens. J Appl Toxicol. 2013; 33: 1433-1441.
- Sir JH, Barr AR, Nicholas AK, Carvalho OP, Khurshid M, Sossick A, et al. A primary microcephaly protein complex forms a ring around parental centrioles. Nat Genet. 2011; 43: 1147-1153.
- Wang HG, Rapp UR, Reed JC. Bcl-2 targets the protein kinase Raf-1 to mitochondria. Cell. 1996; 87: 629-638.
- Alavi A, Hood JD, Frausto R, Stupack DG, Cheresh DA. Role of Raf in vascular protection from distinct apoptotic stimuli. Science. 2003; 301: 94-96.
- Jugdutt BI, Jelani A, Palaniyappan A, Idikio H, Uweira RE, Menon V, et al. Aging-related early changes in markers of ventricular and matrix remodeling after reperfused ST-segment elevation myocardial infarction in the canine model: effect of early therapy with an angiotensin II type 1 receptor blocker. Circulation. 2010; 122: 341-351.
- Gabriel SB, Schaffner SF, Nguyen H, Moore JM, Roy J, Blumenstiel B, et al. The structure of haplotype blocks in the human genome. Science. 2002; 296: 2225-2229.
- Xue B, He L. An expanding universe of the non-coding genome in cancer biology. Carcinogenesis. 2014; 35: 1209-1216.
- von Manstein V, Yang CM, Richter D, Delis N, Vafaizadeh V, Groner B. Resistance of Cancer Cells to Targeted Therapies Through the Activation of Compensating Signaling Loops. Curr Signal Transduct Ther. 2013; 8: 193-202.
- Xia H, Hui KM. Mechanism of cancer drug resistance and the involvement of noncoding RNAs. Curr Med Chem. 2014; 21: 3029-3041.
- Rismanchi S, Yadegar O, Muhammadnejad S, Amanpour S, Taghizadeh-Jahed M, Muhammadnejad A. Expression of vimentin filaments in canine malignant mammary gland tumors: A simulation of clinicopathological features of human breast cancer. Biomed Rep. 2014; 2: 725-728.
- Patruno R, Marech I, Zizzo N, Ammendola M, Nardulli P, Gadaleta C, et al. c-Kit expression, angiogenesis, and grading in canine mast cell tumour: a unique model to study c-Kit driven human malignancies. Biomed Res Int. 2014; 2014: 730246.
- Sharples AP, Al-Shanti N, Lewis MP, Stewart CE. Reduction of myoblast differentiation following multiple population doublings in mouse C2 C12 cells: a model to investigate ageing? J Cell Biochem. 2011; 112: 3773-3785.
- Buttiglieri S, Ruella M, Risso A, Spatola T, Silengo L, Avvedimento EV, et al. The aging effect of chemotherapy on cultured human mesenchymal stem cells. Exp Hematol. 2011; 39: 1171-1181.
- Axelrad MD, Budagov T, Atzmon G. Telomere length and telomerase activity; a Yin and Yang of cell senescence. J Vis Exp. 2013; : e50246.
- Davis T, Skinner JW, Faragher RG, Jones CJ, Kipling D. Replicative senescence in sheep fibroblasts is a p53 dependent process. Exp Gerontol. 2005; 40: 17-26.
- Kiefer PE, Wegmann B, Bacher M, Erbil C, Heidtmann H, Havemann K. Different pattern of expression of cellular oncogenes in human non-small-cell lung cancer cell lines. J Cancer Res Clin Oncol. 1990; 116: 29-37.
- Kang Z, Xu F, Zhang QA, Wu Z, Zhang X, Xu J, et al. Oncogenic mutations in extramammary Paget's disease and their clinical relevance. Int J Cancer. 2013; 132: 824-831.
- Swanson KD, Winter JM, Reis M, Bentires-Alj M, Greulich H, Grewal R, et al. SOS1 mutations are rare in human malignancies: implications for Noonan Syndrome patients. Genes Chromosomes Cancer. 2008; 47: 253-259.
- Jeanblanc M, Ragu S, Gey C, Contrepois K, Courbeyrette R, Thuret JY, et al. Parallel pathways in RAF-induced senescence and conditions for its reversion. Oncogene. 2012; 31: 3072-3085.
- German P, Szaniszlo P, Hajas G, Radak Z, Bacsi A, Hazra TK, et al. Activation of cellular signaling by 8-oxoguanine DNA glycosylase-1-initiated DNA base excision repair. DNA Repair (Amst). 2013; 12: 856-863.