
Research Article
J Drug Discov Develop and Deliv. 2022; 8(1): 1042.
Synthesis and Characterization of a Novel Polymorph of Glimepiride
Jadhav S1,2*, Gosar A1, Mandawad G2* and Patil M1
1Indoco Remedies Ltd, R92/93, TTC Industrial Area, Rabale, India
2Maharashtra Udaygiri College, Udgir.413517.SRTM University, Nanded, India
*Corresponding author: Shivaji Jadhav, Indoco Remedies Ltd., R92/93, TTC Industrial Area, Rabale, Maharashtra, Navi Mumbai 400701, India
Gajanan Mandawad, Maharashtra Udaygiri College, Udgir.413517. SRTM University, Nanded, India
Received: October 17, 2022; Accepted: November 17, 2022; Published: November 24, 2022
Abstract
Polymorphism in drug substances is a phenomenon that leads to a change in the efficacy of drugs due to changes in their physicochemical properties, particularly solubility and bioavailability. Glimepiride has two polymorphs described in the literature Glimepiride form I and Glimepiride form II.A new crystalline form-III was prepared by recrystallization from a Glimepiride form-I by using an N-methyl-2-pyrrolidone solvent system and ethyl acetate and acetone solvent mix 50/50 v/v. The solubility and melting properties of the novel polymorph are significantly different from the reported two polymorphs. A novel polymorph has been evaluated by using an X-ray diffractometer and differential scanning calorimeter, Thermogravimetric techniques, and image analysis. The crystal structure of Glimepiride form III is more thermodynamically stable than the previously reported form I and form II. A new crystalline form of glimepiride with a characteristic melting point of 276.2°C, a distinguished marker 2θpeak at 6.7°, 30.5°, and marker peak of form I and II in the region of 2θ 8-12.5 not observed in diffractogram of novel Glimepiride polymorph III which resulting in a unique Diffractogram pattern of form III.IR spectra show that the absorption band shows at 3325.28cm-1 which is different than reported for Glimepiride polymorph I absorption at 3290 and 3370 cm-1 and for Glimepiride form II absorption at 3370 and 3100 cm-1
Keywords: Polymorphism; Crystallography; DSC; XRD; Image analysis
Introduction
Glimepiride is the latest second-generation sulfonylurea for the treatment of type 2 diabetes mellitus [1]. Glimepiride is a sulphonylurea agent that stimulates insulin release from pancreatic β-cells and may act via extrapancreatic mechanisms. It is administered once daily to patients with type 2 (non-insulin-dependent) diabetes mellitus in whom glycemic is not controlled by diet and exercise alone, and may be combined with insulin in patients with secondary sulphonylurea [2]. Sulfonylureas are used mainly based on their low cost, well-established glucose-lowering action and a longstanding experience in clinical practice [3]. Both the prevalence and incidence of type 2 diabetes are increasing worldwide, particularly in developing countries, in conjunction with increased obesity rates and the modernization of lifestyle [4].
Even after the potency of the API (Active Pharmaceutical Ingredient) is established, the solid-state properties of the API present significant challenges to a formulation scientist. The crystallization process significantly impacts crystal forms, which further affects the physiochemical properties of pharmaceutical solids. The API can be present in different crystalline forms, which impacts the formulation process and stability of the drug product. The API exhibits different polymorphs if the internal structure of a crystal is different. However, if the internal structure remains similar and only the external appearance is different, then the API has the different crystal habits of the same polymorph [5]. Glimepiride has poor aqueous solubility, classified as Biopharmaceutics Classification System (BCS) class II, leading to poor dissolution and limiting drug absorption. Many approaches have been explored to increase the solubility of glimepiride, including the preparation of polymorphs, cyclodextrin inclusion complexes, co-crystals, and solid dispersions [6]. The drug presents two polymorphic forms (Glimepiride form I and Glimepiride form II) described in the literature, and according to in vitro data, Glimepiride form II is about 3.5 times more soluble and releases 2 times the drug amount than Glimepiride form I in the physiological pH range [7].
Glimepiride exhibits very poor solubility at 37°C (< 0.004 mg/ mL) in acidic and neutral media and relatively high permeability (30.4×6 cm/s) through CaCo-2 cell monolayers (Frick et al. 1998). Thus, Glimepiride is categorized as a Class 2 drug by the Biopharmaceutics Classification System (Amidon et al. 1995) [8]. Infrared studies showed that the increase in the dissolution profile is related to the intermolecular interactions (hydrogen bonds), which were dependent on composition [9]. Solid drug delivery systems are crucial formulations for the oral route. In such systems, particle size and polymorphism have a strong impact on drug dissolution and on drug absorption [10].
In the present work, novel crystalline polymorphs of the glimepiride synthesis and characterization are thoroughly described, thus providing useful insights into their physicochemical properties, like sphericity, compactness, roundness extent, circularity, solidity, L/W Ratio, W/L aspect ratio, particle size distribution, specific surface area. Special attention has been paid to the experimental conditions for the synthesis of novel polymorph III with two different methods and its reproducibility along with characterization by developing new analytical methods for the determination of polymorph.
Materials and Methods
Materials
All solvents used in the synthesis and analysis, including N-methyl- 2-Pyrrolidone, Acetone, Ethyl alcohol, and acetonitrile (Merck, India), were HPLC grade. Glimepiride used, was taken from Indoco Remedies Ltd.
General methods for the Synthesis of a Novel Polymorph of Glimepiride
Method I: Synthesis was carried out by glimepiride polymorph I was taken into N-methyl-2-Pyrrolidone as a solvent medium under constant stirring using a magnetic stirrer with a hot plate (Deepali, Mumbai, India)at about 60-75°C for 1hour and then the sample was kept at a deep freezer at a temp of about sign before 10°C to -15°C for 24 hours. The crystalline novel form was obtained by filtering N-methyl-2Pyrrolidone through a Whatman filter paper No.41 with a 20μm-pore size. And subsequent drying at 105°C in the oven for 8 hours. All synthetics steps involved in recrystallization were repeated to ensure the reproducibility of the novel polymorph. Particle size was down by using the instrument Retsch MM400 ball mill operating at 25 Hz frequency using zirconium balls placed in jars along the glimepiride polymorph.
Method II: Synthesis was carried out by glimepiride polymorph I was taken into Acetone, Ethyl alcohol 50:50 V/V as a solvent medium, and pH was adjusted to 9 with aqueous ammonia under constant stirring using a magnetic stirrer with a hot plate (‘Deepali, Mumbai, India) at about 25°C for 1 hour and then the sample was kept at a deep freezer at a temp of about sign before 10°C to -15°C for 8 hours. The crystalline novel form was obtained by filtering reaction mass through Whatman No.41 filter paper with a 20μm pore size. And subsequent drying at 105°C in the oven for 8 hours. All synthetics steps involved in recrystallization were repeated to ensure the reproducibility of the novel polymorph. Particle size was down by using the instrument Retsch MM400 ball mill operating at 25 Hz frequency using zirconium balls placed in jars along the synthesized glimepiride polymorph.
General Procedure for the Analysis of a Novel Polymorph of Glimepiride
Differential Scanning Calorimetry (DSC) was carried out using the instrument Model 3+ Mettler-Toledo Switzerland GmbH with a refrigerated system and software STARe was used to identify the melting point and enthalpy of a novel polymorphic and Nitrogen was used at a flow rate of 50 mL/min to purge the sample cell. Approximately 2-3 mg of a sample was taken in a crimped aluminum pan. To scan the sample, the sample is heated at 10°C per minute over a temperature range of 25°C – 400°C. The instrument was calibrated using high-purity indium and znic for temperature and heat flow calibration.
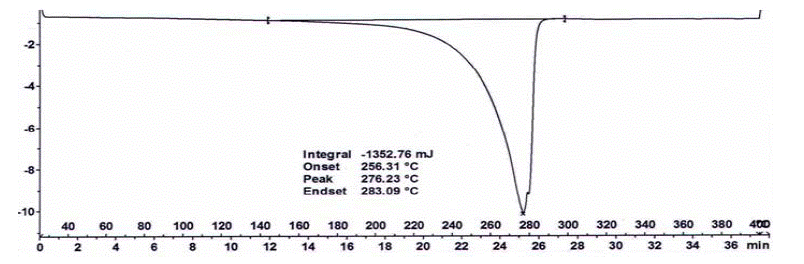
Figure 1: Glimepiride Form III DSC Thermogram.
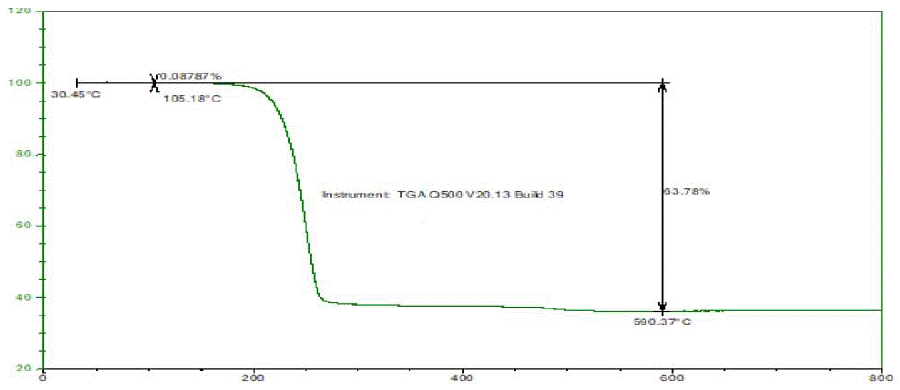
Figure 2: TGA Thermogram of Glimepiride Form III.
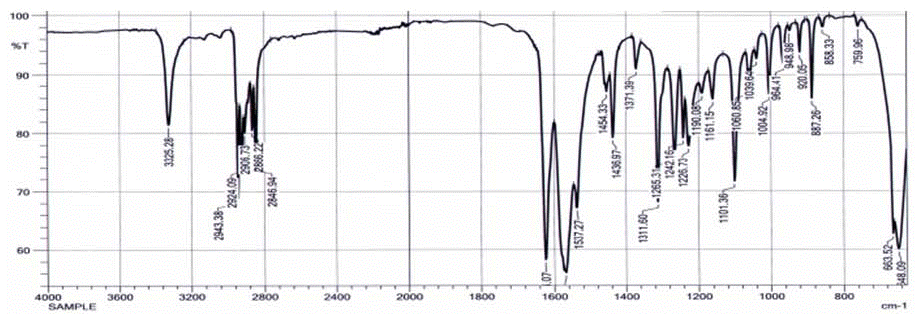
Figure 3: IR Spectra of Glimepiride Form III.
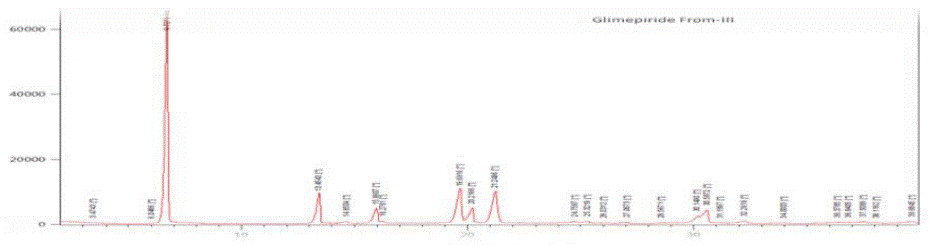
Figure 4: XRPD Diffractogram of Glimepiride Form III.
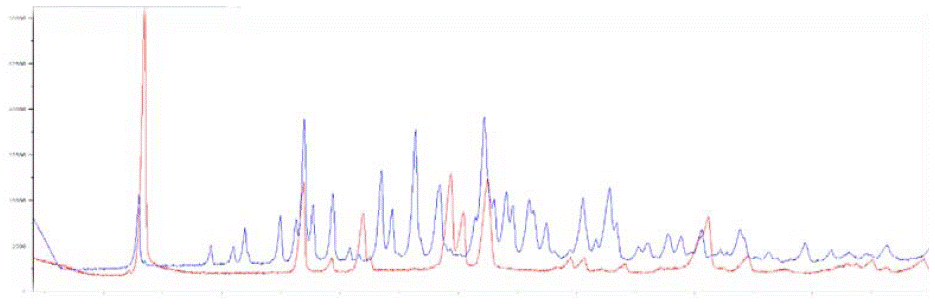
Figure 5: Overlay XRPD Diffractogram of Glimepiride Form I and form III.
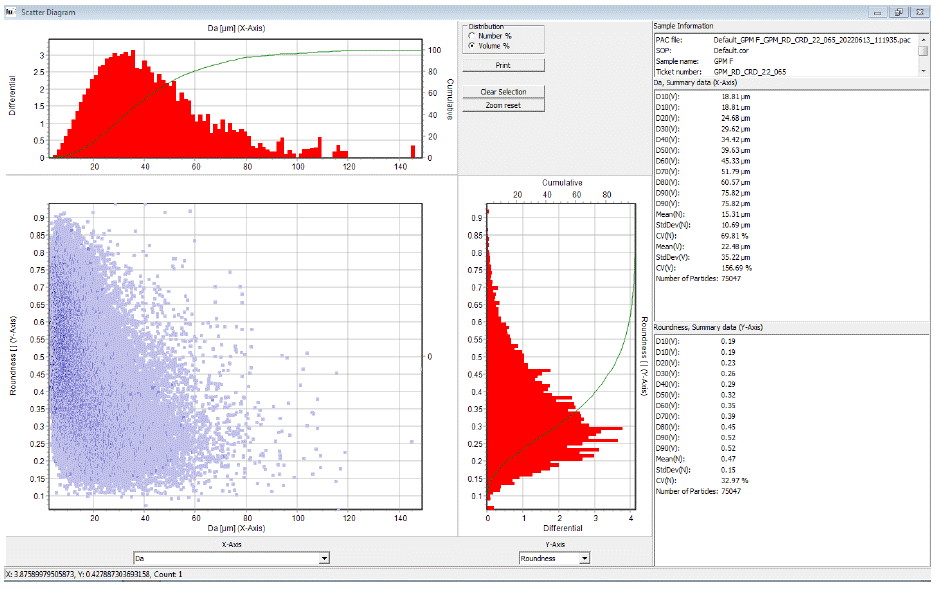
Figure 6: Microtrac image analysis histogram.
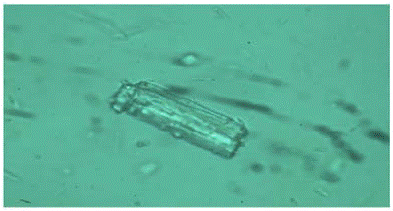
Figure 7: Microscopic images of Glimepiride Polymorph III 100X.

Figure 8: Microscopic images of Glimepiride Polymorph III 10X.
Thermogravimetric analyzer (TGA) was carried out using the instrument Q1000 from Waters TA, US, working on Explorer. Approximately 6mg of sample was heated in a platinum crucible at a rate of 10°C/min with a range from 25°C–800°Cwhile being purged with nitrogen at a rate of 50 mL/min.
Infrared spectroscopy (IR) was carried out using the instrument Affinity I-S from Shimadzu, Japan working with Lab solution software. Approximately 1-2 mg of sample was placed on the ATR diamond crystal and spectra were obtained in the range from 650cm-1 to 4000cm-1.
X-ray powder diffraction (XRPD) was carried out using the instrument PANalytical X’Pert Pro, Malvern PANalytical, Netherlands Approximately 300 mg of sample powder was taken, an even layer was created in a back-loading holder, and samples were analyzed using instrument parameters, Cu-Kα radiation (λ = 1.54 Ǻ) at 40 kV, 45 mA X-ray moving through a nickel filter used to carry out PXRD study. In a continuous scan mode, data were collected using a step size of 0.0131303 and time per step of 49.725 for an angular range 2θ of 2° to 40°.
Particle size distribution (PSD) was carried out using Malvern master size 2000S Malvern Instruments Ltd., Worcestershire, UK. A size range of 0.02 μm to 2000 μm was used for analysis with dualwavelength measurement and single-lens detection. About 1gm of the sample was transferred into the hopper. Instrument parameters like sample RI 1.5, material absorption 0.14, background measurement time of 5 seconds, measurement time of 5 seconds, Vibration feed rate 40%, air pressure 2 bar, and obscuration range kept at 1-6%, to generate results for particle size distribution and specific surface area of the novel polymorph.
Particle morphology (dynamic image analysis) was carried out by using the image analyzer Microtrac, USA, Part AN SI used as software. Transfer approximately 200 mg of the sample into 250 mL of a glass beaker. Add 3-4 drops of water as a dispersant and make a paste by using a glass rod. Then add 10–20 mL of dispersant. Stir well and externally sonicate for 10 seconds, then carry out the analysis with 15,000 particles captured.
Microscopic (static image) analysis was carried out using microscope Carl Zeiss Japan and used software Caliperpro –India with magnification power 100X,10X.
Thermo Dynamic Solubility (UPLC) Waters, USA, High- Performance Liquid Chromatography (UPLC) was used to determine dynamic solubility. Equipped with a quaternary gradient pump, an auto-sampler, and UV/PDA Detector with software Masslynx 4.2, Model-Acquity, Injection volume- 100.0 μL, Column Temperature 35°C, Flow Rate 1.0 mL/min,wavelength-228nm Columns: YMCTriart Dimension 150mm x 4.6mm, 5μm. Volumetric Flasks: 10 mL, 25 mL, 50 with stoppers, Class A. HPLC Vials with Septa Caps: 2mL capacity Membrane Filters: 0.45 mm Nylon Membrane Filters, 47 mm diameter, Millipore. Filter Assembly: A glass filtration assembly consists of a filtration funnel that can hold a 47-mm membrane filter, a metal clamp, and a suction flask. The analytical balance used was a Sartorius and Milli-Q-Water was used for analysis, Acetonitrile by Merck.
Mobile phase Preparation: - Transferred 0.5214 gm of sodium dihydrogen phosphate into 1 L Bottle. The pH of 2.50 with orthophosphoric acid, and added 500 ml Acetonitrile mixed well and degassed by sonication.
Solvent mixture / Diluent: Prepared a mixture of Acetonitrile and water (4:1).
Standard Preparation:-Weigh accurately 10.20 mg Glimepiride WS into a 100 volumetric flask. Dissolved in 25 ml with diluent and made up to the volume with diluent.
Results and Discussion
Glimepiride Form III synthesis was carried out by using glimepiride polymorph I by two unique procedures I and II and found that these two methods are resulting in the formation of novel polymorph III which is reproducible and consistent. An analytical study of polymorph III was performed by using different techniques like IR, DSC, and XRD and discussed in detail.
The DSC thermogram of Glimepiride Form III showed an endotherm at 276 °C and enthalpy is -938.18 J/g. The Reported literature value of Glimepiride Form I, an endotherm at 216 °C,and Form II shows an endotherm at 213°C.
The TGA thermogram of Glimepiride Form III showed there is no loss up to 105°C shows that the compound is not hydrated or solvated means free from water and solvent used for the recrystallization. Further weight loss of about 63.78% at 590 °C.
FTIR analysis for Form I and III were performed. Experimental and literature data of form I, II indicates that there is a change in spectral Region 3000-3500 cm-1and as described in the literature for form I and II that changes are due to differences of inter and intra hydrogen bonding observed and the same phenomenon also observed for Form III which shows absorption at 3325.28 cm-1.
The melting point for Glimepiride form I and II is 216°C and 213°C respectively as stated in the literature [8,5], while the polymorph Glimepiride III shows melting at 276°C. These comparative melting point values are given in Table 2, as supplementary Information. It was demonstrated previously that the DSC thermogram of polymorphic form III differs in pattern and event concerning Glimepiride form I and Glimepiride form II. Hence, it can be concluded that there is a polymorphic transformation occurring when applied processes I and II.
Sr.no.
2θ
d-spacing
1
3.47
25.41
2
6.72
13.12
3
13.45
6.58
4
14.65
6.04
5
15.99
5.54
6
16.27
5.44
7
19.69
4.5
8
20.21
4.39
9
21.25
4.17
10
24.76
3.59
11
25.32
3.51
12
26.03
3.42
13
27.07
3.29
14
28.57
3.12
15
30.15
2.96
16
30.59
2.92
17
32.26
2.77
18
36.38
2.47
19
36.84
2.43
20
37.51
2.39
21
38.11
2.35
22
39.68
2.25
Table 1: Glimepiride polymorph III 2θ and d spacing value.
Sr#
Instrument
Polymorph-I
Reported literature [8,5]Polymorph-II
Reported literature in [8,5]Polymorph-III Experimental data
1
IR (cm-1)
3290, 3370
3330,3100
3325.28
2
DSC (°C)
216
213
276.55
3
XRD (2θ)
6.46, 9.52, 10.48, 10.95
7.84, 10.47,11.95,12.4
6.72, 30.658 marker peak of Form III
Marker peak of form I absent 6.46, 9.52, 10.48, 10.95,12.4
Marker peak of form II absent 7.84,10.47,11.95
Table 2: Literature data form I and II and experimental data of Form III [8].
The XRD Diffractogram of Glimepiride Form III revealed a marker peak at 2θ 6.7, whereas Form I revealed a marker peak at 2θ 6.40, and glimepiride form II shows a peak at 2θ 7.84. All these peaks are distinct from each other.
The particle size of glimepiride Form III results for particle size distribution and of the polymorphmean value is 12.4 μmD10-2.8 μm, D50-12.4 μm, and D90-51. 6μm.specific surface area is 1.14m²/g.
The morphology of Glimepiride Form III was examined using an image analyzer from Microtrac. The photographic result shows a fine spherical shape, and smooth surface, and the particle size was within the Fig. Particle size distribution by Microtrac image analyzer.
Microscopic analysis revealed that particles are rod-shaped when analyzed using 100X, 10X, magnification used for fig nos. 1 and 2 respectively.
Thermodynamic solubility data of Glimepiride form III was performed by using UPLC where injected test solution against the STD solution of glimepiride Polymorph -I and the blank run, recorded the chromatograms. Observed results are recorded in Table 4.
Sr#
Parameter
Results (represent the mean value)
1.
Sphericity
0.60
2.
L/W Ratio
2.70
3.
W/L aspect ratio
0.46
4.
Elipse ratio
0.40
5.
Compactness
0.56
6.
Roundness
0.32
7.
Extent
0.59
8.
Circularity
0.36
9.
Solidity
0.78
10.
Concavity
0.22
11
Convexity
0.83
Table 3: Histogram of Glimepiride Form III.
Buffer
mg/mL
HCL Buffer 1.2
0.0036
Acetate Buffer
0.0063
Phosphate Buffer 6.8
0.0202
Phosphate Buffer 7.4
0.0368
Table 4: Thermodynamic Solubility Data of Glimepiride Form III.
Conclusions
The result obtained from the evaluation of glimepiride polymorph III shows that the polymorphic synthesis method is reproducible, accurate, and robust. The various analytical methods were employed to test the glimepiride polymorph III and confirmed its novelty in that it shows a different diffraction pattern along with a unique marker peak and a different melting point from the rest of the published polymorphs. Glimepiride III polymorph shows IR spectra at 3325.28 cm-1 and the DSC thermogram melt shows at 276.55°C along with enthalpy is -938.18J/g while XRD Diffractogram 6.72, 30.658 marker peak of Form III and marker peak of Form I and Form II 6.46, 9.52, 10.48, 10.95,12.4 and 7.84, 10.47,11.95 respectively are absent in Diffractogram of Glimepiride Form III. Solubility of Glimepiride Form III was highest at pH Phosphate Buffer 7.4 is 0.04mg/mL and particle size by Malvern Mastersizer 2000 mean were 12.4 μm and the specific surface area is 1.14m²/g.
A polymorph stability study shows novel glimepiride polymorph III is stable over a period of the time of six months. An Analytical study was done using XRPD, DSC, and IR and found that different test results are consistent and reproducible over time. The application of the novel form further needs to be evaluated for bioavailability and pharmacokinetics details.
Acknowledgment
The authors wish to extend their gratitude to Indoco Remedies Ltd. for providing all kinds of support. The author wishes to thank all our colleagues who provided technical assistance during the research and data collection.
References
- McCall AL. Clinical review of glimepiride. Expert Opinion on Pharmacotherapy. 2001; 2: 699-713.
- Langtry HD, Balfour JA. Glimepiride: A review of its use in the management of type 2 diabetes mellitus. Drugs. 1998; 55: 563-84.
- Rosenstock J, Kahn SE, Johansen OE, Zinman B, Espeland MA, et al. Effect of linagliptin vs glimepiride on major adverse cardiovascular outcomes in patients with type 2 diabetes: the CAROLINA randomized clinical trial. Jama. 2019; 322: 1155-66.
- Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, et al. Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes care. 2012; 35: 1364-79.
- Datir SR, Kumar D, Bele MH. Modified crystal habits of glimepiride to improve manufacturing processability. Journal of Crystal Growth. 2022; 592: 126711.
- Park H, Seo HJ, Hong SH, Ha ES, Lee S, et al. Characterization and therapeutic efficacy evaluation of glimepiride and L-arginine co-amorphous formulation prepared by supercritical antisolvent process: Influence of molar ratio and preparation methods. International journal of pharmaceutics. 2020; 581: 119232.
- Viana AL, Doriguetto AC, Viana OM, Ruela AL, Freitas JT, et al. Pharmacokinetics and pharmacodynamics of glimepiride polymorphs. International Journal of Pharmaceutics. 2018; 553: 272-80.
- Endo T, Iwata M, Nagase H, Shiro M, Ueda H. Polymorphism of glimepiride: crystallographic study, thermal transitions behavior and dissolution study. STP pharma sciences. 2003; 13: 281-6.
- Cruz-Angeles J, Videa M, Martínez LM. Highly soluble glimepiride and irbesartan co-amorphous formulation with potential application in combination therapy. AAPS Pharm Sci Tech. 2019; 20: 144.
- Sandri G, Bonferoni MC, Rossi S, Caramella CM, Ferrari F. Effects of particle size, surface nature and crystal type on dissolution rate. InParticles and Nanoparticles in Pharmaceutical Products. Springer, Cham. 2018; 303-328.