
Research Article
J Drug Discov Develop and Deliv. 2022; 8(1): 1039.
Extraction, Physicochemical Characterization and Pharmacological Evaluation of Alginate from a Mediterranean Species of Brown Algae Dictyopteris membranaceae
Ayadi R¹*, Msahli A², Hamdi A¹, Majdoub H² and Bouraoui A¹
1Department of Drug Development, Faculty of pharmacy, University of Monastir, Tunisia
2Department of Medicinal Chemistry Laboratory, Faculty of Sciences, University of Monastir, Tunisia
*Corresponding author: Rihab A, Department of Drug Development, University of Monastir, Monastir, 5000, Tunisia
Received: March 02, 2022;Accepted: March 24, 2022; Published: March 31, 2022
Abstract
Brown seaweeds have attracted the attention of research. Alginates, one of the components of brown seaweed, are the subject of our study. In this work, we present the extraction, the physico-chemical characterization and the pharmacological evaluation of alginate from the brown seaweed: Dictyopteris membranaceae. The extraction of alginates from brown seaweed was conducted using Rioux et al. method with few modifications. The characterization of alginates was then preceded by infra-red spectroscopy. The content of different components was determined using Ultraviolet-visible spectroscopy. Molecular properties were determined using size exclusion chromatography. The results of our study showed that the yield of extraction of alginate from Dictyopteris membranacea is 7% of dry weight. Protein, total sugar and alginic acid content were determined using colorimetric assays. The results are comparable to those reported in literature. The infra-red spectrum confirmed the nature of our extract. The molecular weight is 77823 Daltons. Additionally, our extract presented a higher gastroprotective activity at 200mg/Kg than sucralfate administered at the same dose. Our results suggest that alginate from Dictyopteris membranaceae could be used as a gastroprotective agent.
Keywords: Sodium alginates, Dictyopteris membranaceae
Introduction
The marine world is of enormous scientific interest as it houses living organisms with unique structures [1,2], metabolic pathways, reproductive systems, sensory mechanisms and defenses due to their adaptation to unprecedented extreme environmental conditions [3]. Mediterranean coral reefs bring together a heterogeneous set of marine organisms. Among which we find algae. These are made up of a large group of marine plants of spectacular biological importance allowing them to be an attraction for exploitation in research and development. It has also turned out that algae have a ubiquitous presence. They are found on rocks, dead corals and stones [4]. The subject of our study is Dictyopteris membranaceae, brown seaweed up to 10cm in height with a flattened, slender yellowish-brown thallus. Dictyopteris membranaceae contains a considerable amount of content, reports have shown variation in percentages which is linked to the period of harvest and also the geographic location of the species. Alginate is one of the most popular polysaccharides in Dictyopteris membranaceae which can come up to 18.93% of dry weight [5]. Alginate is a negatively charged random or alternating linear copolymer [6]. It is composed of residues of (1→4) α-L-glucuronic (G) and β-D-mannuronic (M) (Figure 1). The proportion and their linear arrangement varies from one species of algae to another, from the part of the algae, and even from the time of harvest [7,8].

Figure 1: Structure of Alginate [27].
Alginates have several applications thanks to their biological and mechanical properties. Due to its biocompatibility and freezing properties, alginates are widely used in the design of various tissue structures designed for biomedical research [9]. Alginates have been reported to increase the production of short chain fatty acids, decrease the breakdown and absorption of triglycerides through inhibition of pancreatic lipases, and decrease the absorption of glucose. They are thus potentially active for the treatment of diseases constituting the metabolic syndrome: type 2 diabetes, arterial hypertension and dyslipidemia [10]. Besides, they are widely used as a material in wound healing dressings, as a dental impression material, and in the treatment of gastrointestinal reflux [11]. Considering its biocompatibility, low toxicity and structure diversity, our study focuses on the extraction, physicochemical characterization and the pharmacological evaluation of the gastroprotective activity of alginate from the brown seaweed: Dictyopteris membranaceae.
Materials and Methods
Sample collection
Dictyopteris membranaceae was collected from rocky areas of the Tunisian coast, at a depth between 1 and 3 meters deep. Then the samples were rinsed and dried in the shade; crushed, sieved and then stored at -20°C until use. The species were identified by the team of the National Institute of Science and Technology of the Sea of Salamboo (INSTM).
Extraction of sodium alginates
100g of the seaweed powder are put in cartridges. The extraction is carried out for 4 hours in petroleum ether using Soxhlet. The cartridges are then dried in an oven at 40°C and the delipidated powder is then recovered.
The extraction of the alginates was carried out according to the Rioux et al. method. Rioux, Turgeon & Beaulieu with some modifications [12]: 30g of each seaweed powder was treated with 12g of CaCl2 in the presence of hydrochloric acid for 3 hours at 70°C. This step is followed by centrifugation (4500rpm for 20 minutes) with retrieval of the pellet. The latter is then brought into contact with 9g of Na2CO3 for 24 hours. This step was repeated twice to improve the yield of the extraction. This is followed by a centrifugation step (4500rpm for 20 minutes). The supernatant is collected to precipitate an aqueous solution of ethanol (ethanol/distilled water 3: 1 V/V). After the third centrifugation step (4500rpm for 20 minutes). The pellet is dissolved in distilled water. The pellet is subsequently dialyzed and freeze-dried.
Physico-chemical characterization of alginates
Colorimetric assays were performed in order to determine protein, total sugar and uronic acid composition of our extract. The protein content was determined using Oliver H. Lowry method [13]. Total sugar content was determined using Dubois and al. method [14]; and uronic acid composition was determined using the carbazole method [15]. The identification of the extract was determined using Fouriertransform infrared spectroscopy (Perkin Elmer Spectrum 2 ATRFTIR). The determination of molecular properties was determined by size-exclusion chromatography with triple detection: multiangle light scattering detector (MALS) (Down HELEOS II, Wyatt Technology, Ca, USA), viscometrical detector (Viscostar II, Wyatt Technology, Ca, USA) and a refractometric index detector (RID 10 Ai Shimadzu, Japan).
Pharmacology
Animals: Wistar rats of either sex, weighing 150-180 g were purchased from Pasteur Institute (Tunis, Tunisia). Housing conditions and in vivo experiments were approved according to the guidelines established by the European Union on Animal Care (CCE Council 86/609).
Gastroprotective activity: The gastroprotective activity of sodium alginates of Dictyopteris membranaceae was evaluated by the HCl/EtOH method which induced gastric ulcer [16]. Rats fasted for 24h were divided into four groups. The control group received an oral dose of saline solution (NaCl 9g/l, 5ml/kg), the test groups sodium alginates (100 and 200mg/kg orally), the reference group received sucralfate (200mg/kg orally) as a reference drug. After 30min, 1ml/100g of 150mM Ethanol/HCl solution was orally given to all groups. Animals were sacrificed 1h after ulcerogenic agent administration and their stomachs were removed and opened along the great curvature for ulcer lesions estimation. The lesion index defined as the summative length of the lesions along the stomach was determined.
Statistical analysis
Data are presented as the mean ± standard error of the mean (s.e.m). Statistical analysis was performed using Student’s t-test. The significance of difference was considered to include values of P<0.05.
Results
Yield of extraction
Our results show that the yield of extraction of sodium alginate from D. membranaceae using the Rioux et al. method with some modifications is 7% of dry weight.
Chemical analysis
Colorimetric assays: The colorimetric assays conducted on the sodium alginates extracted from D. membranaceae determined protein, total sugar and uronic acid content. We found a protein percentage of 13.32%, a total sugar percentage of 20% and the content of uronic acid was 70.23%.
Fourier-transform infrared spectroscopy (FT-IR): The FT-IR spectrum of our extract showed is presented in Figure 2. The exact absorption peaks are given in Table 1. Sodium alginate extracted from Dictyopteris membranaceae showed different absorption bands: intense band at 1605.83cm-1, a large band at 3385.43cm-1, two intense bands at 1047.07cm-1 and 2982.89cm-1. Finally, three weak bands at 803.88, 897.67 and at 944.40 cm-1.
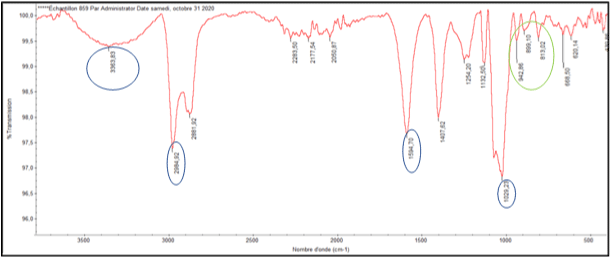
Figure 2: FT-IR spectrum of sodium alginates extracted from D. membranaceae: Polysaccharide confirmation (blue circles), sodium alginate confirmation (green circle).
Analysis by multiple detector size exclusion chromatography (SEC/MALS/VD/DRI) is used to determine the average molecular mass and to provide information on the molecular characteristics of our extract. Detectors response upon elution of sodium alginate from D. membranaceae is shown in Figure 3. Our sample was eluted at a volume between 15 and 20 mL. The data relating to the mean values of alginate from this species are given in Table 2.
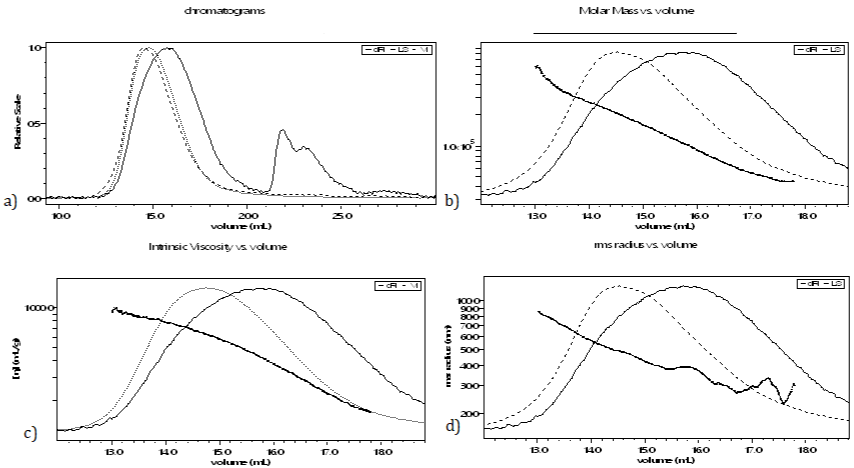
Figure 3: Elution profiles of alginate from Dictyopteris membranaceae by SEC/MALS/VS/DRI in an aqueous solution of LiNO3 0.1mol/L; a) Chromatogram of
multiple detection; b) Evolution of the molar mass as a function of the elution volume; c) Evolution of viscosity as a function of the elution volume; d) Evolution of
the hydrodynamic radius as a function of the elution volume.
Gastroprotective activity
The evaluation of the gastroprotective activity of the sodium alginate extracted from Dictyopteris membranaceae was carried out by measuring the ulcer index (IU), induced by the oral administration of the Ethanol/HCl solution 24 hours fasted Wistar rats. We compared the gastroprotective activity of our extract in the absence (control batch) and in the presence of an antiulcer treatment (reference batch and test batch). The results are shown in Table 3. Oral administration of the EtOH/HCl solution to the animals of the control group produced mucosal damage characterized by the presence of several red hemorrhagic bands of different sizes along the gastric glandular axis. We found that sucralfate, used as a reference drug at a dose of 200mg/ Kg protected the gastric mucosa with a percentage of 29.4%. This activity exceeded that of alginate from Dictyopteris membranaceae at a dose of 100mg/Kg, which protected the stomach with a percentage of 22.6%. However, the gastroprotective activity of alginate at a dose of 200mg/Kg is greater than that provided by sucralfate at the same dose with a percentage of 37.8%.
Discussion
Yield of extraction
The extraction yield for Dictyopteris membranaceae is low compared to the literature. In this work, we found a yield of 7% compared to the work of Abid and al. who reported a yield of extraction of 66.72% [5]. The large difference in these results might be due to differences in either the season of harvest, or the part of the algae on which the extraction is done, and finally, the addition of yield improving agents could be the reason of the noticeable difference [8].
Chemical analysis
Colorimetric assays: The protein content determination in the extract using colorimetric assay can be indication of potential impurities in our product. Our results showed a protein percentage of 13.32%. The analysis of protein content in Dictyopteris delicatula extract showed a lower content ranging from 0.1 to 0.7% [17]. The difference between this value and that found in our study could be due to the difference in species level, harvest season, extraction method as well as purification process. Studies have also shown that depending on the species of algae and the harvest season, the protein content can be up to 50% [18]. These results show that, one hand brown algae have good nutritional quality. On the other hand, the purification process adopted in our method is not sufficient to eliminate all the proteins whose presence in the extract constitutes a compromise for the biocompatibility of alginates [19].
Our results showed a total sugar content of 20.05%. However, reports from literature show higher percentages: 87.29% and 67.87% for two types of extracts obtained from Dictyopteris membranaceae. Other reports work found a total sugar content of 80.4% in an extract of Dictyopteris justii [20].
The uronic acid content in our extract is consistent with literature. Other work showed percentages of 69.81% which is very close to our results (70.23%).
Fourier-transform infrared spectroscopy (FT-IR): The infrared spectrum shows an intense band at 1594.70cm-1 reflecting the asymmetrical stretching of carboxylate (COO-) confirming the high content of uronic acid in our extract [21,22]. Besides, the spectrum showed 3 peaks at an interval between 950 and 750 cm-1, giving additional confirmation of the presence of uronic acid presence. A wide band is observed between 3300 and 3390 cm-1 assigned to the presence of free OH groups. The spectrum also showed the most intense band at 1047.07cm-1, corresponding to the C=O group [23]. Finally, an intense band is shown at 2982.89cm-1 indicating stretching vibrations of C-H links.
Size exclusion chromatography (SEC): The results show that the mass average molecular mass (Mm) of our extract is 104000g.mol-1. These results agree with those found in literature [12]. Raymond and al. reported that the Mw of alginates ranges between 20000 and 240000 [24]. The dispersity (Đ) is greater than 1 which means that the sample is not uniform. This result is compared favorably to the literature, since typically, alginate have a dispersity ranging between 1.5 and 3 [25]. Alginate from algae is a naturally occurring polydisperse polymers, contrary to bacterial alginate that are naturally monodisperse [26].
Gastroprotective activity
Gastric ulcer is a fairly common disease [27]. Proton pump inhibitors (PPI) are the most widely prescribed drugs for its treatment [2]. Although effective, PPIs are many reported adverse effects 28 and can be the subject to numerous drug interactions [29]. It is therefore necessary to find alternative treatment that are at least as effective and better yet, safer.
In our study, we compared the gastroprotective activity of sodium alginate extracted from Dictyopteris membranaceae with that of sucralfate. The chemical structure of the latter occurs as a mineral complex of sucrose sulfate and aluminum hydroxide. Its gastroprotective efficacy is due to the fact that it acts at several levels, for example, it inhibits peptic enzymes, forms a physical protective barrier, promotes tissue growth and regeneration, activates macrophages, prostaglandins and cyclooxygenases [30].
The gastroprotective activity of polysaccharides extracted from brown algae has been reported in numerous studies [31]. That of alginates extracted from Dictyopteris membranaceae could be due to their ability to form gels: this characteristic allows them to adhere to epithelial cells and thus protect the gastric mucosa [5]. In addition, it may be due to their antioxidant activity [5] as the ethanol-induced ulcer involves reactive oxygen derivatives in their pathophysiology [32]. This means that alginate inhibits the formation of lipid peroxidation products by reducing the degree of imbalance in the balance of prooxidant/antioxidant molecules induced by ulcerogenic products [33]. The results of our study showed that at the same dose of 200mg/Kg, sodium alginate from extracted from Dictyopteris membranaceae provides better gastroprotective activity than sucralfate with a percentage of 37.8% against 29.4% respectively. This suggests that, acting at the same level, sodium alginate is pharmacologically more active than sucralfate. Moreover, sodium alginate has more levels of action than sucralfate.
Conclusion and Perspective
Brown algae are considered among the richest classes in terms of therapeutic and nutritional potential.
In our study, we extracted alginates from Dictyopteris membranaceae. Then, we carried out a physicochemical characterization of the obtained extract by colorimetric assays, infrared spectroscopy in order to identify the alginate and finally size exclusion chromatography.
The pharmacological evaluation of the gastroprotective activity of sodium alginate showed that provides better gastroprotective activity than sucralfate when given at the same dose.
Further studies are needed to improve the extraction efficiency of alginates so that their pharmacological and biological potential can be further explored.
References
- Zubia M, Fabre MS, Kerjean V, et al. Antioxidant and antitumoural activities of some Phaeophyta from Brittany coasts. Food chemistry. 2009; 116: 693-701.
- Ammar HH, Hafsa J, Le Cerf D, Bouraoui A, Majdoub H. Antioxidant and gastroprotective activities of polysaccharides from the Tunisian brown algae. J Tunisian Chem Soc. 2016; 18: 80-88.
- Das S. Biotechnological exploitation of marine animals. Animal Biotechnology. Elsevier. 2014: 541-562.
- Bajpai VK. Antimicrobial bioactive compounds from marine algae: A mini review. 2016.
- Abid MD, Lajili S, Ammar HH, et al. Chemical and biological properties of sodium alginates isolated from tow brown algae Dictyopteris Membranaceae and Padina Pavonica. Trends Journal of Sciences Research. 2019; 4: 62-67.
- Goh CH, Heng PWS, Chan LW. Alginates as a useful natural polymer for microencapsulation and therapeutic applications. Carbohydrate Polymers. 2012; 88: 1-12.
- Draget KI, Taylor C. Chemical, physical and biological properties of alginates and their biomedical implications. Food Hydrocolloids. 2011; 25: 251-256.
- Torres MR, Sousa AP, Silva Filho EA, et al. Extraction and physicochemical characterization of Sargassum vulgare alginate from Brazil. Carbohydrate research. 2007; 342: 2067-2074.
- Sarker B, Boccaccini AR. Alginate utilization in tissue engineering and cell therapy. Alginates and Their Biomedical Applications. Springer. 2018: 121- 155.
- Kumar SA, Brown L. Alginates in metabolic syndrome. Alginates and their Biomedical Applications. Springer. 2018: 223-235.
- Draget KI, Smidsrød O, Skjåk-Bræk G. Alginates from algae. Polysaccharides and polyamides in the food industry: properties, production, and patents. 2005: 1-30.
- Ammar HH, Lajili S, Sakly N, et al. Influence of the uronic acid composition on the gastroprotective activity of alginates from three different genus of Tunisian brown algae. Food chemistry. 2018; 239: 165-171.
- López CVG, García MdCC, Fernández FGA, Bustos CS, Chisti Y, Sevilla JMF. Protein measurements of microalgal and cyanobacterial biomass. Bioresource technology. 2010; 101: 7587-7591.
- Pawlaczyk I, Czerchawski L, Kuliczkowski W, et al. Anticoagulant and antiplatelet activity of polyphenolic-polysaccharide preparation isolated from the medicinal plant Erigeron canadensis L. Thrombosis Research. 2011; 127: 328-340.
- Bitter T. A modified uronic acid carbazole reaction. Anal Biochem. 1962; 4: 330-334.
- de Souza Almeida ES, Cechinel Filho V, Niero R, Clasen BK, Balogun SO, de Oliveira Martins DT. Pharmacological mechanisms underlying the anti-ulcer activity of methanol extract and canthin-6-one of Simaba ferruginea A. St-Hil. In animal models. Journal of ethnopharmacology. 2011; 134: 630-636.
- Magalhaes KD, Costa LS, Fidelis GP, et al. Anticoagulant, antioxidant and antitumor activities of heterofucans from the seaweed Dictyopteris delicatula. International journal of molecular sciences. 2011; 12: 3352-3365.
- Vassilev SV, Baxter D, Andersen LK, Vassileva CG. An overview of the chemical composition of biomass. Fuel. 2010; 89: 913-933.
- Dusseault J, Tam SK, Ménard M, et al. Evaluation of alginate purification methods: effect on polyphenol, endotoxin, and protein contamination. Journal of Biomedical Materials Research Part A: An Official Journal of the Society for Biomaterials, The Japanese Society for Biomaterials, and The Australian Society for Biomaterials and the Korean Society for Biomaterials. 2006; 76: 243-251.
- Teodosio Melo KR, Gomes Camara RB, Queiroz MF, et al. Evaluation of sulfated polysaccharides from the brown seaweed Dictyopteris justii as antioxidant agents and as inhibitors of the formation of calcium oxalate crystals. Molecules. 2013; 18: 14543-14563.
- Bi F, Mahmood SJ, Arman M, Taj N, Iqbal S. Physicochemical characterization and ionic studies of sodium alginate from Sargassum terrarium (brown algae). Physics and Chemistry of Liquids. 2007; 45: 453-461.
- Mathlouthi M, Koenig JL. Vibrational spectra of carbohydrates. Advances in carbohydrate chemistry and biochemistry. 1987; 44: 7-89.
- Fenoradosoa TA, Ali G, Delattre C, et al. Extraction and characterization of an alginate from the brown seaweed Sargassum turbinarioides Grunow. Journal of applied phycology. 2010; 22: 131-137.
- Rowe RC, Sheskey P, Quinn M. Handbook of pharmaceutical excipients. Libros Digitales-Pharmaceutical Press. 2009.
- Rehm BH. Alginates: biology and applications. 2009; 13. Springer.
- Moradali MF, Ghods S, Rehm BH. Alginate biosynthesis and biotechnological production. Alginates and their biomedical applications. Springer. 2018: 1-25.
- AbdelAziz EY, Tadros MG, Menze ET. The effect of metformin on indomethacin-induced gastric ulcer: Involvement of nitric oxide/Rho kinase pathway. European Journal of Pharmacology. 2021; 892: 173812.
- Sheen E, Triadafilopoulos G. Adverse effects of long-term proton pump inhibitor therapy. Digestive diseases and sciences. 2011; 56: 931-950.
- Ogawa R, Echizen H. Drug-drug interaction profiles of proton pump inhibitors. Clinical pharmacokinetics. 2010; 49: 509-533.
- McCarthy DM. Sucralfate. New England Journal of Medicine. 1991; 325: 1017-1025.
- Amornlerdpison D, Peerapornpisal Y, Taesotikul T, Noiraksar T, Kanjanapothi D. Gastroprotective activity of Padina minor Yamada. Chiang Mai J Sci. 2009; 36: 92-103.
- Mizui T, Doteuchi M. Lipid peroxidation: a possible role in gastric damage induced by ethanol in rats. Life sciences. 1986; 38: 2163-2167.
- Krylova S, Khotimchenko YS, Zueva E, et al. Gastroprotective effect of natural non-starch polysaccharides. Bulletin of experimental biology and medicine. 2006; 142: 454-457.