
Review Article
Austin Emerg Med. 2021; 7(1): 1069.
The Effect of Hydroxychloroquine on Mortality and Pneumonia Development in SARS-Cov-2 Positive Mildly Symptomatic Outpatients without Findings of Pneumonia
Kara A1*, Guner R2, Erdinç S3, Korukoglu G4, Tezer H5, Gul A6, Hayran M7, Temel F8 and Topal S8
1Department of Pediatrics, Hacettepe University School of Medicine, Turkey
2University of Health Sciences, Ankara Metropolitan Training and Research Hospital, Turkey
3University of Health Sciences, Ankara Training and Research Hospital, Turkey
4Turkish Ministry of Health, Virology Reference Laboratory, Ankara, Turkey
5Department of Pediatrics, Gazi University School of Medicine, Turkey
6Istanbul Universty, Istanbul Medical School, Turkey
7Department of Preventive Oncology, Hacettepe University School of Medicine, Turkey
8Turkish Ministry of Health, General Directorate of Public Health, Turkey
*Corresponding author: Ates Kara, Hacettepe University School of Medicine, Ihsan Dogramaci Children’s Hospital, Department of Pediatrics, Sihhiye/ Ankara, Turkey
Received: January 23, 2021; Accepted: February 23, 2021; Published: March 02, 2021
Abstract
Although a period longer than 10 months has passed since the detection of the first cases in and more than 40 million people have been diagnosed with COVID-19 worldwide, there is still no well-accepted and proven treatment choice for the novel coronavirus disease. This study aimed to retrospectively investigate cases in whom treatment had started due to detected as positive during screening and also having shown signs including fever, cough, shortness of breath, excessive malaise, fatigue or loss of smell-taste, without any findings of pneumonia between March 11, 2020, when the first cases were detected in Turkey, and the beginning of May, 2020.
A total of 19.276 SARS-CoV-2 PCR positive outpatients, within the first 48 hours of detection and had no findings in lung auscultation or radiology, were detected from the data of Health Information System. 9559 patients were males (49.6%) and 9717 were females (50.4%). An underlying disease considered in the risk group for COVID-19 was found in 1789 of the patients (8.8%). An underlying disease was present in 9.4% using hydroxychloroquine and in 9% not using hydroxychloroquine. 43 deaths (0.2%) were detected among all cases. Mortality in cases using and not using hydroxychloroquine was respectively 5 (in 12.293 cases) and 38 (in 6.983 cases).
It was confirmed that pneumonia developed in 2.080 of the patients (10.8%). This number was found as 1286 (10.5%) in cases using HQ and as 794 (11.4%) in cases not using HQ. In conclusion, since this study confirmed that hydroxychloroquine used in outpatients presenting in the early period without any symptoms of pneumonia can ensure survival and prevent pneumonia development particularly in young adults, we may speculate that the early use of hydroxychloroquine in mildly symptomatic patients results in a cost-effective and potent treatment.
Keywords: COVID-19; SARS-CoV-2; Treatment; Hydroxychloroquine
Introduction
The signs of COVID-19 pandemic started to emerge in the city of Wuhan in China with patients who presented with symptoms of viral respiratory tract infection and in whom no agent could be detected in December 2019. A short time after the evaluation of the first cases and detecting that these cases were associated with a live animal market, the agent was confirmed to be a coronavirus and was documented to possess similarities with another coronavirus, namely SARS-CoV, detected as an agent in 2002. A specific antiviral treatment has not been found against the coronaviruses causing SARS and MERS that became widely known in the 2000s and those showing seasonal prominence or being dominantly seen in the childhood period [1-3].
Discovered first in the 1930s, chloroquine has been widely used in malaria (for protection and treatment purposes) and in autoimmune disease like rheumatoid arthritis and systemic lupus erythematosus [4-7]. Its antecedent, an herbal form, had been used by the South American Natives centuries ago. Following the realization that this herbal powder obtained from the bark of the tree referred to as Cinchona was effective in malaria, it was brought to Europe in the seventeenth century, and this form had been used in the treatment of malaria for a very long time. In the meantime, it was discovered coincidentally that the powder was also useful in rheumatoid arthritis and systemic lupus erythematosus and could be used in treatment. Thanks to the studies, continuing after the Second World War hydroxychloroquine was produced following its modification through hydroxylation. Hydroxychloroquine is now preferred more due to the fact that it has similar effect and indication spectrum but less side-effect frequency [7]. Hydroxychloroquine reaches its peak plasma concentration in 3-4 hours, and chloroquine reaches the same concentration in half an hour [8,9]. Half-life of chloroquine and hydroxychloroquine is long, they can remain in the body for days and even weeks, and urinary excretion may continue up to 3 months [10,11].
There are a limited number of studies on the antiviral activity of chloroquine and hydroxychloroquine, and they were started to be used experimentally in the SARS-CoV-2 pandemic since it had already been established to have in-vitro activity in the epidemics caused by SARS-CoV-1 and MERS CoV [12-15]. It is considered that chloroquine and hydroxychloroquine have more than one effect mechanism on SARS-CoV-2. Their first effect is blocking the cellular ingestion of the host cell receptor, Angiotensin Converting Enzyme 2 (ACE2), by inhibiting its glycosylation [15-17]. Second, chloroquine and hydroxychloroquine disrupt the stability of intracellular pH and organelles by penetrating into endosome and lysosome and prevents the reproduction of the virus within the cell and infection by suspending protein catabolism, endocytosis, and exocytosis necessary for the replication and infection of the virus [18]. It has also been proven by previous studies that the anti-inflammatory and immunemodulator effects of these drugs increase antiviral activity in-vivo [19]. In studies conducted for the in-vitro activity of chloroquine, it has been shown that chloroquine and hydroxychloroquine are extremely effective in decreasing viral replication and could easily reach EC50 (50% of maximum-effective concentration) level with standard dosage [15,16,20]. EC50 level of chloroquine at the 48th hour showing in vitro activity against SARS-CoV-2 in Vero E6 cells has been determined as 1.13μM [16]. While the infection degree of chloroquine and hydroxychloroquine in Vero E6 cells was 0.01, their level of activity against SARS-CoV-2 was found respectively as EC50 2.71μM and 4.51μM [16]. Moreover, their antiviral activities increase thanks to the well-spread of these agents to tissues, and more specifically the lungs [15]. While chloroquine has been found more effective in vitro against the SARS-CoV-1 infection in previous studies, hydroxychloroquine has been proven to be more effective in studies conducted on the SARS-CoV-2 infection [15,21].
The first case in Turkey was confirmed in March 11, 2020, and the Coronavirus Scientific Advisory Board formed by the Ministry of Health published a diagnostic, treatment, and follow-up protocol upon its detection and was started to be used widely in the country. Within this framework, it was aimed to evaluate the possible effects of hydroxychloroquine by retrospectively analyzing the data of the patients followed until the second week of April 2020.
Materials and Methods
Primary healthcare is provided and followed by family practitioners in Turkey. Healthcare institutions with beds are comprised of those of the Ministry of Health, universities, private institutions, and foundations. Majority of the healthcare services are given by the state and a healthcare insurance system covering all citizens is enforced. As of the onset of the pandemic, all drugs used in inpatient and outpatient clinics in all treatment facilities have been offered free of charge by the Ministry of Health. Recommendations for treatment have been implemented pursuant to the COVID-19 (SARS-CoV-2 Infection) Guideline comprising all case evaluation and treatment recommendations published by the Coronavirus Advisory Board of the Ministry of Health of Turkey. Within this perspective, this study retrospectively investigated cases in whom treatment had swiftly started due to having being evaluated as having had contact, detected as positive during screening and also having shown signs including fever, cough, shortness of breath, excessive malaise, fatigue or loss of smell-taste, but not any findings of pneumonia between March 11, 2020, when the first cases were detected in Turkey, and the beginning of May, 2020.
In terms of case evaluation, mortality and pneumonia development were chosen as the main criteria, and 30-day survival and development of adverse effects were also discussed. In order to have a detailed distribution of age groups evaluating symptoms and course of the disease, the patients were grouped as 0-1 year, 2-11 years, 12-14 years, 15-18 years, 19-29 years, 30-39 years, 40-49 years, 50-59 years, 60-69 years, and 70 years and older. A secondary grouping was made for mortality evaluation and pneumonia development as follows: 15 years and younger, 15-39 years, 40-59 years, and 60 years and older. Patient records were accepted according to the physician’s notes, who examined and noted down patient’s history. When symptoms were investigated in the records, fever was confirmed upon measuring a temperature of 38°C and higher with contact-free thermometer in all patients. Furthermore, the records were scanned for the presence of cough, shortness of breath, loss of taste-smell, headache, malaise, muscle pain, and diarrhea, and whether these symptoms developed following the first positivity detection or not.
Since the use of hydroxychloroquine is recommended as 2x200 mg for 5 days pursuant to the Ministry of Health COVID-19 Guideline, all cases using hydroxychloroquine were evaluated as having received this dosage accordingly. In the follow-up of the cases, information regarding the presence of pneumonia development within the 14 days following PCR positivity was extracted from follow-up and medical records. Similarly, mortality until the 30th day of PCR positivity was also scrutinized.
Results
A total of 19.276 SARS-CoV-2 PCR positive outpatients, who were confirmed during filiation (contact tracing) and presented with no complications or were within the first 48 hours of complications and who had no findings in lung auscultation or radiology, were detected from the data of Health Information System. Nine thousand five hundred and fifty-nine patients were males (49.6%) and 9717 were females (50.4%). Other demographic data are presented in (Table 1).
In terms of underlying diseases, an underlying disease considered in the risk group for COVID-19 was found in 1789 of the patients (8.8%). An underlying disease was present in 1161 patients (9.4%) using Hydroxychloroquine (HQ) and in 628 patients (9%) not using hydroxychloroquine. The distribution of underlying diseases in the whole group and in the patients who used and did not use hydroxychloroquine was found respectively as follows: diabetes mellitus (DM) 476 (2.5%), 330 (2.7%), 146 (2.1%), hypertension 748 (3.9%), 502 (4.1%), 246 (3.5%), asthma 872 (4.5%), 544 (4.4%), 328 (4.7%), chronic renal failure 17 (0.1%), 11 (0.1%), 6 (0.1%), and cancer 66 (0.3%), 41 (0.3%) 25 (0.4%).
In terms of hydroxychloroquine use, it was found that 12.293 (63.8%) patients used hydroxychloroquine. In terms of mortality, 43 deaths (0.2%) were detected among all cases. Mortality in cases using and not using hydroxychloroquine was established respectively as 5 (in 12.293 cases) and 38 (in 6.983 cases, 0.5%). The age groups in which mortality occurred were as follows: one mortality in the 15-39 years group not using HQ, four mortalities in the 40-59 years group not using HQ, 38 mortalities in the 60 years and older group, of whom 33 did not use HQ and 5 did. In terms of comorbidity, while comorbid diseases were not found in 31 of the patients who died (31 deaths in 8.633 cases without comorbidities, 0.2%), a comorbid disease was seen in 12 of them (12 patients among 1084 cases with comorbid diseases, 0.7%). Details are given in (Table 2). In terms of pneumonia development, it was confirmed that pneumonia developed in 2.080 of the patients (10.8%). This number was found as 1286 (10.5%) in cases using HQ and as 794 (11.4%) in cases not using HQ. Pneumonia development in the age groups as per HQ use is given in (Table 3 and Figure 1).
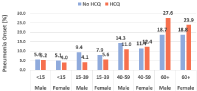
Figure 1: Effect of HQ on pneumonia onset.
Age group
Number
Sex
Hydroxychloroquine Use
Number (n)
Percentage (%)
Cumulative Percentage (%)
Male n (%*)
Female n (%*)
No n (%*)
Yes n (%*)
0 month-1 year
75
0.4
0.4
34 (45.3)
41 (54.7)
41 (54.7)
34 (45.3)
2-11 years
805
4.2
4.6
410 (50.9)
395 (49.1)
335 (41.6)
470 (58.4)
12–14 years
411
2.1
6.7
210 (51.1)
201 (48.9)
141 (34.3)
270 (65.7)
15-18 years
648
3.4
10.1
317 (48.9)
331 (51.1)
202 (31.2)
446 (68.8)
19-29 years
3835
19.9
30
1895 (49.4)
1940 (50.6)
1380 (36.0)
2455 (64.0)
30-39 years
4074
21.1
51.1
2068 (50.8)
2006 (49.2)
1443 (35.4)
2631 (64.6)
40-49 years
3550
18.4
69.5
1765 (49.7)
1785 (50.3)
1299 (36.6)
2251 (63.4)
50-59 years
2778
14.4
83.9
1362 (49.0)
1416 (51.0)
1048 (37.7)
1730 (62.3)
60-69 years
1964
10.2
94.1
983 (50.1)
981 (49.9)
714 (36.4)
1250 (63.6)
70 years and older
1136
5.9
100
515 (45.3)
621 (54.7)
380 (33.5)
756 (66.5)
*Row Percentage.
Table 1: Distribution of the age groups.
Male
Female
All Patients
Deaths
% Dead
Total N
Deaths
% Dead
Total N
Deaths
% Dead
Total N
Age Group
<15
Hydroxychloroquine Use
Absent
0
0.00%
269
0
0.00%
294
0
0.00%
563
Present
0
0.00%
460
0
0.00%
424
0
0.00%
884
Total
0
0.00%
729
0
0.00%
718
0
0.00%
1447
15-39
Hydroxychloroquine Use
Absent
0
0.00%
1494
1
0.10%
1485
1
0.00%
2979
Present
0
0.00%
2711
0
0.00%
2711
0
0.00%
5422
Total
0
0.00%
4205
1
0.00%
4196
1
0.00%
8401
40-59
Hydroxychloroquine Use
Absent
2
0.20%
1159
2
0.20%
1188
4
0.20%
2347
Present
0
0.00%
1968
0
0.00%
2013
0
0.00%
3981
Total
2
0.10%
3127
2
0.10%
3201
4
0.10%
6328
60+
Hydroxychloroquine Use
Absent
21
4.00%
524
12
2.10%
570
33
3.00%
1094
Present
2
0.20%
974
3
0.30%
1032
5
0.20%
2006
Total
23
1.50%
1498
15
0.90%
1602
38
1.20%
3100
All ages
Hydroxychloroquine Use
Absent
23
0.70%
3446
15
0.40%
3537
38
0.50%
6983
Present
2
0.00%
6113
3
0.00%
6180
5
0.00%
12293
Total
25
0.30%
9559
18
0.20%
9717
43
0.20%
19276
Comorbidity
Absent
19
0.20%
8854
12
0.10%
8633
31
0.20%
17487
Present
6
0.90%
705
6
0.60%
1084
12
0.70%
1789
All Patients
25
0.30%
9559
18
0.20%
9717
43
0.20%
19276
Table 2: 14-day mortality by age group, gender, comorbidity and HCQ use in COVID-19 patients monitorized with home care.
Male
Female
Total
Pneumonia count
Pneumonia percent
Total N
Pneumonia count
Pneumonia percent
Total N
Pneumonia count
Pneumonia percent
Total N
Hydroxychloroquine Use
Absent
15
5.60%
269
15
5.10%
294
30
5.30%
563
Present
24
5.20%
460
17
4.00%
424
41
4.60%
884
Total
39
5.30%
729
32
4.50%
718
71
4.90%
1447
15-39
Hydroxychloroquine Use
Absent
140
9.40%
1494
118
7.90%
1485
258
8.70%
2979
Present
111
4.10%
2711
153
5.60%
2711
264
4.90%
5422
Total
251
6.00%
4205
271
6.50%
4196
522
6.20%
8401
40-59
Hydroxychloroquine Use
Absent
166
14.30%
1159
135
11.40%
1188
301
12.80%
2347
Present
216
11.00%
1968
249
12.40%
2013
465
11.70%
3981
Total
382
12.20%
3127
384
12.00%
3201
766
12.10%
6328
60+
Hydroxychloroquine Use
Absent
98
18.70%
524
107
18.80%
570
205
18.70%
1094
Present
269
27.60%
974
247
23.90%
1032
516
25.70%
2006
Total
367
24.50%
1498
354
22.10%
1602
721
23.30%
3100
All ages
Hydroxychloroquine Use
Absent
419
12.20%
3446
375
10.60%
3537
794
11.40%
6983
Present
620
10.10%
6113
666
10.80%
6180
1286
10.50%
12293
Total
1039
10.90%
9559
1041
10.70%
9717
2080
10.80%
19276
Comorbidity
Absent
880
9.90%
8854
797
9.20%
8633
1677
9.60%
17487
Present
159
22.60%
705
244
22.50%
1084
403
22.50%
1789
All Patients
1039
10.90%
9559
1041
10.70%
9717
2080
10.80%
19276
Table 3: Pneumonia onset by age group, gender, comorbidity and HCQ use in COVID-19 patients monitorized with home care.
Table 4 summarizes factors constituting risks for pneumonia development and the effect of HQ. Factors that might have an impact on mortality and the effect of HQ are presented in (Table 5).
p-value
Odds Ratio (OR)
Lower
Upper
Hydroxychloroquine effect*
<0.001
14.39
5.54
37.4
Age Group (ref: <40)**
<0.001
40-59
0.113
5.88
0.66
52.72
60+
<0.001
116.5
15.87
856.11
Comorbidity presence
0.253
1.49
0.75
2.97
Gender (Male vs Female)
0.817
1.12
0.43
2.91
Hydroxychloroquine & Male Gender Interaction
0.36
2.44
0.36
16.46
*HCQ effect is described as the risk of mortality when HCQ is not used. I.e. the protective effect of HCQ use.
**Due to the very low number of events for mortality, a 3-tiered age grouping was used and no HCQ-age group interaction is introduced in the model.
Table 4: Factors associated with 14-day mortality in COVID-19 patients.
p-value
Odds Ratio (OR)
95% CI for OR
Lower
Upper
Hydroxychloroquine effect*
0.097
1.128
0.979
1.3
Comorbidity presence
<0.001
1.762
1.547
2.007
Age Group (ref: <15)
<0.001
15-39
0.02
1.359
1.05
1.758
40-59
<0.001
2.58
2.001
3.327
60+
<0.001
4.817
3.714
6.248
Gender (Male vs Female)
0.074
1.092
0.992
1.202
Hydroxychloroquine & Male Gender Interaction
0.021
1.253
1.035
1.518
Hydroxychloroquine & Age Interaction (ref:<15)
<0.001
Hydroxychloroquine additional effect for 15-39
0.081
1.582
0.945
2.649
Hydroxychloroquine additional effect for 40-59
0.854
0.953
0.574
1.584
Hydroxychloroquine additional effect for 60+
0.041
0.584
0.348
0.979
*HCQ effect is described as the risk of pneumonia when HCQ is not used. I.e. the protective effect of HCQ use.
Table 5: Laboratory values at hospital admission.
Discussion
There are a limited number of randomized-control and observational studies evaluating treatment with chloroquine and Hydroxychloroquine (HQ) in the COVID-19 disease [22-24]. In these studies, data on the efficacy of chloroquine and hydroxychloroquine are conflicting. These studies are criticized for not having undergone a peer-review, having small sample sizes and flawed methods [6]. Our study with a retrospective design can be considered suggestive with the presence of a relatively high number of patients. In our data, hydroxychloroquine use was continued for five days. When groups using and not using hydroxychloroquine were evaluated, the range of patient age was 0-101 years in the group using HQ and was 0-100 years in the group not using HQ. Mean and median age values of the patients using and not using HQ were found respectively as 40.5±18 and 39 years and 40.1±17.9 and 39 years. Considering that age has a significant impact on the course of the disease, both groups were evaluated similar. In addition, female to male ratio in patients using and not using HQ was determined as 49.3/49.7 and 49.7/49.3 and was accepted similar.
When age groups, route of transmission, and onset of symptoms of our patients were evaluated, the numbers were given separately for 0-1 year, 2-11 years, 12-14 years, 15-18 years, 19-29 years, 30-39 years, 40-49 years, 50-59 years, 60-69 years, and 70 years and older. 12-14 years and 15-18 years age groups were formed due to the fact that these two age groups presented with more adult characteristics. However, in group comparisons, the groups were formed in terms of literature data and clinical course as 15 years and younger (n: 1447), 15-39 years (n: 8401), 40-59 years (n: 6328) and 60 years and older (n: 3100). Age groups and characteristics are given in (Table 1).
In a short report published in February 2020, chloroquine was used in more than a hundred patients, and it was stated that chloroquine improved pulmonary findings in a shorter time compared to the control group, and virus clearance was ensured. However, no other detailed study was published regarding the study and control groups on this subject [22]. In a randomized control study performed during the early periods of the pandemic with 30 patients, the group receiving hydroxychloroquine (400mg/day, 5 days) and conventional treatment and the other group receiving only conventional treatment were compared, and a significant difference was not found in clinical results like fever and pulmonary imaging alterations and in 7 day viral clearance (87% vs. 95% p: >0.05) [23]. In another randomized control study comprising of 62 patients with mild symptoms (not receiving oxygen support despite findings in computerized tomography), earlier clinical improvement (cough: 2.0 days vs. 3.1 days; fever: 2c.2 days vs. 3.2 days) was shown in the group receiving standard treatment (antiviral agents, antibacterial agents, immunoglobulin, and corticosteroid) and hydroxychloroquine (400mg/day, 5 days) compared to the group receiving only the standard treatment [24]. A more significant improvement was confirmed in pneumonia findings radiologically (80% vs. 55%, p: <0.04) [24]. However, this study was published without peer review.
The recommendation of the Ministry of Health in Turkey was to use hydroxychloroquine the instant that the patient was suspected of COVID-19 possibility®. In this study, we retrospectively compared patients in whom hydroxychloroquine was started in the early period during sample collection and PCR positivity was confirmed thereafter and who did not show any clinical or radiological pulmonary findings and those that met the same conditions but did not receive treatment. Among the comparison criteria, we determined two main outcomes as targets: mortality and pneumonia development. (Table 2 and Table 3) respectively evaluates mortality and pneumonia development. Mortality in the group using hydroxychloroquine was seen in 5 patients in the 60 years and older age group; however, mortality in the group not using hydroxychloroquine was seen in one patient in the 15-39 years age group, four patients in the 40-59 years age group, and 38 patients in the 60 years and older age group (Table 2). In terms of first 14-day mortality, hydroxychloroquine use had a significant mortality-reducing effect in the 60 years and older age group. However, despite the advantageous impression of the 40-59 years age group due to low mortality rate, the effect could not be achieved as much. Since it is known that sex and the presence of comorbid diseases generally affect mortality, the early start of hydroxychloroquine was shown to have a positive effect on survival when the effects of these factors were eliminated (Table 4). Early use of hydroxychloroquine in patients aged 15 years and older provides an advantage for survival close to 14.4 folds as odds ratio. When pneumonia development was evaluated (Table 5), it was seen that pneumonia developed more distinctively in young adults and young male adults. It was also established that its protective impact decreased in patients aged 60 years and older. The preventive-protective effect of early use of hydroxychloroquine against pneumonia development in patients aged 15 years and older is remarkable.
Conclusion
In conclusion, since this study confirmed that hydroxychloroquine used in outpatients presenting in the early period without any symptoms of pneumonia and in the evaluation of those having been in contact with a patient can ensure survival and prevent pneumonia development particularly in young adults, we believe that early use of hydroxychloroquine in mildly symptomatic patients results in a costeffective and potent treatment. The study has some serious limitations including not having a prospective but a retrospective study design and no randomization. The effect of early use of hydroxychloroquine in well-defined, mildly-symptomatic patients will not be surprising considering the possible effect mechanism of hydroxychloroquine.
Ethical Approval
Approved by Ministry of Health Ankara Municipal Hospital Ethical Committee.
References
- Off-label use of medicines for COVID-19. 2020.
- Pastick KA, Okafor EC, Wang F, Lofgren SM, Skipper CP, Nicol MR, et al. Review: Hydroxychloroquine and Chloroquine for Treatment of SARS-CoV-2 (COVID-19). Open Forum Infect Dis. 2020; 7.
- Concordia Pharmaceuticals Inc. Plaquenil Hydroxychloroquine Sulfate Tablets. USP. 2013.
- Ducharme J, Farinotti R. Clinical pharmacokinetics and metabolism of chloroquine. Focus on recent advancements. Clin Pharmacokinet. 1996; 31: 257-274.
- Sullivian DJ. Chichona alkoloids: Quinine and quinidine. In: Staines HM, Krishna S, eds. Treatment and Prevention of Malaria: Antimalarial Drug Chemistry, Action and Use. 12th edition. Basel: Springer. 2012: 45-68.
- Shippey EA. Wagler VD, Collamer AN. Hydroxychloroquine: An old drug with new relevance. Cleve Clin J Med. 2018; 85: 459-467.
- Vincent MJ, Bergeron E, Benjannet S, Erickson BR, Rollin PE, Ksiazek TG, et al. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol J. 2005; 69.
- Yao X, Ye F, Zhang M, Cui C, Huang B, Niu P, et al. In Vitro Antiviral Activity and Projection of Optimized Dosing Design of Hydroxychloroquine for the Treatment of Severe Acute Respiratory Syndrome Coronavirus 2 (SARSCoV- 2). Clin Infect Dis. 2020; 71: 732-739.
- Wang M, Cao R, Zhang L, Yang X, Liu J, Xu M, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019- nCoV) in vitro. Cell Res. 2020; 30: 269-271.
- Yan Y, Zou Z, Sun Y, Li X, Xu KF, Wei Y, et al. Anti-malaria drug chloroquine is highly effective in treating avian influenza A H5N1 virus infection in an animal model. Cell Res. 2013; 23: 300-302.
- Al-Bari MA. Chloroquine analogues in drug discovery: new directions of uses, mechanisms of actions and toxic manifestations from malaria to multifarious diseases. J Antimicrob Chemother. 2015; 70: 1608-1621.
- Schrezenmeier E, Dorner T. Mechanisms of action of hydroxychloroquine and chloroquine: implications for rheumatology. Nat Rev Rheumatol. 2020; 16: 155-166.
- Colson P, Rolain JM, Lagier JC, Brouqui P, Raoult D. Chloroquine and hydroxychloroquine as available weapons to fight COVID-19. Int J Antimicrob Agents. 2020; 55: 105932.
- Biot C, Daher W, Chavain N, Fandeur T, Khalife J, Dive D, et al. Design and synthesis of hydroxyferroquine derivatives with antimalarial and antiviral activities. J Med Chem. 2006; 49: 2845-2849.
- https://covid19bilgi.saglik.gov.tr/tr/covid-19-rehberi.html
- Gao J, Tian Z, Yang X. Breakthrough: Chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci Trends. 2020; 14: 72-73.
- Chen J, Liu D, Liu L, Liu P, Xu Q, Xia L, et al. A pilot study of hyrdoxychloroquine in treatment of patients with coronavirus disease-19 (COVID-19). Journal of Zhejiang University. 2020; 49.
- Chen Z, Hu J, Zhang Z, Jiang S, Han S, Yan D, et al. Efficacy of hydroxychloroquine in patients with COVID-19: results of a randomized clinical trial. MedRxiv. 2020.
- Gautret P, Lagier JC, Parola P, Hoang VT, Meddeb L, Mailhe M, et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020: 105949.
- Gautret P, Lagier JC, Parola P, Hoang VT, Meddeb L, Sevestre J, et al. Clinical and microbiological effect of a combination of hydroxychloroquine and azithromycin in 80 COVID-19 patients with at least a six-day follow up: A pilot observational study. Travel Med Infect Dis. 2020; 101663.
- Molina JM, Delaugerre C, Le Goff J, Mela-Lima B, Ponscarme D, Goldwirt L, et al. No evidence of rapid antiviral clearance or clinical benefit with the combination of hydroxychloroquine and azithromycin in patients with severe COVID-19 infection. Med Mal Infect. 2020.
- Tang W, Cao Z, Han M, Wang Z, Chen J, Sun W, et al. Hydroxychloroquine in patients with COVID-19: an open-label, randomized, controlled trial. MedRxiv. 2020.
- Mahevas M, Tran VT, Roumier M, Chabrol A, Paule R, Guillaud C, et al. No evidence of clinical efficacy of hydroxychloroquine in patients hospitalized for COVID-19 infection with oxygen requirement: results of a study using routinely collected data to emulate a target trial. medRxiv. 2020.
- Chiotos K, Hayes M, Kimberlin DW, Jones SB, James SH, Pinninti SG, et al. Multicenter initial guidance on use of antivirals for children with COVID-19/ SARS-CoV-2. J Pediatric Infect Dis Soc. 2020.