
Special Article - Antisense Drug Research and Development
J Drug Discov Develop and Deliv. 2016; 3(1): 1021.
Functionalizing Morpholino Oligos for Antisense Drug Research and Development
Li Yong-Fu*
Gene Tools, LLC, USA
*Corresponding author: Li Yong-Fu, Gene Tools, LLC, 1001 Summerton Way, Philomath, OR97370, USA
Received: January 28, 2016; Accepted: April 27, 2016; Published: May 02, 2016
Abstract
A Morpholino oligo itself is difficult to physically detect in a biological assay system, and is also poorly cell-permeable in cultures and living animals. This article provides an overview of the state of the art for solving these problems using corresponding functionalizations. Modification at the ends of a Morpholino oligo gives functional groups or functional entities; the former are useful for further conjugation with custom-designed molecules, whereas the latter may provide fluorophores for optical detection or delivery-enabling moieties for in vivo antisense activity studies. The combination of both ends, in particular with double functionalization at the 3’-end, yields myriad opportunities for more diverse applications. In addition, a photo-cleavable linker inserted into a Morpholino oligo enables control of gene expression in a spatially and temporally controllable manner.
Keywords: Morpholino oligo; Functionalization; End-modification; Conjugation
Abbreviations
RNA: Ribonucleic Acid; mRNA: messenger RNA; siRNA: small interfering RNA; PNA: Peptide Nucleic Acid; S-DNA: Phosphorothioate-Linked Deoxyribonucleic Acid; LNA: Locked Nucleic Acid; UV: Ultraviolet
Introduction
Drug discovery is arriving at an advanced stage where drug design and development is becoming simpler, made possible through antisense molecules. This advancement arises from the small number of bases of oligonucleotides involved in the hydrogen-bonded bridges of Watson-Crick base pairing, giving predictable interactions with their complementary strands of nucleic acids. As advances in oligo structure have improved their efficacy, specificity and non-toxicity, antisense technology has thus entered into drug research and development as drug candidates themselves. Antisense oligomers can bind and deactivate defective mRNAs when aberrant proteins are made in the body, or usefully alter the mRNAs to treat the genetic diseases when a required protein cannot be produced [1].
Morpholino oligos are among those antisense oligomers including siRNA, PNA, S-DNA, and LNA, but stand out as superior [2] for several reasons: (1) they are free of off-target effects because their uncharged backbone does not interact electrostatically with proteins; (2) each is specific for a particular nucleic acid sequence because the oligo must bind about 14 contiguous bases or more to significantly block a gene transcript, and (3) they are completely stable in biological systems because they are resistant to cleavage by nucleases [3]. However, like other types of antisense oligomers, Morpholino oligos themselves face two practical problems: (1) they are invisible by optical microscopy in biological assay systems, and (2) they cannot easily pass through the cell membrane to reach their intracellular targets.
The power and advantages of assessing intracellular processes at their most fundamental level has propelled the science of Morpholinos into bioconjugate chemistry where particular chemical groups are required to be created to effect coupling, to be modified to realize sensitive detection, or to be functionalized to get delivery in cell culture and animal studies. The success of conjugation schemes depends on the presence of the correct chemical groups. Every chemical modification or conjugation process involves the reaction of one functional group with another, resulting in the formation of a covalent bond. The creation of bioconjugate reagents with spontaneously reactive or selectively reactive functional groups forms the basis for simple and reproducible cross-linking of target molecules. Of the hundreds of reagent systems described in the literature or offered commercially, the most common chemical bond formations can be reduced to a couple dozen or so primary reactions. In this article, amide, carbamate, and triazine linkage are the most preferred derivatization strategies to functionalize Morpholino oligos. Primary and secondary amino groups and Click coupling components (alkyne and azide) are the most preferred functional groups for further conjugation.
This article is designed to provide a general overview of what is available for functionalizing Morpholino oligos including the reactive group installation, fluorophore attachment, cell-permeable moiety conjugation, and photo-switch assembly, all of which are illustrated with short descriptions of their properties and use along with a visual representation of the chemistry of bond formation. This is not meant to be an exhaustive discussion on the theory or mechanism behind each functionalization, nor is it a review of every application in which each functionalization has been used in chemistry or biology. On the last section of advanced modification, the main purpose is to open reader’s minds to what can be available and how to make the full use of the chemistry for developing multi-functional Morpholino oligos for unique or diverse applications.
Chemical Functionalization for Bioconjugation
Chemical attachment of a functional component to a Morpholino oligo forms the basis for constructing a reactive moiety. Unfortunately, the methods developed to cross-link other biological molecules such as proteins often do not apply to Morpholino oligos. The major reactive sites on proteins involve primary amines, sulfhydryls and others that are relatively easy to derivatize. Morpholino oligos contain none of these functional groups. There are particular sites that can be modified on the bases or the linkage between subunits to produce derivatives able to couple with a second molecule. However, modifications on these sites may impact the pairing of a Morpholino oligo with its complementary target sterically and/or electronically. Therefore, the ends of the Morpholino oligo have been the sites of choice for the introduction of functionalization.
5’-end OR 3’-end single modification
In the solid phase synthesis of Morpholino oligos, functionalization at the 5’-end of the oligo takes advantage of the tri-functional triazine moiety whereby installation of both the functional group and the site for oligo elongation can be accomplished. 3’-End modification uses the secondary amine of the last Morpholino subunit as a reactive site for functionalization.
Amino groups are probably the most versatile functional moiety for post-synthetic derivatization. They are reactive towards isothiocyanate, isocyanate, acylazide, activated ester, sulfonyl chloride, aldehyde, epoxide, carbonate, arylating agent, imidoester, and anhydride. Among those, the acylation of amine with activated ester is rapid and occurs in high yield to give a stable amide bond. The primary amine can be installed at the 5’-end (5A, Figure 1), or 3’-end (3A, Figure 2).
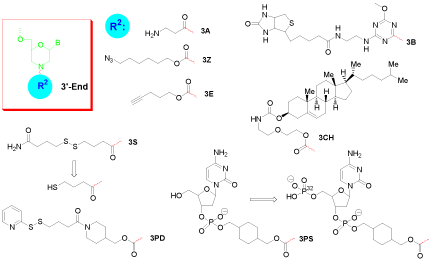
Figure 2: 3’-End single modification of morpholino oligo.
Click coupling is a class of biocompatible reactions intended to join substrates of choice with specific biomolecules. Among those, azide and alkyne cycloaddition is the most powerful method for bioconjugation [4]. With robust stabilities under the conditions of oligo synthesis, azides (5Z, Figure 1; 3Z, Figure 2) and alkynes (5E, Figure 1; 3E, Figure 2) can be installed at any end of a Morpholino oligo.
Biotin (or Vitamin H) is a small biologically active molecule, acting as a co-enzyme in living cells. With its highly specific affinity towards streptavidin, it is used in various biotechnology assays for quality and quantity testing. Biotinylated oligonucleotides can be used to attach specifically to streptavidin-enzyme conjugates, to streptavidin-protein conjugates, to streptavidin coated surfaces or to streptavidin-dye conjugates. Biotin can be coupled to a Morpholino oligo at the 3’-end (3B, Figure 2), and an additional accompanying amino group (3AB, Figure 3) provides a reactive site for further needed conjugation [5].
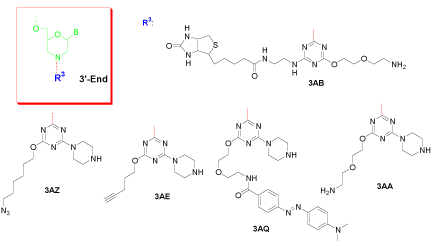
Figure 3: 3’-End double modification of morpholino oligo.
Cholesterol is a lipid molecule which is an essential structural component of all animal cell membranes that is required to maintain both membrane structural integrity and fluidity. Attachment of cholesterol (3CH, Figure 2) at the 3’-end of a Morpholino oligo may alter the lipophilicity of the conjugate and impact the interaction with the cell membrane.
The sulfhydryl group is a popular target in many modification strategies. It can react with haloacetyl and alkyl halide derivatives, maleimides, aziridines, acryloyl derivatives, and thiol-disulfide exchange reagents. The sulfhydryl group is provided in a protected form (disulfide amide, 3S, Figure 2) and needs to be generated freshly from reduction of disulfide before being used for further conjugation. In addition to its utility to couple thiol-reactive molecules to the Morpholino oligos, it can also be used for direct immobilization of oligos on solid supports (i.e. attachment to gold surfaces [6]).
Complementary to the sulfhydryl, a pyridyl dithio group (3PD, Figure 2) is the most popular type of thiol-disulfide exchange functional moiety used in the construction of a cross-linker. A pyridyl disulfide will readily undergo an interchange reaction with a free sulfhydryl to yield a single mixed disulfide product.
Conjugation of deoxycytidine 3’-phosphate with the 3’-end of a Morpholino oligo yields a 5’-hydroxyl group of the nucleotide (3PS, Figure 2), a substrate for phosphorylation by enzymatic reaction using terminal transferase [7]. This procedure is applicable for the 32P-labelling of Morpholino oligos.
3’-End double modification
Double modification at the 3’-end of a Morpholino oligo employs the controllable reactivity of cyanuric chloride. This tri-functional entity can accommodate two different functional groups with the third to form the covalent bond with the final Morpholino subunit. Figure 3 shows its utility. For example, 3AB contains a biotin on one side and a primary amine on the other side for further conjugation; On one side 3AZ contains an azide and 3AE contains an alkyne, useful for further Click reactions, while both have a secondary amine on the other side for whatever subsequent coupling is necessary; 3AQ installed Dabcyl together with a secondary amine for continued attachment; and 3AA provides two different but hard-to-distinguish amines for attaching two identical pieces to the 3’-end of a Morpholino oligo.
Visual Functionalization for Optical Detection
Fluorescent dyes are widely used in many applications, including as tracers for localization of biological structures by fluorescence microscopy, for quantification of analytes by fluorescence immunoassay, for flow cytometric analysis of cells, and for measurement of physiological states of cells. Their primary advantages over other types of absorption dyes include the orders of magnitude greater detectability of fluorescence emission over light absorption, the visibility of emission at a wavelength distinct from the excitation, the generally low level of fluorescence background in most biological samples, and the measurable intrinsic spectral properties of fluorescence polarization, life-time and excited state energy transfer.
Similar to most types of biomolecules, Morpholino oligos are difficult to be physically detected in a biological assay system and the fluorescent dye provides the method for detection and / or quantification of the interaction. With the most recent addition of Gene Tools Blue (3GB, Figure 4) to the commercially available Morpholino fluorescent labels (fluorescein and lissamine), the visible range has been covered from 400 nm to 600 nm emission wavelength. This range of available fluorescent conjugates allows the choice of a fluorescent dye which is significantly different from the background fluorescence, for example to avoid interference with autofluorescence or with green fluorescent protein. In addition, with a range of available fluorescent emissions, oligo conjugates can be quantified independently when several fluorescent conjugates are used simultaneously.
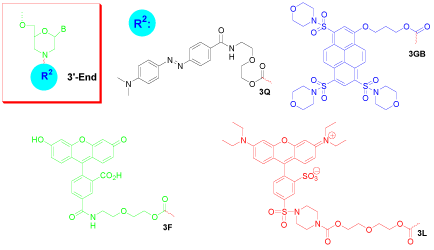
Figure 4: 3’-End fluorophore modification of morpholino oligo.
Dabcyl is a non-fluorescent dye predominantly used as a quencher for molecular beacons or probes. If Dabcyl is coupled to an oligonucleotide in close proximity to a fluorophore, it absorbs much of the emitted light of the fluorophore. Enlarging this distance (i.e. by melting of a beacon’s stem) results in an increased emission signal when the fluorophore is excited. The Dabcyl modification can be introduced to either the 5’-end (5Q, Figure 1) or 3’-end (3Q, Figure 4), and can be added with an additional accompanying amino group (3AQ, Figure 3) to provide a reactive site for further conjugation.
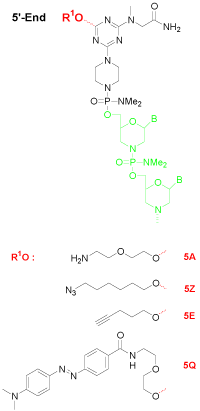
Figure 1: 5’-End modification of morpholino oligo.
Gene Tools Blue (3GB, Figure 4) is a new addition to the fluorophores for labeling Morpholino oligos or other biomolecules. The high polarity of this dye makes it useful for biological and physiological applications thanks to its good water solubility. This fluorophore has excitation maximum at 421 nm and emission maximum at 465 nm. The extinction coefficient of the molecule is about 31,000 M-1 cm-1. The dye has greater Stokes shift (44 nm) than other dyes with similar spectral properties. The molecular structure of the dye is non-ionic, facilitating its entry into cells for intracellular detection. The spectral properties of the fluorescent dye are sufficiently different in wavelengths and intensity from fluorescein that Gene Tools Blue is remarkably useful for avoiding interference from green fluorescent protein expression in biological systems or for permitting simultaneous use of several dyes for multi-labeling applications with minimum interference.
Fluorescein (3F, Figure 4) is perhaps the most popular of all fluorescent labeling agents. Fluorescein has an effective excitation wavelength of about 501 nm and its emission wavelength of about 524 nm. Although its flurorescent intensity fades when it is dissolved in buffers, exposed to light, or stored for extended periods, the fluorophore usually provides excellent detectability in assay systems, making it an important fluorophore for confocal laser-scanning microscopy and flow cytometry applications.
The Lissamine form of rhodamine B (3L, Figure 4) has a maximal absorptivity at 575 nm, and its emission maximum occurs at 593 nm, emitting red luminescence. It has been used in numerous applications, including multiple-labeling techniques in microscopy. Lissamine is relatively insoluble in water. The aqueous solubility of some of its Morpholino conjugates might be affected.
Cell-Permeable Functionalization for in vivo Biological Studies
Morpholinos provide all the desired properties of stability, nuclease resistance, high efficacy, long-term activity, water solubility, low toxicity and exquisite specificity. However, due to their high mass (usually 6,000 to 10,000 Daltons), Morpholinos lack the ability to permeate freely into mammalian cells, thereby precluding their therapeutic benefit from treatment of viral, bacterial or parasitic diseases, splice-modifying treatment for genetic defects, or treatment of a broad range of other currently intractable diseases.
To facilitate antisense drug research and development, Morpholino oligos require functionalization that can transport oligos into the cytosol/nuclear compartment of cells of living animals. A cellpenetrating peptide can be conjugated with a Morpholino oligo for in vivo studies [8,9]. A similar and commercially-available approach is to covalently conjugate a dendrimeric octa-guanidine moiety (Vivo- Porter) to the 3’-end of a Morpholino oligo to give a delivery-enabled Morpholino oligo (Vivo-Morpholino [10], Figure 5).
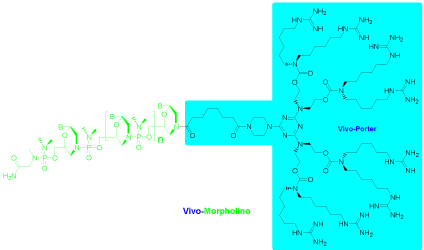
Figure 5: Vivo-Porter conjugate of morpholino oligo.
Outstanding results can be achieved systemically with intravenous (i. v.) injection of the Vivo-Morpholino, and modest systemic delivery achieved by intraperitoneal (i. p.) injection. Delivery to most major organs has been tested for quantifiable knockdown or exon-skipping in liver, small intestine, colon, muscle, lung and stomach tissues, and lesser but quantifiable delivery in the spleen, heart, skin and brain [11]. Efficient localized delivery has also been achieved by injecting a Vivo-Morpholino directly into the area of interest.
Photolytic Functionalization for Spatial- Temporal Control of Gene Activity
Although Morpholino oligos are commonly used antisense technology for gene regulation, their function has been limited by a lack of temporal and spatial control. Developmental biologists had a particular problem with the knockdown of embryonic lethal genes. For certain embryonic genes, suppressing the expression of a gene throughout embryonic development produces an early lethal phenotype, precluding analysis of the gene’s function later in development. To solve this problem, a photolytic subunit (Photo- Linker, Figure 6a), taking up roughly the same space in the molecule as an unmodified Morpholino subunit, has been incorporated near the middle of the oligo to yield a Photo-Morpholino [12]. The Photo- Morpholino can be cleaved by 365 nm UV light, generating shorter fragments that have little binding affinity to their target. Thus, two modes of action can be designed and implemented.
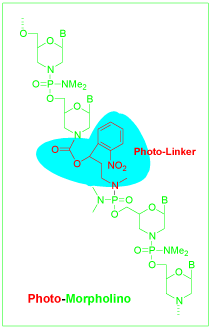
Figure 6a: Photo-morpholino structure.
(A) An antisense-Photo-Morpholino can be used to bind strongly to its RNA target and, at the desired time and place, can be cleaved into fragments with light to restore (an ON switch, Figure 6b) the normal activity of the RNA.
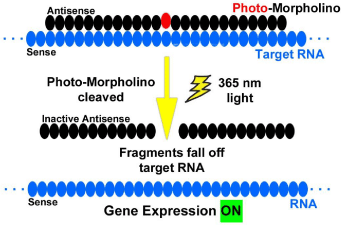
Figure 6b: Antisense photo-morpholino strategy.
(B) A sense-Photo-Morpholino can be bound to an unmodified antisense-Morpholino. The RNA target will maintain normal expression in the presence of the biologically-inactive Morpholino pair. By cleaving the bound sense-Photo-Morpholino with light, the unmodified antisense-Morpholino is released to bind to its RNA target (an OFF switch) and alter gene expression (Figure 6c).
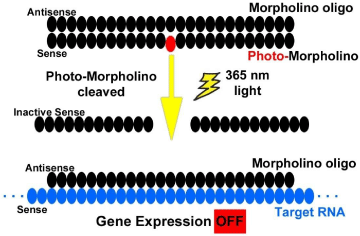
Figure 6c: Sense photo-morpholino strategy.
Advanced Functionalization for Multiple Modifications
5’-End AND 3’-end-single modification
The dual modifications at both 5’-end and 3’-end expand the scope of functionalization of a Morpholino oligo. The combination of 5’-end (entries 1-4, Table 1) with 3’-end (entries 5-12 and 18-22, Table 1) modifications gives in theory 52 possible double-functionalized entities, designated by combining entries in the Symbol column of the table.
Entry
Position
Symbol
Structure Location
Note
1
5’-end
5A
Figure 1
5’-Amine
2
5’-end
5Z
Figure 1
5’-Azide
3
5’-end
5E
Figure 1
5’-Alkyne
4
5’-end
5Q
Figure 1
5’-Dabcyl
5
3’-end
3A
Figure 2
3’-Amine
6
3’-end
3Z
Figure 2
3’-Azide
7
3’-end
3E
Figure 2
3’-Alkyne
8
3’-end
3B
Figure 2
3’-Biotin
9
3’-end
3CH
Figure 2
3’-Cholesterol
10
3’-end
3S
Figure 2
3’-disulfide amide (3’-Surfhydryl precursor)
11
3’-end
3PD
Figure 2
3’-Pyridyldithio
12
3’-end
3PS
Figure 2
3’-Phosphate substrate
13
3’-end
3AB
Figure 3
3’-Amine and biotin
14
3’-end
3AZ
Figure 3
3’-Amine and azide
15
3’-end
3AE
Figure 3
3’-Amine and alkyne
16
3’-end
3AQ
Figure 3
3’-Amine and dabcyl
17
3’-end
3AA
Figure 3
3’-Amine and amine
18
3’-end
3Q
Figure 4
3’-Dabcyl
19
3’-end
3GB
Figure 4
3’-Gene Tools blue
20
3’-end
3F
Figure 4
3’-Fluorescein
21
3’-end
3L
Figure 4
3’-Lissamine
22
3’-end
V
Figure 5
Vivo-Porter (for Vivo-Morpholino conjugate)
23
middle
P
Figure 6a
Photo-Linker (for Photo-Morpholino assembly)
24
3’-end
ODA
Figure 8
On-column diamine
Table 1: Functionalizing Moieties for Morpholino Oligos.
One particular example is the combination of 5’-Dabcyl (a quencher) and 3’-Gene Tools Blue (a fluorophore) to assemble a molecular beacon with a Morpholino oligo (Figure 7). The fluorophore is internally quenched in the hairpin shaped molecule, but its fluorescence is restored when it binds to a target nucleic acid sequence. This system can be used as a probe that can report the presence of specific nucleic acids in homogenous solutions.
Although a majority of the combinations are feasible to assemble, certain caution has to be exercised with regard to the compatibility of the processes. For example, 5’-amine modified Vivo-Morpholino cannot be obtained according to the current process, since the ammonolytic deprotection followed by the guanidinylation required during synthesis of the octa-guanidine dendrimer inevitably converts the 5’-end amine into a guanidine as well.
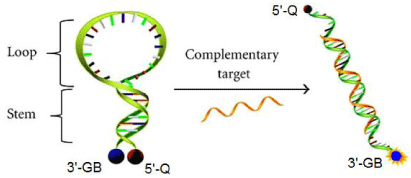
Figure 7: Molecular beacon probe of morpholino oligos.
5’-End AND 3’-end-double modification
The combination of both 5’-end and 3’-end-double modification yields a tri-functionalized Morpholino oligo. The combinations that can be designed are based on the combination of 5’-end (entries 1-4, Table 1) with 3’-end double modification (entries 13-17, Table 1), which in theory gives 20 triple functionalized entities. These are designated by combining the entries from the Symbols column of the table.
Advanced modifications
Advanced double modification at 3’-end requires a trifunctional cross-linker of the triazine chemistry, wherein the basic design strategy takes advantage of cyanuric chloride to build three different reactive groups in one molecule. Their reactivities can be manipulated to install two amino groups protected orthogonally (Figure 8 and entry 24, Table 1), and leave the third chloride to couple with the final subunit of a Morpholino oligo while still on the synthesis resin. The chemistry on the solid support allows the convenient assembly of a vast variety of combinations.
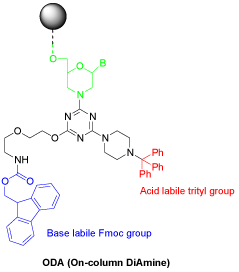
Figure 8: An orthoganally protected diamine for advanced 3’-end double
modification.
Removal of the Fmoc group under basic conditions generates the primary amine for further conjugation, whereas acidic detritylation exposes the secondary amine which is reactive for continued modification. This orthogonality of protecting groups facilitates the orchestration of assembling conjugates needed for multiple purposes. The conjugates to assemble are no longer limited to the moieties listed in Table 1. More special or custom-tailored conjugates can be made for more unique, more powerful or more diverse applications.
Advanced tri-functionalization of a Morpholino oligo is a yet more complex process where both ends of the Morpholino oligo are modified, with one reactive group at the 5’-end and two reactive groups at the 3’-end, a structure reminiscent of the three legged Isle of Man teapot in Great Britain (Figure 9).

Figure 12: Advanced triple modification of morpholino oligo (on the template of
British majolica three legged isle of man teapot).
The teapot (a Morpholino oligo) equipped with three legs is empowered to “walk” in multiple directions. This triple modification capability opens up the doors for designing a Morpholino oligo multi-functionalized with a variety of properties. For example, one can install a 5’-end azide for further Click reaction with a specialty conjugate, and 3’-end branched with a lissamine dye and a Vivo-Porter for the corresponding intracellular optical imaging and assay and in vivo biological gene regulation. On top of those, we may even insert a photolytic “chip” (the Photo-Linker, entry 23, Table 1) to make the teapot (the Morpholino oligo) fragile under UV light exposure, i.e. a Photo-Morpholino with additional triple end-functionalizations.
Chemically modified oligos have been immensely helpful in understanding mechanistic aspects of numerous biological interactions and processes. With the continuous effort to improve and innovate functionalizing techniques, Morpholino oligos will fulfill their promise for antisense applications in diagnostics and therapeutics.
Acknowledgement
The author is very grateful to Dr. James E. Summerton for his insightful suggestions, Dr. Jon D. Moulton for his critical proofreading of the manuscript, and Dr. Jeremy Bushman and Weiyi (Andrew) Li for their valuable discussions to improve the quality of paper.
References
- Li YF. To discover, develop and deliver a right drug: a showcase for antisense technology. J Drug Discov Develop and Deliv. 2014; 1: 3.
- Summerton JE. Morpholino, siRNA, and S-DNA compared: impact of structure and mechanism of action on off-target effects and sequence specificity. Curr Top Med Chem. 2007; 7: 651-660.
- Hudziak RM, Barofsky E, Barofsky DF, Weller DL, Huang SB, Weller DD. Resistance of morpholino phosphorodiamidate oligomers to enzymatic degradation. Antisense Nucleic Acid Drug Dev. 1996; 6: 267-272.
- Griepenburg JC, Rapp TL, Carroll PJ, Eberwine J, Dmochowski IJ. Ruthenium-Caged Antisense Morpholinos for Regulating Gene Expression in Zebrafish Embryos. Chem Sci. 2015; 6: 2342-2346.
- Dou SP, Virostko J, Greiner DL, Powers AC, Liu GZ. Quantitative Correlation of in Vivo Properties with in Vitro Assay Results: The in Vitro Binding of a Biotin-DNA Analogue Modifier with Streptavidin Predicts the in Vivo Avidin- Induced Clearability of the Analogue-Modified Antibody. Mol Pharmaceutics. 2015; 12: 3097-3103.
- Tercero N, Wang K, Gong P, Levicky R. Morpholino monolayers: preparation and label-free DNA analysis by surface hybridization. J Am Chem Soc. 2009; 131: 4953-4961.
- Kozlov IA, Nielsen PE, Orgel LE. A method for the 32P labeling of peptides or peptide nucleic acid oligomers. Bioconjug Chem. 1998; 9: 415-417.
- Amantana A, Moulton HM, Cate ML, Reddy MT, Whitehead T, Hassinger JN, et al. Pharmacokinetics, biodistribution, stability and toxicity of a cellpenetrating peptide-morpholino oligomer conjugate. Bioconjugate Chem. 2007; 18: 1325-1331.
- Betts C, Saleh AF, Arzumanov AA, Hammond SM, Godfrey C, Coursindel T, et al. Pip6-PMO, A New Generation of Peptide-Oligonucleotide Conjugates with Improved Cardiac Exon Skipping Activity for DMD Treatment. Molecular Therapy - Nucleic Acids. 2012; 1: e38.
- Li YF, Morcos PA. Design and synthesis of dendritic molecular transporter that achieves efficient in vivo delivery of morpholino antisense oligo. Bioconjug Chem. 2008; 19: 1464-1470.
- Morcos PA, Li Y, Jiang S. Vivo-Morpholinos: a non-peptide transporter delivers Morpholinos into a wide array of mouse tissues. Biotechniques. 2008; 45: 613-614, 616, 618 passim.
- Tallafuss A, Gibson D, Morcos P, Li Y, Seredick S, Eisen J, Washbourne P. Turning gene function ON and OFF using sense and antisense photomorpholinos in zebrafish. Development. 2012; 139: 1691-1699.