
Special Article - Antisense Drug Research and Development
J Drug Discov Develop and Deliv. 2016; 3(2): 1022.
Structure Activity Study of Clinically Observed Adverse Events and Oligomer Chemistry
Iversen PL*
Department of Environmental & Molecular Toxicology, Oregon State University, USA
*Corresponding author: Iversen PL, Department of Environmental & Molecular Toxicology, Oregon State University, Agricultural Life Sciences Bldg, Corvallis, Oregon 97331, USA
Received: January 28, 2016; Accepted: April 29, 2016; Published: May 03, 2016
Abstract
The number and diversity of new nucleic acid based therapeutics in clinical trial illustrates the remarkable flexibility of this approach to therapy. The use of synthetic oligomers has the advantage of control of dose and duration of action over gene therapy and Cas9/CRISPR approaches that are developed in parallel. Further, synthetic compounds may contain a variety of modifications to fine tune their therapeutic purpose and define their mechanism of action. The expansion in diversity of nucleic acid therapeutics may also reflect the approaches to barriers observed in previous clinical trials. The objective of this review is to provide new insight into the clinical adverse event data reported for nucleic acid based therapeutics in advanced development, with the goal of establishing a comprehensive framework for evaluating the current implications, and future direction, of therapeutic antisense technologies. A pattern of adverse events, some sufficiently severe to require discontinuation of treatment, include Flu-Like Symptoms (FLS), Injection Site Reactions (ISR), kidney abnormalities, elevated liver enzymes, and thrombocytopenia are frequently observed with oligomer chemistries that have negatively charged phosphate linkages and naturally occurring sugars. Anti-Drug Antibodies (ADA) have been reported in high percentages following chronic treatment with oligomer chemistries designed to enhance duration of tissue residence time. However, these common adverse events are not observed with Phosphorodiamidate Morpholino Oliogmers (PMO) containing morpholino sugars and no negative charge in the phosphate linkage.
Keywords: Antisense therapy; Maximum tolerated dose; Clinical trials; Oligonucleotide toxicity
Abbreviations
ADA: Anti-Drug Antibodies; PTT: Partial Thromboplastin Times; BCL2: B-Cell Leukemia-Lymphoma Gene 2; DAMPS: DNA Damage-Associated Molecular Patterns; DNA: Deoxyribonucleic Acid; FDA: Food and Drug Agency of the United States; FLS: Flu- Like Symptoms; G4: highly structured guanosine quartets; GRO: Guanosine Rich Oligomer; hsCRP: high sensitivity C-Reactive Protein; ISR: Injection Site Reactions; MTD: Maximum Tolerated Dose; NDA: New Drug Application; PMO: Phosphorodiamidate Morpholino Oliogmers; Poly I:C: Polyriboinosinic- Polyribocytidylic Acids; PSO: Phosphorothioate Oligonucleotides; PSO-2-OMe: Phosphorothioate 2’O-methyl RNA; PSO-2-MOE: Phosphorothioate 2’O-methoxyethyl RNA; RNA: Ribonucleic Acid; ROS: Reactive Oxygen Species; TLR: Toll-Like Receptor; TNFα: Tumor Necrosis Factor Alpha; ULN: Upper Limit of Normal
Introduction
Oligonucleotide drug development must be viewed through a lens, which recognizes that humans are well equipped to recognize both foreign and endogenous DNA/RNA in the circulation. This protective recognition arises either as part of an innate, microbial DNA immune response or in response to cellular danger signaled by DNA Damage-Associated Molecular Patterns (DAMPs) [1,2]. Now that synthetic analogues of DNA and RNA are being developed as therapeutics for both acute and chronic treatment regimens, it is critical that we clarify the extent to which these emerging therapeutics result in chronic or inappropriate activation of these nucleic-acid sensors.
Information available to guide optimal therapeutic oligomer chemistry has transitioned from what chemical structures can be made to properties in biological systems and then on to efficacy in animal models. Recently, advanced therapeutic development of oligonucleotides has provided a wealth of information from well controlled clinical trials. Human data are the most pertinent information as, in some cases, the preclinical efficacy reports are discordant with human efficacy observations such as the case with TKM-100802 which was highly effective in nonhuman primates [3] but its use in patients from the recent Ebola outbreak were not effective [4] and off-target effects including activation of inflammatory cytokines were notable outcomes [5]. In addition, detailed summaries are only recently publicly available for Ampligen, mipomersen, drisapersen, and eteplirsen which provide comprehensive evaluation of efficacy and safety from multiple human studies (Figure 1).
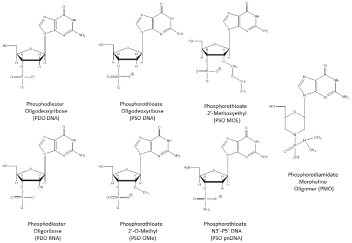
Figure 1: Oligomer Chemistry Structures.
Oligomer Chemistries Evaluated in Human Clinical Trials
Phosphodiester Deoxyribonucleotides (DNA)
Unmodified DNA is too unstable to be a useful oligonucleotide therapeutic with the exception of highly structured Guanosine quartets (G4). A completely unmodified deoxyribose oligomer with random sequence is degraded in 3-7 minutes in serum while T30175, a G4 DNA, is not fully degraded in five hours [6] and AS1411, a second G4 DNA oligomer, was not completely degraded in cell culture media in five days [7]. GRO29A (also AGRO100 and AS1411) is an unmodified Guanosine Rich Oligomer (GRO) 26-mer (5’-TTT GGT GGT GGT GGT TGT GGT GGT GGT GG-3’) that forms a stable G4 structure. Mice bearing A549, non-small cell lung cancer cells were administered 5, 10 and 40 mg/kg intravenous doses of AS1411 for five consecutive days and reduced tumor growth was observed at 10 and 40 mg/kg. No significant preclinical toxicity was observed with single IV doses of 100 mg/kg in rats and in dogs after 4 days of continuous infusion of 10 mg/kg/day [8]. GRO29A was discovered in 1997, nucleolin was identified as the target in 1998, in vivo efficacy studies were reported in 1999, hit to lead optimization leading to AS1411 in 2000, preclinical toxicology studies were completed by the end of 2002, phase I trials were conducted in 2003 and 2005, and phase II studies began in 2007 and 2008 [9]. A phase II study in 35 patients with renal cell carcinoma that had failed at least 1 tyrosine kinase inhibitor were administered AS1411 at 40 mg/kg/day by continuous intravenous infusion on days 1-4 of a 28 day cycle for two cycles [10]. AS1411 revealed limited activity in that 1/35 patients (2.9%) were observed with a durable response. Adverse events are summarized in (Table 1) included fatigue (22.9% grade 1, 5.7% grade 2) and anemia (8.6 grade 2, 2.9% grade 3). Abnormal laboratory findings included lymphocytes (65% grades 1-4), platelets (14.3% grade 1-2 only), creatinine (65.7% grade 1-2), AST (62.9% grade 1-2), gamma GT (40% grades 1-3), and ALT (20% grade 1-2). Advanced development of AS1411 for cancer is likely to be replaced by enhanced delivery strategies [11], conjugation with doxorubicin [12] or evaluation in HIV-1 patients [13].
Chemistry
Compound
Dose (mg/kg)
Route-Regimen
Common Adverse Events
Phosphodiester DNA
AS1411
40
i.v. infusion- Days 1-4
Fatigue 28.6%
Platelets 14.3%
Creatinine 65.7%
AST 62.9%
Phosphodiester RNA
Ampligen
~5.7
i.v. 30 minute infusion twice weekly
Flu-like symptoms 86%
Thrombocytopenia 3.1%
Creatinine 1.9%
SGPT 1.2%
Infusion reactions 31%
Phosphorothioate N3’-P5’
Imetelstat
9.4
i.v. 2 hour infusion
Days 1 and 8 q21 days
Flu-like symptoms
Thrombocytopenia 48%
Neutropenia 23%
AST 11.5%
Phosphorothioate DNA
Genesense
(Oblimersen)
3
i.v. 5 day continuous infusion
Fatigue 30%
Thrombocytopenia 17.5%
Creatinine 5%
ALT 5%
Infusion reactions 100%
Phosphorothioate 2’-O-methyl-RNA
Drisapersen
6
Subcutaneous injection
Fatigue
Thrombocytopenia 7%
Renal abnormalities 60.9%
Liver enzymes (AST) 2.5%
Injection site reactions 78.7%
Anti-drug antibodies (ADA) 29%
Phosphorothioate 2’-MOE-RNA
Kynamro
(Mipomersen)
3
Subcutaneous injection weekly
Flu-like symptoms 30%
Renal abnormalities 9%
Liver enzymes (ALT) 18.4%
Injection Site Reactions 87.4%
Anti-drug antibodies (ADA) 71%
Neoplasms 3.2%
Phosphorodiamidate Morpholino Oligomers
Eteplirsen
30
i.v. infusion weekly
Flu-like symptoms 0%
Renal abnormalities 0%
Liver enzymes 0%
Injection site reactions 0%
PMOplus
AVI-7288
12.5
i.v. infusion daily
Flu-like symptoms 0%
Renal abnormalities 0%
Liver enzymes 0%
Injection site reactions 0%
Table 1: Oligomer related adverse events noted in clinical trials.
Phosphodiester Ribonucleotides (RNA)
Polyriboinosinic-polyribocytidylic acids (Poly I: C) were recognized for their therapeutic potential to induce interferon in 1967 [14]. Optimization of this double-stranded RNA structure for interferon induction involved insertion of a mispairing uridine [15] in Poly (I): Poly (C12U) now called Ampligen. Thirteen healthy volunteers between 20 and 46 years old were administered 200 mg (80mL) and 600 mg (400 mL) by slow intravenous infusion. No immunological parameters were different from placebo at the 200 mg dose and only neopterin was different (p = 0.06) at the 600 mg dose [16]. High neopterin levels are associated with production of reactive oxygen species (ROS) and an active inflammatory response [17]. The AEs included fatigue (38% 5/13), flu-like symptoms (38% 5/13), and infusion reactions (30.8% 4/13). An Ampligen NDA was submitted to the FDA in 2007 and accepted for review in 2008 but in November of 2009 the Complete Response Letter did not approve the application. An NDA for studies with 400 mg dose administered twice weekly by 30 minute intravenous infusion was then submitted to the FDA in June of 2012 addressing concerns from the earlier review. The list of adverse events are summarized in (Table 1) included infusion reactions (31%), liver function abnormalities (primarily SGPT 3x ULN; 1.2%), leukopenia (1.9%), flu-like symptoms (86%), thrombocytopenia (3.1%), elevated creatinine (1.9%) and development of autoimmune disease (0.27%).
The concern that Ampligen might contribute to autoimmune disease characterized by immune responses to RNA has been uniquely addressed. Ampligen does not induce TNFα, a key cytokine observed in autoimmune responses, in contrast to poly IC and related RNA compounds [18] which appears to be due to a specific lack of interaction with cytoplasmic MDA5 [19]. Ampligen binding TRL-3 leads to signal transduction through TRIF and not MyD88 as do other RNA sensors which lead to production of TNFα [20]. Remarkable differences between species in the maximum tolerated doses of Ampligen (rintatolimod) have been observed with rabbits MTD of 1.25 mg/kg, dog and rat MTD of 12.5 mg/kg, and cynomologus monkey MTD of 100 mg/kg [21]. The MTD for an unrelated RNA oligomer therapeutic was 1.25 indicated in (Table 2) (Figure 2) [22].
Chemistry
Sequence 5’-3’
MTD (mg/kg)
Reference
PDO-RNA
ALN-VSP
Ampligen
PolyI:polyC12U
1.25
5.7
Tabernero et al., 2013
Ampligen briefing doc. 2012
PSO-DNA
Oblimersen
ISIS 3521
ISIS 5132
GTI-2040
MG98
d(TCT CCC AGC GTG CGC CAT)
d(GTT CTC GCT GGT GAG TTT CA)
d(TCC CGC CTG TGA CAT GCA TT)
d(GGC TAA ATC GCT CCA CCA AG)
d(TCT ATT TGA GTC TGC CAT TT)
3
6
5
5
4.9
Genesense Briefing Doc 2006
Villalona-Calero et al., 2004
Tolcher et al., 2002
Leighl et al., 2009
Plummer et al., 2009
PSO-2’OMe
ISIS 14803
AEG35156
Drisapersen
GTG CTC ATG GTG CAC GGT CT
GC TGA GTC TCC ATA TTG CC
UCA AGG AAG AUG GCA UUU CU
6
3
6
McHutchison et al., 2006
Schimmer et al., 2009
Goemans et al., 2011
PSO-2’MOE
ISIS 183750
OGX-427
Mipomersen
ISIS 104838
TGTCA TAT TCC TGG A TCCTT
GGG ACG CGG CGC TCG GTC AT
GCCUC AGT CTG CmTT Cm GCACC
GCmTGATTTAGAGAGAGGTCmCmCm
14
9.1
2.8
6
Hong et al., 2011
Kamada et al., 2007
Mipomersen briefing doc 2012
Sewell et al., 2002
PSO-N3’-P5’
Imetelstat
TAG GGT TAG ACA A
9.4
Thompson et al., 2013
PMO
AVI-4126
AVI-4557
Eteplirsen
ACGTTGAGGGGCATCGTCGC
CTGGGATGAGAGCCATCACT
CTTACAGGCTCCAATAGTGGTCAGT
>1.5
>7.5
>50
Iversen et al., 2003
Iversen et al., in preparation
Mendell et al., 2013
PMOplus
AVI-7100
AVI-7537
AVI-7288
CGG T+TA GAA GAC +TCA +TCT TT
GCC +ATG GT+T TT+T TC+T C+AG G
GAATATTAAC+AI+AC+TGAC +A+AGTC
>12
>12,5
>12.5
In preparation
Heald et al., 2014
Heald et al., 2015
Table 2: Oligomer maximum tolerated dose summary.
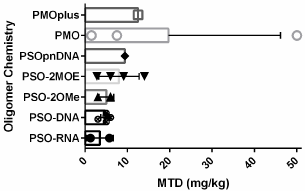
Figure 2: Maximum Tolerated Dose. Data from multiple oligomers with
similar chemistries but unrelated sequences are provided as individual points.
Bars represent the mean of the chemistry group and the error bars indicate
the standard deviation for each oligomer chemistry group.
N3’-P5’ thio-phosphoramidate oligonucleotides (pn DNA)
The N3’→P5’ oligomer chemistry was initially reported as telomerase inhibitors capable of inducing premature cell senescence in HME50-5E cells at 500 nM [23] and then refined as allosteric inhibitors of hTR (GRN137159) with an IC50 of 0.5 nM [24]. A development candidate, GRN163 (5’-TAGGGTTAGACAA-3’), with IC50 of 0.045 nM was identified that not only inhibited telomerase but induced tumor cell apoptosis [25]. Addition of a palmate lipid to the 5’-end of GRN163 (GRN163L) led to 7-fold greater potency in several cell lines [26]. In vivo activity in an A549 lung cancer mouse model was demonstrated with 5 to 15 mg/kg GRN163L administered intraperitoneally every 3 days for 3 weeks [27]. A phase I clinical trial revealed partial responses in osteosarcoma and Ewing sarcoma accompanied by dose limiting thrombocytopenia and neutropenia at doses of 7.7 to 9.4 mg/kg administered intravenously [28]. Further observations in a phase II study in patients with non-small-celllung cancer at 9.4 mg/kg by intravenous infusion on days 1 and 8 of a 21-day cycle revealed thrombocytopenia and neutropenia but no significant improvement in progression free survival or overall survival [29].
Phosphorothioate Deoxyribonucleotides (PSO)
BCL2 (B-cell leukemia-lymphoma gene 2) is linked to neoplasia by enhancing cell survival through inhibition of apoptosis. An 18 base phosphorothioate deoxyribonucleotide, G3139, targeting Bcl-2 was initially reported to induce apoptosis in a human leukemia cell line [30]. In vivo efficacy was demonstrated in human melanoma mouse model [31] and in a mouse lymphoma xenograft model using a 2-week infusion to produce complete tumor regression in 83 percent of the mice [32]. An NDA for Genesense plus dacarbazine in patients with advanced metastatic melanoma was submitted to the FDA in 2003 and reviewed in 2004 [33]. The ODAC did not recommend approval based on limitation of the 7-month patient follow-up leading to resubmission in 2005 with 24-month followup. A 5 to 7-day continuous infusion of 3-7 mg/kg/day in 40 patients in a phase I study and 26 patients at the MTD of 3 mg/kg/day in a phase II study led to fever (32.5%), fatigue (30%), hypotension (20%) and anemia, thrombocytopenia, and night sweats (17.5%). Catheter related events were observed in the pivotal studies which included 18 patients with pain, bruising, redness, 11 patients developed infections at the catheter site, and 2 patients were reported with thrombolytic events at the catheter. Other findings included elevated ALT (5%) and creatinine (5%). MTD for unrelated oligomers made of similar chemistry ranged from 3 to 6 mg/kg (Table 2) [34-37].
Phosphorothioate 2’-O-methyl-RNA (PSO-2-OMe)
Gene deletions in the X-linked dystrophin gene that disrupt the translation reading frame result in Duchenne Muscular Dystrophy. An NDA for Drisapersen (a 20 base 2’-O-methyl phosphorothiaote), was very recently submitted to the FDA for the induction of exon skipping of exon 51 of the dystrophin pre-mRNA. Preclinical efficacy of the 2’-O-methyl phosphorothioate RNA provided encouraging results in the mdx mouse model [38] (Heemskrek et al., 2010). A phase I/II study involved weekly abdominal subcutaneous injections of drisapersen (0.5, 2.0, 4.0, and 6.0 mg/kg dose levels) for 5 weeks in 12 patients revealed injection site reactions (75%), proteinuria (100%), but no thrombocytopenia or elevated liver enzymes [39]. A clinical safety database from 9 clinical trials from 326 patients with DMD, 312 patients that received at least 1 dose, and 302 patients that were treated in repeat dose studies of 6 mg/kg administered subcutaneously was summarized in the FDA briefing document [40]. Adverse events included thrombocytopenia (7%), renal abnormalities (60.9%), and injection site reactions (78.7%). Renal abnormalities included proteinuria, urine protein/creatinine ratio, and membranous glomerulonephritis associated with complement C1q accumulation. Less common AEs involved elevations in liver enzymes including gamma-GT (2.5%), AST (2.5%), and alopecia. Permanent discontinuation of treatment was observed in 4.5% of the repeat dose treated patients. Anti-Drisapersen Antibodies (ADA) was observed in 32 of 109 patients evaluated (29.4%) during 48 weeks of treatment. Five patients with severe thrombocytopenia were tested for anti-platelet antibodies, four were positive (80%) in = 1 time point. The MTD for oligomers with similar chemistry but unrelated sequences range from 3-6 mg/kg. (Table 2) [41,42].
Phosphorothioate 2’-methoxyethyl-RNA (PSO-MOE)
Mipomersen is a 20 base phosphorothioate 2’-methoxyethyl RNA 5-10-5 gapmer motif targeting expression of apoB-100 to lower LDL-C in individuals with Homozygous Familial Hypercholesterolemia (HoFH). Mipomersen was approved by the FDA but failed to gain approval in Europe (EMEA) because of the high discontinuation rate. Pooled phase III trials found 55% (77/141) discontinued treatment primarily due to adverse events [43]. Safety evaluations identified: (i) Injection Site Reactions (ISR) was reported in 87.4% (228/261) of mipomersen-treated individuals and in pooled phase 3 trials, 28% (13/47) discontinued because of ISR. (ii) Flu-Like Symptoms (FLS) were observed within 2 days of treatment and were reported by 30% of mipomersen-treated individuals with 15% discontinued because of FLS. (iii) Inflammatory and immunological issues which include high sensitivity C-Reactive Protein (hsCRP), complement activation (Bb and C5a), inflammatory markers (IL-1β, IL-6, IL-13, IFN-α, IFN-β, MCP-1, and MIP-1α. Anti-Mipomersen Antibodies (ADA) was observed in 71% (102/142) mipomersen-treated individuals evaluated. The appearance of antibodies increased from 4% at 13 weeks to 33% by week 50 and ultimately reached 71% thus a slow time to development of ADA. (iv) Renal issues including proteinuria was observed in 9% (23/256) mipomersen treated individuals. (v) Hepatic enzymes were increased including AST 15.6% (22/141) and ALT 18.4% (26/141) in mipomersen-treated individuals, peak elevation was observed after 26 weeks of treatment. (vi) Neoplasms were observed in preclinical carcinogenicity studies in mice in which significant increases in hepatocellular adenoma at 60 mg/ kg/week and in rats in which malignant fibrous histiocytoma and malignant fibrosarcoma at 10 mg/kg/week. There were neoplasms in 3.2% (24/749) mipomersen-treated individuals versus 0.9% (2/221) neoplasms in the placebo individuals. The MTD for oligomers with similar chemistry but unrelated sequences range from 6-14 mg/kg (Table 2) [44-46].
Efficacy was observed in the primary endpoint, decreases in LDL-C from baseline, of 21.4% (p<0.001) in the 34 patient ISIS 301012-CS5, 48.4% (p<0.001) in the 39 patient MIPO3500108, 33.2% (p<0.001) in the 83 patient ISIS 301012-CS7, and 32.4% (p<0.001) in the 105 patient ISIS 301012-CS12.
Phosphorodiamidate Morpholino Oligomers (PMO)
PMO chemistry and early clinical activity has been reviewed [47,48]. Initial clinical studies suggested PMOs were well tolerated [49]. Eteplirsen is currently under development by Sarepta Theraeputics for the treatment of DMD with genetic mutations correctable by skipping exon 51. Preclinical evaluation of eteplirsen at maximum feasible doses of 320 mg/kg once weekly for 12 weeks by either i.v. or s.c. administration in cynomolgus monkeys resulted in no drug-related effects [50,51]. In a phase II study in young boys with DMD and mutations correctable by skipping exon 51 etepliresen were given by weekly i.v. infusions of placebo, 30 or 50 mg/kg/wk eteplirsen for 24 weeks, and placebo group could switch to 30 or 50 mg/kg/wk etepliersen for 24 weeks of open-label treatment [52]. Muscle biopsies revealed 23% positive dystrophin fibers in the 30 mg/kg/wk group compared to zero in the placebo group at 24 weeks (p<0.002). Further, a 67.3 meter benefit was observed in ambulationevaluable treated patients compared to placebo patients (p<0.001). No severe adverse events were encountered, and no injection site reactions were observed.
AVI-7288 and AVI-7537 are under development at Sarepta for the treatment of Marburg and Ebola virus infections. The program involved integration of a limited number of modifications to the phosphorodiamidate backbone to create the PMO plus oligomer chemistry [53]. AVI-7288 targets the translation initiation site for the virally encoded NP protein, and daily i.v. administration of 15 mg/kg has produced 100% survival in a Marburg Musoke lethal challenge model in cynomolgus monkeys. Dose-escalation studies in healthy volunteers have been conducted for four PMOplus agents [54] and repeat dose studies have recently been reported for AVI-7288 [55].
Conclusion
The purpose in comparing therapeutic oligonucleotides composed of different chemistries is to identify chemical elements that may be responsible for adverse events in human clinical trials. Adverse events in common to multiple oligomer chemistries include Flu- Like Symptoms (FLS), thrombocytopenia, Injection Site Reactions (ISR), kidney abnormalities, and liver enzyme elevations. These adverse events are easily identified in clinical settings which provides for prudent therapeutic management including discontinuation of treatment. However, the time delay in Anti-Drug Antibodies (ADA) presents a greater challenge in interpretation of the medical significance of ADA and the as yet unidentified accompanying T-cell responses.
Actions on coagulation and platelets
Clinical observations of individuals treated with phosphorothioates reveal a prolongation in clotting time and thrombocytopenia. Nucleotide-based aptamer anticoagulants are well known independent of phosphorothioate chemistry [56-59]. Sequence-specific phosphorothioate anticoagulants have been described [60] and at least two 2’-O-methoxyethyl oligomers are in clinical trial. However, sequence non-specific effects indicate that as little as two phosphorothioate linkages are sufficient to significantly Prolong Partial Thromboplastin times (aPTT) [61]. The G-quartet appears to be a potent inhibitor of aPTT relative to monomeric phosphorothioate structures [62]. Phosphorothioates containing 2’-modifications retain anticoagulant properties but are perhaps less potent than unmodified sugars [63-65]. The biochemical mechanism for inhibition of coagulation involves binding to thrombin thus preventing fibrinogen binding and inhibition of the tenase complex prior to Factor Va involvement [66,67]. The anticoagulant properties of phosphorothioates may exclude their therapeutic use for hemorrhagic viral infections and was a key dose-limiting toxicity for Imetelstat [28]. Neither PMO nor PSO-MOE chemistries are associated with prolongation in clotting time, thrombocytopenia, or complement activation directing attention to the role of the heavily modified sugars in evading these adverse events (Figure 1). PMO do not interfere with PT or aPTT (Figure 3) suggesting limited interaction with thrombin or the tenase complex.
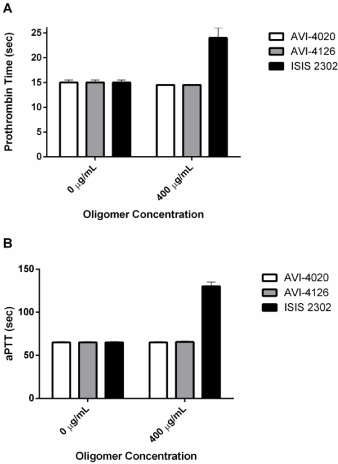
Figure 3: Oligomer Induced Coagulopathy. Human blood coagulation times
were measured in the presence (400 ug/mL) or absence (0 ug/mL) of three
oligomers, 2 PMOs and 1 PSO. Panel A indicates partial thrombin time in
seconds and panel B indicates partial thrombin time in seconds.
Immune stimulation
The CpG motif is highly effective in production of hybridization- independent immune responses but virtually all phosphorothioate oligonucleotides are active [68,69]. The immune stimulation is the result of direct cellular activation leading to hyperplasia in the spleen and lymph nodes accompanied by production of IL-6, IL- 12, and interferon γ [70]. Oligomer induced immune responses are complex as a C-rich oligomer, MT01- ACCCCCTCT, can prevent CpG immune stimulation [71]. The nucleic acid induced immune response in humans involves but is not limited to fatigue, chills, and flu-like symptoms as has been observed for AS1411, poly (I:C12U), oblimersen, imetelstat, drisapersen, and mipomersen but not eteplirsen (Table 1) (Figure 2). A marker of the cellular activation in macrophages is neopterin, a guanine metabolite observed following administration of poly (I:C12U). High neopterin levels are associated with production of Reactive Oxygen Species (ROS) and an active inflammatory response [16]. The cellular response generating ROS was also observed for phosphorothioate oligonucleotides [72, 73]. ROS can form 8-oxo-G adducts in DNA, RNA and it is also possible in a therapeutic oligonucleotide [74]. The consequences of 8-oxo-G range from a potent activation of a DNA damage sensing pathway with subsequent immune response [75] to mutagenesis [76]. The enhanced stability and longer tissue residence time for drisapersen and mipomersen may predispose oligonucleotides with these chemistries to production of ADA. Investigations with PMO reveal minimal production of ROS, no resulting production of 8-oxo-G, and no immune stimulation (Figure 4). Further, unlike the negatively charged oligomer chemistries, eteplirsen has not produced IJR and FLS. These observations would appear to establish a link between therapeutic oligonucleotide induction of ROS and the frequent observations of injection site reactions and flu-like symptoms. A more speculative link would include production of anti-DNA antibodies and neoplasia.
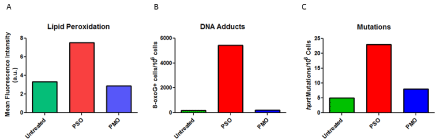
Figure 4: Reactive Oxygen Induced by Oligomers. Oligomers were added to V79 cells in culture at a concentration of 5 uM. Panel A is the measure of lipid
peroxidation determined by flow cytometry presenting the mean fluorescence intensity for the population of cells examined. Panel B is the measure of 8-oxoguanosine
adducts per million V79 cells by ELISA with an antibody to 8-oxo-G. Panel C is the measure of colonies per million cells grown in the presence of
6-thioguanine, a measure of mutations in Hypoxanthine Phosphoribosyl Transferase (hprt) following exposure of V79 cells to 5 uM oligonucleotide indicated.
References
- Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006; 124: 783-801.
- Tang D, Kang R, Coyne CB, Zeh HJ, Lotze MT. PAMPs and DAMPs: signal 0s that spur autophagy and immunity. Immunol Rev. 2012; 249: 158-175.
- Thi EP, Mire CE, Lee AC, Geisbert JB, Zhou JZ, Agans KN, Snead NM. Lipid nanoparticle siRNA treatment of Ebola-virus-Makona-infected nonhuman primates. Nature. 2015; 521: 362-365.
- Arbutus Press Release. “Tekmira provides update on TKM-Ebola-Guinea”. Arbutus Biopharma.
- Kraft CS, Hewlett AL, Koepsell S, Winkler AM, Kratochvil CJ, Larson L, et al. The Use of TKM-100802 and Convalescent Plasma in 2 Patients with Ebola Virus Disease in the United States. Clin Infect Dis. 2015; 61: 496-502.
- Bishop JS, Guy-Caffey JK, Ojwang JO, Smith SR, Hogan ME, Cossum PA, et al. Intermolecular G-quartet motifs confer nuclease resistance to a potent anti-HIV oligonucleotide. J Biol Chem. 1996; 271: 5698-5703.
- Dapic V, Bates PJ, Trent JO, Roger A, Thomas SD, Miller DM. Antiproliferative activity of G-quartet-forming oligonucleotides with backbone and sugar modifications. Biochemistry. 2002; 41: 3676-3685.
- Ireson CR, Kelland LR. Discovery and development of anticancer aptamers. Mol Cancer Ther. 2006; 5: 2957-2962.
- Bates PJ, Laber DA, Miller DM, Thomas SD, Trent JO. Discovery and development of the G-rich oligonucleotide AS1411 as a novel treatment for cancer. Exp Mol Pathol. 2009; 86: 151-164.
- Rosenberg JE, Bambury RM, Van Allen EM, Drabkin HA, Lara PN, Harzstark AL, et al. A phase II trial of AS1411 (a novel nucleolin-targeted DNA aptamer) in metastatic renal cell carcinoma. Invest New Drugs. 2014; 32: 178-187.
- Charoenphol P, Bermudez H. Aptamer-targeted DNA nanostructures for therapeutic delivery. Mol Pharm. 2014; 11: 1721-1725.
- Trinh TL, Zhu G, Xiao X, Puszyk W, Sefah K, Wu Q, et al. A Synthetic Aptamer-Drug Adduct for Targeted Liver Cancer Therapy. PLoS One. 2015; 10: e0136673.
- Metifiot M, Amrane S, Mergny JL, Andreola ML. Anticancer molecule AS1411 exhibits low nanomolar antiviral activity against HIV-1. Biochimie. 2015; 118: 173-175.
- Field AK, Tytell AA, Lampson GP, Hilleman MR. Inducers of interferon and host resistance. II. Multistranded synthetic polynucleotide complexes. Proc Natl Acad Sci USA. 1967; 58: 1004-1010.
- Carter WA, Pitha PM, Marshall LW, Tazawa I, Tazawa S, Ts’o PO. Structural requirements of the rI n -rC n complex for induction of human interferon. J Mol Biol. 1972; 70: 567-587.
- Fuchs D, Baier-Bitterlich G, Wede I, Wachter H. Reactive oxygen and apoptosis. Oxidative stress and the molecular biology of antioxidant defenses. Cold Spring Harbor Laboratory Press, New York. 1966; 139-167.
- Hendrix CW, Margolick JB, Petty BG, Markham RB, Nerhood L, Farzadegan H, et al. Biologic effects after a single dose of poly(I):poly(C12U) in healthy volunteers. Antimicrob Agents Chemother. 1993; 37: 429-435.
- Avril T, Tayrac M, Leberre C, Quillien V. Not all polyriboinosinicpolyribocytidylic acids (Poly I:C) are equivalent for inducing maturation of dendritic cells: implication for alpha-type-1 polarized DCs. J Immunother. 2009; 32: 353-362.
- Gowen BG, Wong MH, Jung KH, Sanders AB, Mitchell WM, Alexopoulou L, et al. TRL3 is essential for the induction of protective immunity against Punta Toro virus infection by the double-stranded RNA (dsRNA), Poly(I;C12U), but not Poly(I:C): Differential recognition of synthetic dsRNA molecules. J Immunol. 2007; 178: 5200-5208.
- Bagchi A, Herrup EA, Warren HS, Trigilio J, Shin HS, Valentine C, et al. MyD88-dependent and MyD88-independent pathways in synergy, priming, and tolerance between TLR agonists. J Immunol. 2007; 178: 1164-1171.
- Mitchell WM, Nicodemus CF, Carter WA, Horvath JC, Strayer DR. Discordant biological and toxicological species responses to TLR3 activation. Am J Pathol. 2014; 184: 1062-1072.
- Tabernero J, Shapiro GI, LoRusso PM, Cervantes A, Schwartz GK, Weiss GJ, et al. First-in-humans trial of an RNA interference therapeutic targeting VEGF and KSP in cancer patients with liver involvement. Cancer Discov. 2013; 3: 406-417.
- Herbert BS, Pongracz K, Shay JW, Gryaznov SM. Oligonucleotide N3’-->P5’ phosphoramidates as efficient telomerase inhibitors. Oncogene. 2002; 21: 638-642.
- Pruzan R, Pongracz K, Gietzen K, Wallweber G, Gryaznov S. Allosteric inhibitors of telomerase: oligonucleotide N3’-->P5’ phosphoramidates. Nucleic Acids Res. 2002; 30: 559-568.
- Asai A, Oshima Y, Yamamoto Y, Uochi TA, Kusaka H, Akinaga S, et al. A novel telomerase template antagonist (GRN163) as a potential anticancer agent. Cancer Res. 2003; 63: 3931-3939.
- Herbert BS, Gellert GC, Hochreiter A, Pongracz K, Wright WE, Zielinska D, et al. Lipid modification of GRN163, an N3’-P5’ thio-phosphoramidate oligonucleotide, enhances the potency of telomerase inhibition. Oncogene. 2005; 24: 5262-5268.
- Dikmen ZG, Gellert GC, Jackson S, Gryaznov S, Tressler R, Dogan P, et al. In vivo inhibition of lung cancer by GRN163L: a novel human telomerase inhibitor. Cancer Res. 2005; 65: 7866-7873.
- Thompson PA, Drissi R, Muscal JA, Panditharatna E, Fouladi M, Ingle AM, et al. A phase I trial of imetelstat in children with refractory or recurrent solid tumors: a Children’s Oncology Group Phase I Consortium Study (ADVL1112). Clin Cancer Res. 2013; 19: 6578-6584.
- Chiappori AA, Kolevska T, Spigel DR, Hager S, Rarick M, Gadgeel S, et al. A randomized phase II study of the telomerase inhibitor imetelstat as maintenance therapy for advanced non-small-cell lung cancer. Ann Oncol. 2015; 26: 354-362.
- Reed JC, Stein C, Subasinghe C, Haldar S, Croce CM, Yum S, et al. Antisense-mediated inhibition of BCL2 protooncogene expression and leukemic cell growth and survival: Comparisons of phosphodiester and phosphorothioate oligodeoxynucleotides. Canc Res. 1990; 50: 6565-6570.
- Jansen B, Schlagbauer-Wadl H, Brown BD, Bryan RN, van Elsas A, Müller M, et al. bcl-2 antisense therapy chemosensitizes human melanoma in SCID mice. Nat Med. 1998; 4: 232-234.
- Cotter FE, Waters J, Cunningham D. Human Bcl-2 antisense therapy for lymphomas. Biochim Biophys Acta. 1999; 1489: 97-106.
- Genesense Injection Advisory Committee Briefing Document: NDA 21-874 01. 2006.
- Villalona-Calero MA, Ritch P, Figueroa JA, Otterson GA, Belt R, Dow E, et al. A phase I/II study of LY900003, an antisense inhibitor of protein kinase C-alpha, in combination with cisplatin and gemcitabine in patients with advanced non-small cell lung cancer. Clin Cancer Res. 2004; 10: 6086-6093.
- Tolcher AW, Reyno L, Venner PM, Ernst SD, Moore M, Geary RS, et al. A randomized phase II and pharmacokinetic study of the antisense oligonucleotides ISIS 3521 and ISIS 5132 in patients with hormone-refractory prostate cancer. Clin Canc Res. 2002; 8: 2530-2535.
- Leighl NB, Laurie SA, Chen XE, Ellis P, Shepherd FA, Knox JJ, et al. A phase I/II study of GTI-2040 plus docetaxel as second-line treatment in advanced non-small cell lung cancer: a study of the PMH phase II consortium. J Thorac Oncol. 2009; 4: 1163-1169.
- Plummer R, Vidal L, Grifin M, Lesley M, de Bono J, Coulthard S, et al. Phase I study of MG98, an oligonucleotide antisense inhibitor of human DNA methyltransferase 1, given as a 7-day infusion in patients with advanced solid tumors. Clin Cancer Res. 2009; 15: 3177-3183.
- Heemskerk H, de Winter C, van Kuik P, Heuvelmans N, Sabatelli P, Rimessi P, et al. Preclinical PK and PD studies on 2’-O-methyl-phosphorothioate RNA antisense oligonucleotides in the mdx mouse model. Mol Ther. 2010; 18: 1210-1217.
- Goemans NM, Tulinius M, van den Akker JT, Burm BE, Ekhart PF, Heuvelmans N, et al. Systemic administration of PRO051 in Duchenne’s muscular dystrophy. N Engl J Med. 2011; 364: 1513-1522.
- Drisapersen Briefing Document, NDA 206031. 2015.
- McHutchison JG, Patel K, Pockros P, Nyberg L, Pianko S, Yu RZ, et al. A phase I trial of an antisense inhibitor of hepatitis C virus (ISIS 14803), administered to chronic hepatitis C patients. J Hepatol. 2006; 44: 88-96.
- Schimmer AD, Estey EH, Borthakur G, Carter BZ, Schiller GJ, Tallman MS, et al. Phase I/II trial of AEG 31356 X-linked inhibitor of apoptosis protein antisense oligonucleotide combined with Idarubicin and cytarabine in patients with relapsed or primary refractory acute myelogenous leukemia. J Clin Oncol. 2009; 27: 4741-4746.
- Mipomersen Sodium Injection 200 mg/mL, FDA Briefing Document, NDA 203568, October 18, 2012.
- Sewell LK, Geary RS, Baker BF, Glover JM, Mant TGK, Yu RZ, et al. Phase I trial of ISIS 104838, a 2’-methoxyethyl modified antisense oligonucleotide targeting tumor necrosis factor-alpha. J Pharmacol Exp Ther. 2002; 303: 1334-1343.
- Hong DS, Kurzrock R, Oh Y, Wheler J, Naing A, Brail L, et al. A phase I dose escalation, pharmacokinetic, and pharmacodynamic evaluation of eIF-4E antisense oligonucleotide LY2275796 in patients with advanced cancer. Clin Canc Res. 2011; 17: 6582-6591.
- Kamada M, So A, Muramaki M, Rocchi P, Beraldi E, Gleave M. Hsp27 knockdown using nucleotide-based therapies inhibit tumor growth and enhance chemotherapy in human bladder cancer cells. Mol Cancer Ther. 2007; 6: 299-308.
- Iversen P. Phosphorodiamidate Morpholino Oligomers. In Antisense Drug Technology: Principles, Strategies and Applications (Ed. Stan Crooke) Marcel Dekker, NY. 2001; 375-389.
- Iversen P. Morpholinos. In Antisense Drug Technology: Principles, Strategies and Applications (Ed. Stan Crooke) Marcel Dekker, NY. 2008; 565-581.
- Iversen, PL, Arora, V, Acker, AJ, Mason, DH, Devi, GR. Efficacy of Antisense Morpholino Oligomer Targeted to c-myc in Prostate Cancer Xenograft Model and a Phase I Safety Study in Humans. Clinical Cancer Research. 2003; 9: 1-10.
- Sazani P, Weller DL, Shrewsbury SB. Safety pharmacology and genotoxicity evaluation of AVI-4658. Int J Toxicol. 2010; 29: 143-156.
- Sazani P, Ness KP, Weller DL, Poage DW, Palyada K, Shrewsbury SB. Repeat-dose toxicology evaluation in cynomolgus monkeys of AVI-4658, a phosphorodiamidate morpholino oligomer (PMO) drug for the treatment of duchenne muscular dystrophy. Int J Toxicol. 2011; 30: 312-321.
- Mendell JR, Rodino-Klapac LR, Sahenk Z, Roush K, Bird L, Lowes LP, et al. Eteplirsen for the treatment of Duchenne muscular dystrophy. Ann Neurol. 2013; 74: 637-647.
- Iversen PL, Warren TK, Wells JB, Garza NL, Mourich DV, Welch LS, t al. Discovery and early development of AVI-7537 and AVI-7288 for the treatment of Ebola virus and Marburg virus infections. Viruses. 2012; 4: 2806-2830.
- Heald AE, Iversen PL, Saoid JB, Sazani P, Charleston JS, Axtelle T, et al. Safety and Pharmacokinetic Profiles of Phosphorodiamidate Morpholino Oligomers with activity against Ebola Virus and Marburg Virus: Results of Two Single Ascending Dose Studies. Antimicrob Agents Chemother. 2014; 58: 6639-6647.
- Heald AE, Charleston JS, Iversen PL, Warren TK, Saoud JB, Al-Ibrahim M, et al. AVI-7288 for Marburg Virus in Nonhuman Primates and Humans. N Engl J Med. 2015; 373: 339-348.
- Griffin LC, Tidmarsh GF, Bock LC, Toole JJ, Leung LL. In vivo anticoagulant properties of a novel nucleotide-based thrombin inhibitor and demonstration of regional anticoagulation in extracorporeal circuits. Blood. 1993; 81: 3271- 3276.
- Dyke CK, Steinhubl SR, Kleinman NS, Cannon RO, Aberle LG, Lin M, et al. First-in-human experience of an antidote-controlled anticoagulant using RNA aptamer technology: Phase 1a pharmacodynamics evaluation of a drug-antidote pair for the control regulation of Factor IXa activitiy. Circulation. 2006; 114: 2490-2497.
- Gilbert JC, DeFeo-Fraulini T, Hutabarat RM, Horvath CJ, Merlino PG, Marsh HN, et al. First-in-human evaluation of anti von Willebrand factor therapeutic aptamer ARC1779 in healthy volunteers. Circulation. 2007; 116: 2678-2686.
- Pagano B, Martino L, Randazzo A, Giancola C. Stability and binding properties of a modified thrombin binding aptamer. Biophys J. 2008; 94: 562-569.
- Cierniewski CS, Babinska A, Swiatkowska M, Wilczynska M, Okruszek A, Stec WJ. Inhibitiion by modified oligodeoxynucleotides of the expression of type-1 plasminogen activator inhibitor in human endothelial cells. Eur J Biochem. 1995; 227: 494-499.
- Wallace TL, Bazemore SA, Kornbrust DJ, Cossum PA. Repeat-dose toxicity and pharmacokinetics of a partial phosphorothioate anti-HIV oligonucleotide (AR177) after bolus intravenous administration to cynomolgus monkeys. J Pharmacol Exp Ther. 1996; 278: 1313-1317.
- Webb MS, Tortora N, Cremese M, Kozlowska H, Blaquere M, Devine DV, et al. Toxicity and toxicokinetics of a phosphorothioate oligonucleotide against the c-myc oncogene in cynomologus monkeys. Antisense. Nucleic Acid Drug Devel. 2001; 11:155-163.
- Agrawal S, Jiang Z, Zhao Q, Shaw D, Cai Q, Roskey A, et al. Mixed-backbone oligonucleotides as second generation antisense oligonucleotides: in vitro and in vivo studies. Proc Natl Acad Sci U S A. 1997; 94: 2620-2625.
- Kandimalla ER, Manning A, Zhao Q, Shaw DR, Byrn RA, Sasisekharan V, et al. Mixed backbone antisense oligonucleotides: design, biochemical and biological properties of oligonucleotides containing 2’-5’-ribo- and 3’-5’-deoxyribonucleotide segments. Nucleic Acids Res. 1997; 25: 370-378.
- Henry SP, Zuckerman JE, Rojko J, Hall WC, Harman RJ, Kitchen D, et al. Toxicological properties of several novel oligonucleotide analogs in mice. Anticanc Drug Des. 1997; 12: 1-14.
- Sheehan JP, Lan HC. Phosphorothioate oligonucleotides inhibit the intrinsic tenase complex. Blood. 1998; 92: 1617-1625.
- Farman CA, Kornbrust DJ. Oligodeoxynucleotide studies in primates: antisense and immune stimulatory indications. Toxicol Pathol. 2003; 31 Suppl: 119-122.
- Caporaso N. The molecular epidemiology of oxidative damage to DNA and cancer. J Natl Cancer Inst. 2003; 95: 1263-1265.
- Fuchs D, Baier-Bitterlich G, Wede I, Wachter H. Reactive oxygen and apoptosis. In: Oxidative stress and the molecular biology of antioxidant defenses. Scandalios J, ed., Cold Spring Harbor Laboratory Press, New York. 1966; 139-167.
- Hendrix CW, Margolick JB, Petty BG, Markham RB, Nerhood L, Farzadegan H, et al. Biologic effects after a single dose of poly(I):poly(C12U) in healthy volunteers. Antimicrob Agents Chemother. 1993; 37: 429-435.
- Henry SP, Templin MV, Gillett N, Rojko J, Levin AA. Correlation of toxicity and pharmacokinetic properties of a phosphorothioate oligonucleotide designed to inhibit ICAM-1. Toxicol Pathol. 1999; 27: 95-100.
- Henry S, Stecker K, Brooks D, Monteith D, Conklin B, Bennett CF. Chemically modified oligonucleotides exhibit decreased immune stimulation in mice. J Pharmacol Exp Ther. 2000; 292: 468-479.
- Klinman DM, Yi AK, Beaucage SL, Conover J, Krieg AM. CpG motifs present in bacteria DNA rapidly induce lymphocytes to secrete interleukin 6, interleukin 12, and interferon gamma. Proc Natl Acad Sci U S A. 1996; 93: 2879-2883.
- Marnett LJ. Oxyradicals and DNA damage. Carcinogenesis. 2000; 21: 361- 370.
- Yang G, Wan M, Zhang Y, Sun L, Sun R, Hu D, et al. Inhibition of a C-rich oligodeoxynucleotide on activation of immune cells in vitro and enhancement of antibody response in mice. Immunology. 2010; 131: 501-512.