
Review Article
J Drug Discov Develop and Deliv. 2014;1(2): 8.
Phospholipid Vesicles for Enhanced Drug Delivery in Dermatology
Željka Vanić*
Department of Pharmaceutical Technology, University of Zagreb, Croatia
*Corresponding author: Željka Vanić, Department of Pharmaceutical Technology, University of Zagreb, A. Kovačića,10000 Zagreb, Croatia
Received: October 10, 2014; Accepted: March 24, 2015; Published: March 25, 2015
Abstract
Phospholipid vesicles (liposomes) have been widely investigated as carriers for enhancing skin delivery of drugs. The similarity between lipid compositions of liposomes with the skin structures enables liposomes to be used as physiologically acceptable drug delivery nanosystems. By manipulation of their physico-chemical properties the effective skin delivery of the encapsulated/ incorporated drug can be achieved. They can be used as carriers to increase the solubility of the poorly soluble drugs or improve the stability of instable compounds. Phospholipid vesicles enable localized skin delivery of lipophilic drugs, enhance the penetration of hydrophilic drugs and help in reducing the drug irritation. Even without entrapped active compound, they have been shown to improve the skin condition by increasing the hydration level of the skin and integrity of the stratum corneum. The potentials and perspectives of different types of phospholipid vesicles have been summarized in this review, including conventional liposomes, deformable liposomes, ethosomes, invasomes and propylene glycol-containing liposomes, with the emphasis on their potential mechanism of action in enhanced skin delivery.
Keywords: Skin; Topical delivery; Conventional liposomes; Deformable liposomes; Ethosomes; Propylene glycol liposomes
Introduction
Application of ointments and lotions onto the skin for cosmetic and therapeutic effects has been used for thousands of years. Nowadays, more than one third of drugs under clinical evaluation are related to delivery into or through the skin [1,2]. Regarding easiness of application and patient's accessibility, delivery via the skin represent an ideal route of drug administration for achieving local (dermal) or systemic effects (transdermal delivery) [3].
Skin is the largest human organ which consists of the three anatomically distinct layers; epidermis, dermis and subcutaneous layer (Figure 1). The natural function of the skin is to protect the body from unwanted influences from the environment. The main barrier of the skin is the outermost layer-the stratum corneum. Often described as "brick and mortar"-like structure, stratum corneum is composed of 10-15 thick layers of dead keratinocytes embedded in intercellular lipid matrix, composed of the ceramides, free fatty acids, triglycerides, cholesterol, cholesterol sulphate and sterol/wax esters [6]. This barrier remains slightly open and permeable to the environment to allow evaporation of water from the living cells of the skin. Substances (drugs) which are applied onto the skin have to pass stratum corneum to reach the target cells in the lower layers of epidermis or dermis. Three main pathways are possible: (i) through the hair follicles and associated sebaceous glands, (ii) through the sweat ducts and (iii) across the continuous stratum corneum comprising transcellular and intercellular pathways [6]. Parameters which affect the delivery of topically applied drugs include the size of molecule, the lipophilicity, type of formulation, presence of penetration enhancers and physical state of stratum corneum [7]. It is well known that lipophilic drugs are easily transported through the skin as compared to hydrophilic drugs, especially those of high molecular weight. Therefore, to achieve sufficient penetration of active substances into or through the skin, the barrier properties of the stratum corneum are often reduced by chemical penetration enhancers or physical methods such as iontophoresis, sonophoresis, electroporation and microneedles [8]. Alternative approach is based on the use of drug delivery nanosystems, which are capable to enhance penetration of hydrophilic drugs into the skin and control release and deposition of lipophilic drugs at the site of action [9].
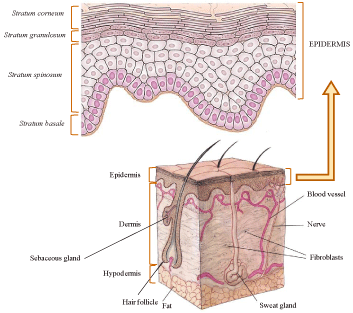
Figure 1: Cross section of the human skin (adapted from [4,5]).
In the context of structural similarity with the skin constituents, biocompatibility and biodegradability, phospholipid-based nanovesicles are especially interesting for skin therapy. This article is focused on their potentials as drug nanocarriers in improved dermal therapy. By using these nanosystems higher concentrations of drugs can be localized at the site of action, thus reducing the systemic drug levels and consequently the systemic side effects. Most of the herewith presented studies still require extensive research in the laboratory conditions, some of them are in the clinical stages and a few are already on the market.
Phospholipid Vesicles (liposomes): General Considerations
Phospholipid vesicles (liposomes) are biodegradable and biocompatible nanovesicles composed of one or more phospholipid bilayers surrounding inner aqueous compartment(s). Compared to the other drug delivery nanosystems, lipid composition of liposomes is quite similar to the intercellular lamellae and keratinocytes. Besides, structure properties enable liposomes to encapsulate or incorporate drugs of different size and lipophilicity. Hydrophilic drugs will be encapsulated into the aqueous compartment(s), lipophilic inside the bilayer, while amphiphilic will partition themselves between these two regions. Liposomes are characterized by their lipid composition, membrane rigidity/elasticity, particle size, surface charge, number of lamellae and inner/outer aqueous phases, characteristics which determine their stability and drug delivery abilities [10]. Phospholipids building liposomes act as a penetration enhancers enabling the penetration of individual lipid components into stratum corneum and subsequently alteration of the intercellular lipid matrix within the skin. Therefore, encapsulation of hydrophilic drugs in liposomes can improve their penetration into the skin. Furthermore, liposomes may provide targeted delivery to skin appendages and can assure localized depot of the lipophilic drug in the skin thus assuring sustained release and minimizing systemic absorption [9]. According to the structure properties, presence of surfactant or co-solvent in their composition, liposomes are categorized as conventional (classic) liposomes, deformable (elastic) liposomes, ethosomes, invasomes and propylene glycol-containing liposomes. Mechanisms of action, i.e. interactions of different types of liposomes with the skin are summarized in several reviews [9-13].
Conventional Liposomes
This term is used for classic liposomes. They are composed of one (unilamellar liposome) (Figure 2), or more concentrically positioned phospholid bilayers (oligo- or multi-lamellar liposomes) enclosing aqueous compartments. The bilayers comprise of neutral phopsholipids or combinations of neutral and surface charged phospholipids. Cholesterol is often present as a membrane constituent to improve the bilayer rigidity of vesicles [14].
Among variety of liposomally encapsulated drugs, several classes of drugs were intensively studied in dermatology: corticosteroids, anesthetics, retinoids and antimicrobials (Table 1).
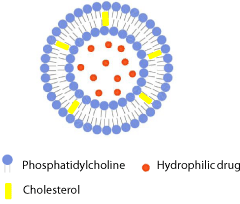
Figure 2: Schematic drawing of conventional liposome.
Indication
Drug
Phospholipid vesicles
Investigation/Status
Reference
Acne
Clindamycin hydrochloride
Conventional liposomes
Clinical study
[28,29]
Tretinoin
Conventional liposomes
Clinical study
[22]
Benzoyl peroxide
Conventional liposomes
Clinical study
[31]
5-aminolevulinic acid
Conventional liposomes
Clinical study
[24]
Azelaic acid
Conventional liposomes, ethosomes
In vitro
[60]
Dermatitis
Triamcinolone acetonide
Conventional liposomes
Deformable liposomes
In vivo (animals)
[15]
[83]
Hydrocortisone
Conventional liposomes
In vivo (animals)
[16,17]
Dexametasone, hydrocortisone
Deformable liposomes
In vivo (animals)
[84]
Bethametasone
Deformable liposomes
In vitro
[44]
Psoriasis
Vitamin D3
Conventional liposomes
In vivo (animals)
[34]
Dithranol
Conventional liposomes
Clinical study
[35]
Metotrexate
Deformable liposomes
Ethosomes
In vitro
[48]
[62]
Cyclosporin A
Ethosomes
In vitro
[85]
Vitiligo
Psoralen
Ethosomes
In vivo (animals)
[70]
Khellin
Conventional liposomes
Case study
[86]
Antimicrobial therapy
Econazole (Pevaryl ®Ò Lipogel)
Conventional liposomes
Registered
[25]
Acyclovir (SupravireÒ)
Ethosomes
Registered
[87]
Miconazole nitrate
Propylene glycol liposomes
In vitro
[72]
Erythromycin
Ethosomes
In vivo (animals)
[88]
Bacitracin
Ethosomes, conventional liposomes
In vitro
[65]
Terbinafine hydrochloride
Deformable liposomes, ethosomes
In vitro
[89]
Actinic keratosis and skin cancer
T4 endonuclease 5 (T4N5 liposomes)
pH-sensitive liposomes
Clinical/Phase III
[80,81]
Celecoxib
Ethosomes, deformable and conventional liposomes
In vitro
[66]
Topical anaesthesia
Lidocaine
Conventional liposomes
Clinical
[19]
Lidocaine, tetaracaine
Deformable liposomes
In vivo (humans)
[90]
Cinchocaine
Propylene glycol liposomes, ethosomes, deformable liposomes, conventional liposomes
In vivo (animals)
[71]
Alopecia
Minoxidil (NanominoxÒ)
Ethosomes
Registered
[87]
Table 1: Examples of phospholipid vesicles investigated for dermal drug delivery.
The pioneering study on the advantages of using conventional liposomes in topical skin therapy was reported by Mezei and Gulasekharam in 1980 [15]. They have proved that liposomal triamcinolone acetonide, when compared to control lotion, provided increased drug deposition in epidermis and dermis, while concentrations of the drug in thalamic region (potential place for sideeffects) and urinary excretions were significantly reduced. Similar effects have been shown using liposome encapsulated hydrocortisone [16,17] and anesthetics such as tetracaine [18] and lidocaine [19]. Higher and sustained concentrations of corticosteroids and anesthetics in the skin assured higher local efficacy, lower frequency of applications and less pronounced side-effects due to lower absorption of lipophilic drugs into the blood [20].
Compared with the other drug delivery nanosystems, skin application of liposomes even without encapsulated active substance ("empty" liposomes) may improve the skin condition by increasing skin hydration and integrity of stratum corneum [21]. This effect is of high importance in dermatology particularly for the treatment of xerosis cutis and atopic dermatitis [20].
Benefits of using liposomes in topical skin therapy can be seen in reduced skin irritation of substances such as retinoides [22]. Liposomal tretinoin gel showed 1.5 fold improvement of drug efficacy and a marked decrease in all side effects during 3 months of acne treatment as compared to conventional formulation [23]. Furthermore, using of liposomes increased the efficacy of treatments even with the significantly lower concentrations of active substances than in conventional formulations. For example, changing of a vehicle for 5-aminolevulinic Acid (5- ALA) in photodynamic therapy from the moisturizing cream to liposome formulation reduced the concentration of active substance by a factor of 40 while still inducing the same effect, and additionally eliminated the necessity for occlusion [24].
Effective therapy of fungal skin infections is of great importance in dermatology. Conventional topical formulations are often limited by poor efficiency in delivering antifungal drugs to the target site in the skin. To improve the local therapy antifungals have been encapsulated in different delivery nanosystems. Econazole was the first antifungal drug incorporated in conventional liposomes. Clinical investigations revealed a hastened onset of effect due to increased concentration of the drug in the stratum corneum while treatment duration could be reduced. Pevaryl® Lipogel was the first approved liposome product and showed the potentials of liposomal systems [25]. Other antimicrobial drugs were also investigated as liposome skin formulations, such as miconazole [26] and fluconazole [27].
The potential of using liposome-encapsulated clindamycin hydrochloride in the treatment of acne has been first demonstrated by Šentjurc and collaborators. Liposome formulation has been shown to significantly decrease the numbers of pustules as compared to control formulation [28]. Similar results have been proven later by Honzak and Šentjurc [29]. The other liposome-incorporated active substances have been reported to enhance the effectiveness of the acne therapy, too. For example, lauric acid-loaded liposomes have been shown to fuse with the membranes of Propionebacterium and release the lauric acid directly into the bacterial membranes [30]. Improved treatment of Propionebacterium acnes with marked reduction in adverse symptoms has also been demonstrated with liposomes containing benzoyl peroxide [31].
Follicular route represent a privileged penetration pathway for liposomes. They enter faster into hair shunts than through the stratum corneum and hereby offer the possibility to create high local concentrations of the active compounds within the follicular duct. Melanin, which is the target for laser hair removal, is not present in all types of hair. As a result, there is a lack of pigment in blond, gray, and white hair that led to the idea of external chromophore application. Clinical study evaluating melanin-encapsulated liposomes demonstrated significantly higher efficacy in the treatment of white and blond hair compared with a control group. However, the clinically observed hair reduction was so weak that additional effort as well as higher costs argues against the application of the tested formulation [32].
Among different dermatological conditions and diseases, treatment of psoriasis is of great relevance. Although major advances in the understanding of the pathogenesis and controlling the disease have been made, the need for safe, cost-effective and disease-effective cures still remains [33]. Conventional liposomes have been proven to improve the skin delivery of the vitamin D3 and dithranol in the treatment of psoriasis. It has been confirmed that by using liposomal formulations of vitamin D3 therapeutic effect can be obtained by lower concentrations than in commercial preparations [34]. In the case of plaque type psoriasis, the entrapment of dithranol in liposomes has promoted its epidermal bioavailability and enabled the dose reduction and the consequent dose-dependent side-effects [35].
For the treatment of Rosacea liposomes containing metronidazole were proposed. Vesicles composed of natural and synthetic lipids were evaluated based on physico-chemical characterization and optimal preparation was incorporated into different dermal vehicles. According to the physical stability and rheological properties liposomes-in-Carbopol gel was considered as the best formulation for the topical treatment of Rosacea [36].
All above presented examples of using conventional liposomes in dermatology report a localized effect whereby vesicles accumulate drugs in stratum corneum or upper layers of epidermis and in the appendages. Lipid composition, method of preparation and thermodynamic state of the bilayers of liposomes were shown to greatly affect skin deposition behavior of liposomes [37]. Generally, conventional liposomes are not expected to penetrate into viable skin [9], although occasional transport processes were reported [38,39]. It is commonly accepted that they remain confined on the skin surface by adhering and fusing with the structures of stratum corneum. Interactions of liposomal lipids with intercellular lipid matrix of the stratum corneum could result in its destabilization. Therefore delivery of drugs into the upper layers of epidermis by conventional liposomes is suggested to be via penetration enhancing effect of liposomal lipids and deposition of small liposomes in hair follicles [9]. To enhance delivery of drugs into deeper skin layers or even transdermally, novel types of liposomes with pronounced elastic bilayers were investigated.
Deformable Liposomes
Deformable liposomes (also known as Transferosomes?, elastic, flexible or ultradeformable, liposomes) represent a novel type of phospholid vesicles designed to improve dermal and especially transdermal drug delivery. Although similar to conventional liposomes in morphology, deformable liposomes function differently. The inventors of these vesicles proposed that the vesicles penetrate through the intact skin but only when applied under non-occluded conditions [40-42]. Deformable liposomes (Figure 3) are composed of phospholipids and an edge activator (single chain surfactant), which destabilizes the phospholipid bilayers of vesicles by increasing their deformability (elasticity). Therefore, these vesicles may squeeze through pores in stratum corneum which are less than 1/10 of the vesicle diameter and transport entrapped drug deeper into the skin [42]. Deformable liposomes require a hydration gradient to encourage skin penetration (non-occlusive condition). Then the osmotic gradient operating from the dry skin surface towards wet viable tissues drives vesicles through stratum corneum. Namely, phospholipids tend to escape dry surroundings, and to remain swollen, they must follow hydration gradient and penetrate into more hydrated layers of skin, i.e. viable epidermis and dermis [1]. It has been confirmed by Honeywell-Nguyen et al. [43] that intact elastic vesicles can penetrate into the stratum corneum under nonocclusive application; however, only few intact vesicles were found in the deeper layer of the horny layer.
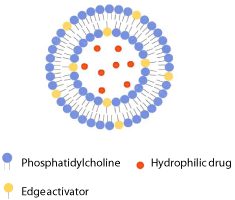
Figure 3: Schematic drawing of deformable liposome.
Despite the considerable interest in the mechanism of drug delivery by deformable liposomes, there is still considerable discussion whether deformable liposomes act as carrier system by penetrating intact through the skin or as penetration enhancers [3]. Recent study by Gillet et al. [44] supports the second mechanism, i.e. role of deformable liposomes and its constituents as penetration enhancer. Bethametasone was released from the vesicles followed by diffusion of free drug molecules through stratum corneum and their partition into the viable skin tissue [44].
Even though deformable liposomes were established to increase transdermal delivery of drugs such as diclofenac, ketotifen, zidovudine, insulin, estradiol etc. [9], several studies have shown that deformable liposomes are appropriate only for improving dermal drug delivery (Table 1). El Maghraby and coworkers [45] investigated potential use of deformable liposomes containing 5-fluorouracil. They have shown better in vitro skin delivery of the drug by deformable vesicles as compared to conventional liposomes. However, due to limited drug partitioning inside the skin the authors suggested that deformable liposomes are not penetrating intact into the skin, as earlier claimed by the Cevc and collaborators [40,42]. Actually, vesicle's components acted as penetration enhancers thus promoting skin deposition of the drug [45,46].
Enhanced dermal deposition of dipotassium ghycyrrhizinate (substance isolated from liquorice root) has been proven with elastic liposomes composed of phosphatidylcholine or hydrogenated lecithin mixed with dipotassium ghycyrrhizinate, the component that was also used as edge activator [47]. Furthermore, the advantages of using deformable liposomes over conventional liposomes in skin drug delivery have been demonstrated in the following study by Trotta's group [48]. They have investigated topical administration of methotrexate, very potent antipsoriatic drug, known to cause numerous side-effects and hepatotoxicity when applied orally. Its topical application is limited due to the fact that the drug is hydrophilic and mostly in dissociated form. However, by encapsulating methotrexate in deformable liposomes containing dipotassium ghycyrrhizinate as edge activator 50% of the administered dose was found in the skin thus suggesting its suitability for the topical treatment of psoriasis [48].
Interesting approach of using elastic liposomes has been recently reported by Cadena and collaborators [49]. They have encapsulated quercitin and resveratrol into deformable liposomes containing sodium deoxycholate as edge activator. Since the both active compounds induce a synergic inhibition of adipogenesis and increase apoptosis in adipocytes while sodium deoxycholate has necrotic effects, this innovative phospholipid nanosystem was proposed as a novel approach for dissolving the subcutaneous fat when applied as a subcutaneous injection. However, their research was based only on the physico-chemical characterization of nanovesicles without in vivo data on the efficacy of the system [49].
Deformable liposomes have been shown to facilitate improved and localized effect of miconazole nitrate in the therapy of deep fungal infections. In vivo antifungal activity using a rat model confirmed efficacy of deformable liposomes in the treatment of Candida albicans induced lesions. When the animals were treated with liposomes composed of soya phosphatidylcholine and Span 80 fast recovery from fungal infection was obtained, compared to conventional liposomes and solution of the free drug [50].
Elastic liposomes are usually prepared by the same methods as conventional liposomes. The most commonly used phospholipids include soybean phosphatidylcholine due to high amount of unsaturated fatty acids and egg phosphatidylcholine [51], although hydrogenated and positively charged lipids might be considered as well [52]. It is also possible to include small amount of ethanol (7%, v/v) in the water phase during liposome preparation [53].
The influence of edge activators on physicochemical properties of elastic liposomes were intensively studied [9,54,55]. Commonly, deformable liposomes are of smaller size than corresponding conventional liposomes and could entrap lower amount of the drug [55,56]. Moreover, due to the presence of unsaturated phosphatidylcholine that has low transition temperature (Tm), lipid bilayers are in liquid-crystal state and liposome membrane is highly permeable to encapsulated drug during storage [51]. Furthermore, edge activator destabilizes bilayers of liposomes and increases permeability of the liposome's membranes that was confirmed by increased in vitro release of entrapped hydrophilic drug, as compared to conventional liposomes [57]. These limitations could be overcome by preparing elastic vesicles from phospholipids with Tm of approximately 30°C that would assure formation of stable elastic liposomes with rigid bilayers during storage at room temperature. However, after application onto the skin, liposomal membrane turns into liquid state as the skin temperature (32°C) is higher than Tm of elastic vesicles, thereby enabling satisfactory balance between elasticity and stability [51].
In 2012, new generation of highly deformable liposomes has been introduced by Cevc [58]. It exploits a different and previously unknown mechanism for controlling bilayer properties: adaptive redistribution of hydrophilic additives (modulators) near a bilayer. This replaces and/or enhances surfactant redistribution within a bilayer and improves bilayer vesicle adaptability, stability, physical interaction with tissue and drug payload. Although these vesicles have been suggested for safe treatment of mild inflammation and pain [58], they are attractive for dermal products, too.
Ethosomes
Ethosomes represent a type of phospholipid vesicles developed by Touitou et al. [39] for improved delivery of drug into or across the skin. They are composed of phospholipids and water as the conventional liposomes, but in addition enclose high ratio of ethanol (usually 20-45%, v/v) (Figure 4). Due to well-known skin permeation enhancing effect of ethanol, ethosomes are also described as skin permeation-enhancing vesicles. They are prepared by dissolving phospholipids and lipophilic drug in ethanol and slow addition of aqueous phase during constant mixing [39]. Compared to conventional liposomes of the same phospholipid composition, ethosomes are of significantly smaller diameter that can be explained by high ethanol content. Ethanol also contributes to the negative net surface charge of the vesicle, which additionally decreases the size of ethosomes [9]. The encapsulation efficiency for lipophilic drugs in ethosomes is higher in comparison to conventional and especially to deformable liposomes. This is a consequence of solubilizing effect of ethanol and the multilamellar morphology of ethosomes reported by Touitou and coworkers [39].
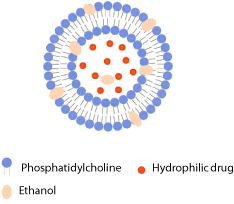
Figure 4: Schematic drawing of ethosome.
Most of the reports on ethosomes are related to transdermal drug delivery [9]. However, they were also studied as drug carriers for improved skin therapy in dermatology providing increased drug solubility and enhancing its penetration through the stratum corneum (Table 1). Thus incorporation of poorly soluble acyclovir into ethosomes led to improved skin bioavailability of the drug. Clinical investigation of ethosomal acyclovir in the treatment of recurrent herpes labialis demonstrated advantages of using ethosomes over commercial product; time necessary for crusting of the lesions and loss of crusts was significantly reduced by applying ethosomal formulation [59]. Esposito and coworkers have studied ethosomes as carriers for skin delivery of azelaic acid. Generally, ethosomes were of smaller size and narrower size distribution than conventional liposomes. Among different ethosome preparations those prepared by the highest ethanol concentration (45%, v/v) were characterized by rapid release of azelaic acid [60].
Phospholipid vesicles containing 10-20% (v/v) of ethanol increased deposition of cyclosporin A into stratum corneum in comparison to conventional liposomes. This study reported on the potential use of ethosomes as carriers to deliver the drugs which are skin-impermeable in the treatment of diseases such as psoriasis [61]. A few years later, Dubey et al. [62] explored ethosomes as a carrier for increased skin delivery of another potent drug for the therapy of psoriasis, metotrexate. In vitro skin permeation studies using human cadaver skin demonstrated that ethosomes were able to enhance metotrexate flux and skin deposition in comparison to the control formulations [62].
For the treatment of alopecia, Touitou's group prepared and evaluated effectiveness of ethosomes in pilosebaceous delivery of minoxidil in vitro. They have shown that the quantity of the drug accumulated into the skin of nude mice after the application of ethosomes was 2.0, 7.0 and 5.0 fold higher as compared to the ethanolic phospholipid dispersion, hydroethanolic solution and ethanolic solution of the drug each containing 0.5% of minoxidil [63].
Ethosomes were also studied for improved topical antimicrobial therapy. Godin et al. [64] investigated in vivo antibacterial effect of ethosomally incorporated erythromycin on a mice model. Ethosomal formulation was shown to be very effective in the healing of S. aureusinduced deep dermal infections, while hydroethanolic solution of erythromycin caused deep dermal and subcutaneous abscesses within 5 days after treatment. In another study, the success of ethosomes to increase delivery of the polypeptide antibiotic bacitracin to deep layers of the skin was reported [65]. In contrast to conventional liposomes, which remained confined on the skin surface, ethosomes allowed a significant penetration of the drug into the deep skin layers.
Novel type of ethosomes, containing an edge activator (Tween 20) in addition to phospholipids and ethanol, has been recently presented by Bragagni and coworkers [66]. Comparison of different phospholipid vesicles examined in their study (conventional liposomes, deformable liposomes and ethosomes), demonstrated potential of novel ethosomal formulation in topical delivery of celecoxib for the prevention of skin cancer.
Ethosomes were also proposed as s vehicle for 5-ALA in photodynamic therapy of non-melanoma cancers. The fluorescence intensity of Protoporphyrin IX (PpIX) was maximal after topical application of ethosomes containing 15% of ethanol and composed of phosphatidylethanolamine, cholesterol and a surfactant (sodium stearate) in comparison to that achieved by 5-ALA in aqueous solution, ethanolic solution and conventional liposomes [67]. In the following study, Fang et al. [68] demonstrated improved delivery of 5-ALA and the formation of PpIX in both normal and hyperproliferative murine skin samples. The expression level of tumor necrosis factor was reduced after the 5-ALA ethosomes were applied to treat hyperproliferative murine skin, suggesting possible application of formulation in the treatment of psoriasis [68].
Ethosomes provide a good platform for enhanced delivery of phytochemicals to the targeted site inside the skin. For example, apigenin loaded ethosomes composed of soy phosphatidylcholine and combination of two alcohols (propylene glyol and ethanol) have been proven to had the strongest effect on reduction of cyclooxygenase-2 levels in mouse skin inflammation model, thus demonstrating promising therapeutic approach for the treatment of UVB-induced skin inflammation [69].
To improve skin permeation and deposition of psoralen for the treatment of vitiligo, Zhang et al. [70] evaluated ethosome formulations. In vitro studies proved significantly greater skin deposition of psoralen by ethosomes while in vivo skin microdialysis showed that the peak concentration and area under the curve of psoralen released from ethosomes were approximately 3 and 2 times higher than those of psoralen from the tincture formulation. The enhanced permeation and skin deposition of psoralen delivered by ethosomes is considered beneficial for reducing toxicity and enhancing the efficacy of long-term psoralen treatments [70].
A proposed mechanism for improved skin drug delivery by using ethosomes lies in dual fluidizing effect of ethanol on the ethosomal lipid bilayers and on the intercellular lipid matrix in stratum corneum. Therefore, the soft ethosomes can penetrate the disturbed skin lipid bilayers creating a pathway through the skin, and fuse with cell membranes in the deeper skin layers releasing encapsulated drug [9,39]. Compared to deformable liposomes which are able to increase skin delivery only when applied non-occlusively, ehosomes are efficient both under non-occlusive and occlusive conditions [10].
Miscellaneous Phospholipid Vesicles in Dermatology
Propylene glycol liposomes
Propylene glycol-containing liposomes (PG liposomes) have been proposed by Elsayed et al. [71] as a new type of phospholipid vesicles for improved skin drug delivery. These vesicles are composed of phospholipids, propylene glycol and water and have been characterized by high entrapment efficiency due to solubilizing effect of propylene glycol (Figure 5).
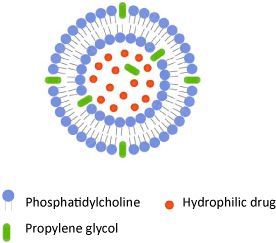
Figure 5: Schematic drawing of propylene glycol-containing liposome.
Preliminary in vivo skin deposition studies using an animal model have shown that PG liposomes were superior to conventional and deformable liposomes as well as to ethosomes in the skin delivery of local anesthetic cinchocaine [71]. PG liposomes were also studied to increase the skin penetration of hydrophilic model drug and have been characterized by increased entrapment efficiency and bigger size compared to deformable and conventional liposomes [56]. For the topical treatment of fungal infections, Elmoslemany et al. [72] have prepared and evaluated miconazole nitrate-loaded PG liposomes. Compared to conventional liposomes, PG liposomes have proven to exhibit better encapsulation efficiency, stability, antifungal activity and enhanced skin deposition [72]. Improved skin delivery by using PG-liposomes is considered to be a consequence of the synergistic effect of propylene glycol on the membrane elasticity of the vesicles and the penetration enhancing effects of both phospholipids and propylene glycol [56].
Invasomes
Invasomes are novel type of elastic phospholipid vesicles introduced by Fahr and his group [73]. They are composed of phosphatidylcholine, ethanol and a mixture of terpenes as penetration enhancers. Invasomes containing 3.3% ethanol and 1% of the terpene mixture (cineole:citral:d-limonene=45:45:10) could significantly enhance the skin penetration and deposition of the highly hydrophobic photosensitizer temoporfin (mTHPC) in comparison to vesicles without terpenes and conventional liposomes [73]. In the following study, the ratio between d-limonene, citral and cineole was varied in the standard terpene mixture and also single terpenes were used. Invasomes containing 1% (w/v) cineole provided the highest skin penetration enhancement of mTHPC indicating that incorporation of a single terpene into invasomes could provide effective delivery of mTHPC [74]. The possible mechanism of enhanced skin delivery by invasomes includes the fragmentation of the part of the vesicles during penetration through the stratum corneum with the release of terpenes and phospholipids. These compounds together with ethanol act as penetration enhancers by fluidizing the intercellular lipids. In addition, they increase the degree of membrane elasticity. Therefore, the perturbed organization of the horney layer's lipids together with the bilayer elasticity of invasomes and the presence of the skin hydration gradient are supposed to facilitate the penetration of small intact invasomes that were not defragmented during their penetration [74].
pH-sensitive liposome
In the middle nineties, Yarosh's group designed and evaluated specific type of liposomes containing a DNA repairing enzyme, i.e. T4 endonuclease 5 (T4N5) for the treatment of actinic keratosis [75,76]. Regarding the composition and mechanism of action, T4N5 liposomes are pH sensitive liposomes, which are stable under neutral pH but become destabilized in acidic pH such as in endosome (inside the cells), where fusion with the endosomal membrane occurs followed by release of the entrapped material into the cytoplasm [77,78]. Applied after UV exposure, T4N5 liposomes were proved to penetrate human skin and efficiently deliver a DNA repairing enzyme into keratinocytes and epidermal Langerhans cells in 15 volunteers with preceding skin cancers [79]. Additionally, it was reported that daily applications of T4N5 liposome lotion during one year to the sun-damaged skin of 30 xeroderma pigmentosa patients with the history of skin cancer or actinic keratosis, significantly lowered the rate of new actinic keratosis and basal cell carcinomas [80]. To the best of my knowledge, this formulation reached the Phase III of clinical investigation [81].
Perspective
Currently there are different directions in studying applicability of phospholipid vesicles in improved delivery of drugs (active substances) in dermatology. Appendages targeting is of great importance in increased skin delivery of drugs, particularly those of higher molecular weight, as previously discussed. On the other hand are investigations of new antimicrobial therapeutics for the treatment of chronic wounds. Different strategies have been proposed based on the special design of vesicles and/or encapsulation of the new substances to combat bacterial resistance [82]. A big challenge represents dermal investigations of immunosupresive and anticancer drugs.
Encapsulation of plant medicines into phospholipid vesicles with increased skin penetration ability is receiving considerable attention in the recent years. A numerous phytochemicals can be incorporated into phospholipid vesicles to improve their delivery into the skin for different purposes in dermatology. Commonly, liposomes provide a plenty of opportunities for innovative research aimed at both increasing efficiency and reducing toxicity of drugs through simple topical application. Since composition of liposomes determines its penetration ability and efficacy of drug delivery, the optimal vesicle composition has to be determined experimentally for each drug (active substance) separately to acquire vesicles of appropriate therapeutical potential.
Conclusion
During the last 3 decades the investigations of phospholipid vesicles for improved delivery of drugs in dermatology have considerably expanded. By designing the different types of vesicles varying in size, surface properties, bilayer composition and membrane rigidity/elasticity delivery of drugs to intended target sites in the skin become feasible. A variety of possible mechanisms in enhancing skin drug delivery have been suggested, depending on the type of vesicles applied. Promising results have been proven with liposomally encapsulated drugs in antimicrobial therapy, treatments of acne, atopic dermatitis, alopecia, vitiligo, psoriasis and xeroderma pigmentosa resulting in the products available on the market or in clinical trials.
Acknowledgment
The author is very grateful to Prof. N. Škalko-Basnet for valuable discussion and suggestions to improve the quality of paper.
References
- Barry BW. Novel mechanisms and devices to enable successful transdermal drug delivery. Eur J Pharm Sci. 2001; 14: 101-114.
- Jepps OG, Dancik Y, Anissimov YG, Roberts MS. Modeling the human skin barrier--towards a better understanding of dermal absorption. Adv Drug Deliv Rev. 2013; 65: 152-168.
- Benson HAE. Transfersomes for transdermal drug delivery. Expert Opin Drug Deliv. 2006; 3: 727-737.
- National Institute of General Medical Sciences(https://www.nigms.nih.gov/Pages/default.aspx).
- Solanas G, Benitah SA. Regenerating the skin: a task for the heterogeneous stem cell pool and surrounding niche. Nat Rev Mol Cell Biol. 2013; 14: 737-748.
- Honeywell-Nguyen PL, Bouwstra JA. Vesicles as a tool for transdermal and dermal delivery. Drug Discov Today Technol. 2005; 2: 67-74.
- Verma DD, Verma S, Blume G, Fahr A. Particle size of liposomes influences dermal delivery of substances into skin. Int J Pharm. 2003; 258: 141-151.
- Delgado-Charro MB, Guy RH. Transdermal Drug Delivery. Hillery AM, Lloyd AW, Swarbrick J, editors. In: Drug Delivery and Targeting for Pharmacists and Pharmaceutical Scientists. Taylor & Francis. 2001; 208-236.
- Elsayed MM, Abdallah OY, Naggar VF, Khalafallah NM. Lipid vesicles for skin delivery of drugs: reviewing three decades of research. Int J Pharm. 2007; 332: 1-16.
- Sinico C, Fadda AM. Vesicular carriers for dermal drug delivery. Expert Opin Drug Deliv. 2009; 6: 813-825.
- El Maghraby GM, Barry BW, Williams AC. Liposomes and skin: from drug delivery to model membranes. Eur J Pharm Sci. 2008; 34: 203-222.
- El Maghraby GM, Williams AC. Vesicular systems for delivering conventional small organic molecules and larger macromolecules to and through human skin. Expert Opin Drug Deliv. 2009; 6: 149-163.
- Romero EL, Morilla MJ. Highly deformable and highly fluid vesicles as potential drug delivery systems: theoretical and practical considerations. Int J Nanomedicine. 2013; 8: 3171-3186.
- Torchilin VP. Recent advances with liposomes as pharmaceutical carriers. Nat Rev Drug Discov. 2005; 4: 145-160.
- Mezei M, Gulasekharam V. Liposomes--a selective drug delivery system for the topical route of administration. Lotion dosage form. Life Sci. 1980; 26: 1473-1477.
- Wohlrab W, Lasch J. Penetration kinetics of liposomal hydrocortisone in human skin. Dermatologica. 1987; 174: 18-22.
- Wohlrab W, Lasch J. The effect of liposomal incorporation of topically applied hydrocortisone on its serum concentration and urinary excretion. Dermatol Monatsschr. 1989; 175: 348-352.
- Gesztes A, Mezei M. Topical anesthesia of the skin by liposome-encapsulated tetracaine. Anesth Analg. 1988; 67: 1079-1081.
- Foldvari M, Gesztes A, Mezei M. Dermal drug delivery by liposome encapsulation: clinical and electron microscopic studies. J Microencapsul. 1990; 7: 479-489.
- de Leeuw J, de Vijlder HC, Bjerring P, Neumann HA. Liposomes in dermatology today. J Eur Acad Dermatol Venereol. 2009; 23: 505-516.
- Artmann C, Roding J, Ghyczy M. Influence of various liposome preparations on skin humidity. Parfum Kosm. 1990; 90: 326.
- Schäfer-Korting M, Korting HC, Ponce-Pöschl E. Liposomal tretinoin for uncomplicated acne vulgaris. Clin Investig. 1994; 72: 1086-1091.
- Patel VB, Misra A, Marfatia YS. Topical liposomal gel of tretinoin for the treatment of acne: research and clinical implications. Pharm Dev Technol. 2000; 5: 455-464.
- Christiansen K, Bjerring P, Troilius A. 5-ALA for photodynamic photorejuvenation-optimization of treatment regime based on normal-skin fluorescence measurements. Lasers Surg Med. 2007; 39: 302-310.
- Naeff R. Feasibility of topical liposome drugs produced on an industrial scale. Adv Drug Deliv Rev. 1996; 18: 343-347.
- Agarwal R, Katare OP. Preparation and in vitro evaluation of miconazole nitrate loaded topical liposomes. Pharm Technol. 2002; 26: 48-60.
- Schwarz JC, Kählig H, Matsko NB, Kratzel M, Husa M, Valenta C. Decrease of liposomal size and retarding effect on fluconazole skin permeation by lysine derivatives. J Pharm Sci. 2011; 100: 2911-2919.
- Škalko N, Čajkovac M, Jalšenjak I. Liposomes with clindamycin hydrochloride in the therapy of acne vulgaris. Int J Pharm. 1992; 85: 97-101.
- Honzak L, Šentjurc M. Development of liposome encapsulated clindamycin for treatment of acne vulgaris. Pflugers Arch. 2000; 440: R44-45.
- Yang D, Pornpattananangkul D, Nakatsuji T, Chan M, Carson D, Huang CM, et al. The antimicrobial activity of liposomal lauric acids against Propionibacterium acnes. Biomaterials. 2009; 30: 6035-6040.
- Patel VB, Misra AN, Marfatia YS. Preparation and comparative clinical evaluation of liposomal gel of benzoyl peroxide for acne. Drug Dev Ind Pharm. 2001; 27: 863-869.
- Sand M, Bechara FG, Sand D, Altmeyer P, Hoffmann K. A randomized, controlled, double-blind study evaluating melanin-encapsulated liposomes as a chromophore for laser hair removal of blond, white, and gray hair. Ann Plast Surg. 2007; 58: 551-554.
- Krueger G, Ellis CN. Psoriasis--recent advances in understanding its pathogenesis and treatment. J Am Acad Dermatol. 2005; 53: S94-100.
- Körbel JN, Sebök B, Kerényi M, Mahrle G. Enhancement of the antiparakeratotic potency of calcitriol and tacalcitol in liposomal preparations in the mouse tail test. Skin Pharmacol Appl Skin Physiol. 2001; 14: 291-295.
- Agarwal R, Katare OP, Vyas SP. Preparation and in vitro evaluation of liposomal/niosomal delivery systems for antipsoriatic drug dithranol. Int J Pharm. 2001; 228: 43-52.
- Škalko N, Čajkovac M, Jalšenjak I. Liposomes with metronidazole for topical use: The choice of preparation method and vehicle. J Liposome Res. 1998; 8: 283-293.
- Fresta M, Puglisi G. Corticosteroid dermal delivery with skin-lipid liposomes. J Control Release 1997; 44: 141-151.
- Mezei M. Biodisposition of liposome-encapsulted active ingredients applied on the skin. Braun-Falco O, Korting HC, Howard I, Maibach MD, editors. In: Liposome Dermatics. Springer-Verlag. 1992; 206-214.
- Touitou E, Dayan N, Bergelson L, Godin B, Eliaz M. Ethosomes - novel vesicular carriers for enhanced delivery: characterization and skin penetration properties. J Control Release. 2000; 65: 403-418.
- Cevc G, Blume G. Lipid vesicles penetrate into intact skin owing to the transdermal osmotic gradients and hydration force. Biochim Biophys Acta. 1992; 1104: 226-232.
- Cevc G, Gebauer D, Stieber J, Schatzlein A, Blume G. Ultraflexible vesicles, Transfersomes, have an extremely low pore penetration resistance and transport therapeutic amounts of insulin across the intact mammalian skin. Biochim Biophys Acta. 1998; 1368: 201-215.
- Cevc G, Schätzlein A, Richardsen H. Ultradeformable lipid vesicles can penetrate the skin and other semi-permeable barriers unfragmented. Evidence from double label CLSM experiments and direct size measurements. Biochim Biophys Acta. 2002; 1564: 21-30.
- Honeywell-Nguyen PL, Wouter Groenink HW, de Graaff AM, Bouwstra JA. The in vivo transport of elastic vesicles into human skin: effects of occlusion, volume and duration of application. J Control Release. 2003; 90: 243-255.
- Gillet A, Lecomte F, Hubert P, Ducat E, Evrard B, Piel G. Skin penetration behaviour of liposomes as a function of their composition. Eur J Pharm Biopharm. 2011; 79: 43-53.
- El Maghraby GM, Williams AC, Barry BW. Skin delivery of 5-fluorouracil from ultradeformable and standard liposomes in-vitro. J Pharm Pharmacol. 2001; 53: 1069-1077.
- El Maghraby GM, Williams AC, Barry BW. Can drug-bearing liposomes penetrate intact skin? J Pharm Pharmacol. 2006; 58: 415-429.
- Trotta M, Peira E, Debernardi F, Gallarate M. Elastic liposomes for skin delivery of dipotassium glycyrrhizinate. Int J Pharm. 2002; 241: 319-327.
- Trotta M, Peira E, Carlotti ME, Gallarate M. Deformable liposomes for dermal administration of methotrexate. Int J Pharm. 2004; 270: 119-125.
- Cadena PG, Pereira MA, Cordeiro RB, Cavalcanti IM, Barros Neto B, Pimentel Mdo C, et al. Nanoencapsulation of quercetin and resveratrol into elastic liposomes. Biochim Biophys Acta. 2013; 1828: 309-316.
- Pandit J, Garg M, Jain NK. Miconazole nitrate bearing ultraflexible liposomes for the treatment of fungal infection. J Liposome Res. 2014; 24: 163-169.
- Chen J, Lu WL, Gu W, Lu SS, Chen ZP, Cai BC. Skin permeation behavior of elastic liposomes: role of formulation ingredients. Expert Opin Drug Deliv. 2013; 10: 845-856.
- González-Rodríguez ML, Rabasco AM. Charged liposomes as carriers to enhance the permeation through the skin. Expert Opin Drug Deliv. 2011; 8: 857-871.
- Elsayed MM, Abdallah OY, Naggar VF, Khalafallah NM. Deformable liposomes and ethosomes: mechanism of enhanced skin delivery. Int J Pharm. 2006; 322: 60-66.
- Jain S, Jain P, Umamaheshwari RB, Jain NK. Transfersomes--a novel vesicular carrier for enhanced transdermal delivery: development, characterization, and performance evaluation. Drug Dev Ind Pharm. 2003; 29: 1013-1026.
- Vanić Ž, Hafner A, Bego M, Škalko-Basnet N. Characterization of various deformable liposomes with metronidazole. Drug Dev Ind Pharm. 2013; 39: 481-488.
- Vanić Ž, Hurler J, Ferderber K, Golja Gašparović P, Škalko-Basnet N, Filipović-Grčić J. et al. Novel vaginal drug delivery system: deformable propylene glycol liposomes-in-hydrogel. J Liposome Res. 2014; 24: 27-36.
- Palac Z, Engesland A, Flaten GE, Škalko-Basnet N, Filipović-Grčić J, Vanić ž. Liposomes for (trans)dermal drug delivery: the skin-PVPA as a novel in vitro stratum corneum model in formulation development. J Liposome Res. 2014; 24: 313-322.
- Cevc G. Rational design of new product candidates: the next generation of highly deformable bilayer vesicles for noninvasive, targeted therapy. J Control Release. 2012; 160: 135-146.
- Horwitz E, Pisanty S, Czerninski R, Helser M, Eliav E, Touitou E. A clinical evaluation of a novel liposomal carrier for acyclovir in the topical treatment of recurrent herpes labialis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999; 87: 700-705.
- Esposito E, Menegatti E, Cortesi R. Ethosomes and liposomes as topical vehicles for azelaic acid: a preformulation study. J Cosmet Sci. 2004; 55: 253-264.
- Verma DD, Fahr A. Synergistic penetration enhancement effect of ethanol and phospholipids on the topical delivery of cyclosporin A. J Control Release. 2004; 97: 55-66.
- Dubey V, Mishra D, Dutta T, Nahar M, Saraf DK, Jain NK. Dermal and transdermal delivery of an anti-psoriatic agent via ethanolic liposomes. J Control Release. 2007; 123: 148-154.
- Meidan VM, Touitou E. Treatments for androgenetic alopecia and alopecia areata: current options and future prospects. Drugs. 2001; 61: 53-69.
- Godin B, Touitou E, Rubinstein E, Athamna A, Athamna M. A new approach for treatment of deep skin infections by an ethosomal antibiotic preparation: an in vivo study. J Antimicrob Chemother. 2005; 55: 989-994.
- Godin B, Touitou E. Mechanism of bacitracin permeation enhancement through the skin and cellular membranes from an ethosomal carrier. J Control Release. 2004; 94: 365-379.
- Bragagni M, Mennini N, Maestrelli F, Cirri M, Mura P. Comparative study of liposomes, transfersomes and ethosomes as carriers for improving topical delivery of celecoxib. Drug Deliv. 2012; 19: 354-361.
- Fang YP, Huang YB, Wu PC, Tsai YH. Topical delivery of 5-aminolevulinic acid-encapsulated ethosomes in a hyperproliferative skin animal model using the CLSM technique to evaluate the penetration behavior. Eur J Pharm Biopharm. 2009; 73: 391-398.
- Fang YP, Tsai YH, Wu PC, Huang YB. Comparison of 5-aminolevulinic acid-encapsulated liposome versus ethosome for skin delivery for photodynamic therapy. Int J Pharm. 2008; 356: 144-152.
- Shen LN, Zhang YT, Wang Q, Xu L, Feng NP. Enhanced in vitro and in vivo skin deposition of apigenin delivered using ethosomes. Int J Pharm. 2014; 460: 280-288.
- Zhang YT, Shen LN, Zhao JH, Feng NP. Evaluation of psoralen ethosomes for topical delivery in rats by using in vivo microdialysis. Int J Nanomedicine. 2014; 9: 669-678.
- Elsayed MM, Abdallah OY, Naggar VF, Khalafallah NM. PG-liposomes: novel lipid vesicles for skin delivery of drugs. J Pharm Pharmacol. 2007; 59: 1447-1450.
- Elmoslemany RM, Abdallah OY, El-Khordagui LK, Khalafallah NM. Propylene glycol liposomes as a topical delivery system for miconazole nitrate: comparison with conventional liposomes. AAPS PharmSciTech. 2012; 13: 723-731.
- Dragicevic-Curic N, Scheglmann D, Albrecht V, Fahr A. Temoporfin-loaded invasomes: development, characterization and in vitro skin penetration studies. J Control Release. 2008; 127: 59-69.
- Dragicevic-Curic N, Scheglmann D, Albrecht V, Fahr A. Development of different temoporfin-loaded invasomes-novel nanocarriers of temoporfin: characterization, stability and in vitro skin penetration studies. Colloid surface B. 2009; 70: 198-206.
- Yarosh D, Bucana C, Cox P, Alas L, Kibitel J, Kripke M. Localization of liposomes containing a DNA repair enzyme in murine skin. J Invest Dermatol. 1994; 103: 461-468.
- Yarosh DB. Liposomes in investigative dermatology. Photodermatol Photoimmunol Photomed. 2001; 17: 203-212.
- Vanić ž, Barnert SR, Schubert R. Fusogenic activity of PEGylated pH-sensitive liposomes. J Liposome Res. 2012; 22: 148-157.
- Peschka-Süss R, Škalko-Basnet N. The association of plain and ligand-bearing neutral and pH-sensitive liposomes with various cells. J Liposome Res. 2000; 10: 43-59.
- Wolf P, Maier H, Mullegger RR, Chadwick CA, Hofmann-Wellenhof R, Soyer HP, et al. Topical treatment with liposomes containing T4 endonuclease V protects human skin in vivo from ultraviolet-induced upregulation of interleukin-10 and tumor necrosis factor-alpha. J Invest Dermatol. 2000; 114: 149-156.
- Yarosh D, Klein J, O'Connor A, Hawk J, Rafal E, Wolf P. Effect of topically applied T4 endonuclease V in liposomes on skin cancer in xeroderma pigmentosum: a randomised study. Xeroderma Pigmentosum Study Group. Lancet. 2001; 357: 926-929.
- Chang HI, Yeh MK. Clinical development of liposome-based drugs: formulation, characterization, and therapeutic efficacy. Int J Nanomedicine. 2012; 7: 49-60.
- Hurler J, Sørensen KK, Fallarero A, Vuorela P, Škalko-Basnet N. Liposomes-in-hydrogel delivery system with mupirocin: In vitro antibiofilm studies and in vivo evaluation in mice burn model. BioMed Research International Volume 2013, Article ID 498485, 8 pages.
- Cevc G, Blume G. Biological activity and characteristics of triamcinolone-acetonide formulated with the self-regulating drug carriers, Transfersomes. Biochim Biophys Acta. 2003; 1614: 156-164.
- Cevc G, Blume G. Hydrocortisone and dexamethasone in very deformable drug carriers have increased biological potency, prolonged effect, and reduced therapeutic dosage. Biochim Biophys Acta. 2004; 1663: 61-73.
- Verma DD, Verma S, McElwee KJ, Freyschmidt-Paul P, Hoffman R, Fahr A. Treatment of alopecia areata in the DEBR model using Cyclosporin A lipid vesicles. Eur J Dermatol. 2004; 14: 332-338.
- de Leeuw J, van der Beek N, Maierhofer G, Neugebauer WD. A case study to evaluate the treatment of vitiligo with khellin encapsulated in L-phenylalanin stabilized phosphatidylcholine liposomes in combination with ultraviolet light therapy. Eur J Dermatol. 2003; 13: 474-477.
- Sudkahar CK, Upadkhyay N, Jain S, Charyulu RN. Ethosomes as non-invasive loom in transdermal drug delivery systems. Sebastian M, Ninan N, Haghi AK, editors. In: Nanomedicine and Drug Delivery. Apple Academic Press Inc. 2013; 1-17.
- Godin B, Touitou E. Erythromycin ethosomal systems: physicochemical characterization and enhanced antibacterial activity. Curr Drug Deliv. 2005; 2: 269-275.
- Zhang JP, Wei YH, Zhou Y, Li YQ, Wu XA. Ethosomes, binary ethosomes and transfersomes of terbinafine hydrochloride: a comparative study. Arch Pharm Res. 2012; 35: 109-117.
- Planas ME, Gonzalez P, Rodriguez L, Sanchez S, Cevc G. Noninvasive percutaneous induction of topical analgesia by a new type of drug carrier, and prolongation of local pain insensitivity by anesthetic liposomes. Anesth Analg. 1992; 75: 615-621.