
Research Article
Austin Emerg Med. 2022; 8(2): 1082.
The Protection of Probiotics on Lung Function in Long-Term Prognosis Amongseptic Children
Wang Y*
Department of Pediatrics, The People’s Hospital of Henan Province, China
*Corresponding author: Yu Wang, Department of Pediatrics, the People’s Hospital of Henan Province, No. 7, Weiwu Road, Zhengzhou 450003, Henan Province, China
Received: June 15, 2022; Accepted: July 18, 2022; Published: July 25, 2022
Abstract
Objectives: This study aimed to investigate whether administration of probiotics alleviated lung function damage in the long-term prognosis of septic children and mechanism of it.
Methods: 100 septic patients were randomly divided into two groups. In addition to standard therapy, they were prescribed oral probiotics or placebo. Another 40 healthy children were enrolled as healthy controls. All septic children were monitored and followed up for nine months. The percentages of NKT cells in blood as well as levels of IL-4, IL-10, IFN-γ, and IgE were assessed dynamically. Proportions of NKT cells in induced sputum were assessed and lung function tests were performed after discharge. Kaplan-Meier analysis was conducted to compare the outcomes between two septic groups. Multivariate linear regression was used to define potential associations of probiotics and percentage of NKT cells.
Results: A total of ninety-five patients survived sepsis and eighty-six children completed the follow-up. For both septic groups, concentrations of IL-4, IL-10, IFN-γ were high at admission and declined eight days thereafter. But IL-4 levels in treatment group decreased more noticeably compared with controls during post-discharge period, accompanied by a significantly larger increase in IL-10 levels. Similarly, the proportion of NKT cells from blood in septic patients dropped gradually as a whole, but parameters in controls got higher than the other group during the observation. Further, percentage of NKT cells of induced sputum from treatment group was lower considerably than controls in follow-up, close to the levels of healthy children.PEF% (peak expiratory flow) and FEV1/ FVC (forced expiratory volume in one second to forced vital capacity)increased obviously in treatment group at the same time. The cumulative event rate of CVA (Cough Variant Asthma) was lower in treatment group than the control, but there was no significant difference between them. Multivariate regression analysis showed probiotic use was negatively correlated with percentage of NKT cells.
Conclusion: Early probiotic application in septic children could promote the recovery of NKT cells and inflammatory factors in long term, which may protect lungs from secondary injury during follow-up.
Keywords: Probiotics; Lungs; Children; Long-Term Prognosis; NKT Cell
Introduction
Pediatric sepsis is a common clinical complication in Pediatric Intensive Care Units (PICUs). Although sepsis mortality has been decreasing because of improvements in intensive care medicine, patients may suffer long term complications that severely impair physiological development. The long-term prognosis of pediatric sepsis is believed to be closely tied to immune disorders [1,2]. Natural Killer T (NKT) cells are a specific subgroup of T-lymphocytes that share similarities with classical natural killer cells. They are regulated by the intestinal flora and play key roles in autoimmune diseases [3]. It was found NKT cells may increase abnormally and their activation can be dysregulated in sepsis for an extended period, dramatically increasing long-term risk of asthma. Therefore, we hypothesized that administration of probiotics to septic children could regulate the intestinal flora and maintain the number of NKT cells, thus protect lungs from sequential injury in long-term prognosis.
Subjects and Methods
Study Participants
One hundred children with new-onset sepsis admitted to the PICU (age 4 to 7 years) were enrolled in the study.
Diagnostic Criteria for Sepsis and Exclusion Criteria
All children met the criteria for sepsis according to the 2012 Surviving Sepsis Campaign with an estimated PICU stay of longer than 72 hours [4].
Exclusion criteria were as follows: [5] (1) congenital heart disease or other congenital deformities; (2) malignancies, diseases of the immune system, or inherited metabolic disorders; (3) history of administration of immunomodulators or immunosuppressors, chemotherapeutic agents, or blood products; (4) chronic lung diseases; (5) history of bronchial asthma; and (6) gasp or wheezing sounds following admission.
Study Design
Pediatric patients with sepsis were randomized into two groups. In addition to standard therapy, patients in the treatment group (n=50) were prescribed probiotics while those in the control group (n=50) were given an oral placebo with the same appearance and taste as the probiotics. The oral probiotics were Acidophilus lactobacillus tablets containing 5×106 colony-forming units/g each of the Chinese and Japanese strains of A. lactobacillus. The patients took tablets orally or by nasal feeding (one tablet three times daily for 30 days consecutively). In addition to probiotics, all septic children received standard concomitant therapy including antibiotics as determining by the attending physician. If the patient was ready for discharge before the study was completed, treatment was permanently discontinued.
Forty healthy children of similar age receiving routine physical examinations were enrolled for comparison. Exclusion criteria were as follows: (1) history of severe cardiopulmonary diseases and other systemic diseases including asthma, pulmonary tuberculosis, pleuritis, recurrent bronchitis, and allergic diseases; (2) history of respiratory tract infection in the past 6 months; and (3) history of administration of probiotics and other immunomodulators.
Randomization was performed using a computer-generated allocation schedule with a six-block design prior to study initiation. A sealed envelope with information on each child’s assigned group was provided. Three previously trained research assistants, who were not part of the study team, prepared medications (probiotic or placebo) for the nutritional unit. Neither the medical nor the nursing staff responsible for monitoring children nor the researchers were aware of treatment group allocation. An independent official on the data safety monitoring board held two sealed envelopes that revealed the randomization sequence without disclosing the treatment groups of other patients.
The clinical outcomes of all patients were observed during hospitalization and patients were followed up for at least 9 months. Episodes of diarrhea and symptoms related to probiotic consumption were also recorded at least daily during the study.
Clinical Outcomes and Measures
Clinical data: All pediatric cases with sepsis were evaluated using Pediatric Risk of Mortality (PRISM III) scores [6] within 24 hours of admission to the PICU. Primary diseases, history of allergy, family history of relevant diseases, and living environment were recorded. Information on other clinical outcome measures including mechanical ventilation, duration of ventilation, and length of stay in the PICU was collected. No limitations were imposed for the type or mode of ventilation.
Follow-up and observation endpoints: All surviving patients were followed up every month after discharge. Patients were asked whether they had a recurrent cough, gasp, shortness of breath, chest tightness, and predisposing factors. Additional questions related to time of onset, pulmonary symptoms upon onset, and treatments received. Patients were also asked about other allergic symptoms. Patients received regular physical examinations including chest X-ray and computed tomography scans to exclude pulmonary infection.
The primary endpoints were the percentages of NKT cells in the peripheral blood and in induced sputum. The secondary endpoints were serum levels of inflammatory factors, lung function indicators, and the incidence of Cough Variant Asthma (CVA) or bronchial asthma [7,8].
Assessment of NKT cell percentages in induced sputum [5,9]: Sputum samples were collected from septic children and healthy children at month6 during follow-up. Before sample collection, the oral cavity and the nasopharyngeal cavity were thoroughly cleaned to reduce contamination of the sputum samples. A solution of 4% sodium chloride (20mL) was added into an ultrasonic atomization inhaler to induce sputum production. The sputum sample was weighed and 1% dithiothreitol was added (4mL for each 1g of sputum) and mixed well. The samples were incubated at 37°C in a water bath for 15 to 20 minutes. The sample was gently oscillated on a vortex mixer once every 5 minutes. After adding 4×phosphate buffer, the oscillation was continued for 5 minutes. The sample was then passed through a 60-μm nylon membrane and centrifuged at 400g× for 5 minutes. The cell components were re-suspended in Phosphate-Buffered Saline (PBS). Total cell counts were determined using a hemocytometer. Cell viability was assessed by Try pan blue staining. Wright’s staining was performed after air drying. Five hundred nucleated cells excluding squamous epithelial cells were counted. Inflammatory cells in the induced sputum were classified and their counts were expressed as percentages. Sputum samples with cell viabilities above 50% and percentages of squamous epithelial cells below 20% were considered acceptable.
The cell suspension (100 μL) was stained with 10μL of fluorescently labeled monoclonal antibodies against CD3 (fluoresce in isothiocyanate) and CD56 (phycoerythrin) (Beckman-Coulter, Brea, CA, USA). The cells were incubated in the dark for 30 minutes and then washed twice with PBS. For the blank control, another 100μL of unstained cell suspension was analyzed flow cytometry (EPICS XL MCL, Beckman-Coulter).
Assessment of percentages of NKT cells and inflammatory cytokine levels: From each septic child, 5mL of anticoagulated blood was drawn under sterile conditions on day 0 and day 8 after admission. Additionally, blood samples were taken from them and healthy child at month 9during follow-up. The percentage of NKT cells in the peripheral blood was assessed by flow cytometry. Supernatant was collected after centrifugation and stored at -80°C. Serum levels of interleukin IL-4, IgE, IL-10, and interferon IFN-γ were measured by enzyme-linked immunosorbent assay.
Measurement of pulmonary function: Lung function tests were performed for septic children and healthy children at month 9 during follow-up. The percentage of peak expiratory flow to predicted value (PEF % pred), percentage of forced expiratory volume in one second (FEV1) to predicted value (FEV1 % pred), and percentage of FEV1 to forced vital capacity (FEV1/FVC) were recorded.
Statistical Analysis
With an average percentage of NKT cells in blood among septic patients, the aim to detect a 40% rise in control group, and a two-sided test with 5% significance and 80% power, the required minimum sample size was 50 patients in each study group. According to the exclusion criteria and consent of parents, 145 children were enrolled totally. Statistical analyses were carried out using STATA 8.0 (Stata Corp, College Station, TX, USA). Normally distributed data were expressed as means ± standard deviations (SDs). When the data were normally distributed, and the variance was homogeneous, a t-test was used to compare differences between means. When data were not normally distributed, the Kruskal-Wallis test was used to assess differences among groups. Differences in dichotomous outcomes were assessed using the chi-square test. Values of P<0.05 were considered statistically significant. Kaplan–Meier analyses with log-rank test were conducted to compare the incidence of outcomes between two septic groups. Multivariate linear regression was used to analyze the influence of multiple factors on percentages of NKT cells in induced sputum.
Ethics
The study was approved by the Ethics Committee of the Medical College of Zhengzhou University (2015-29). All parents of study participants were informed of the aims, requirements, and risks of the study and were advised that they could withdraw from the study at any time. Written informed was obtained prior to study enrolment. The study was registered in the Chinese Clinical Trial Registry (ChiCTR-OCC-15007215).
Results
Participant Characteristics
In treatment group, two patients died of sepsis in hospital and four patients were lost to follow-up. As for the controls, three patients died of sepsis in hospital and five patients were lost to follow-up. A total of 86septic children were included in the final analysis. The study and control groups had a mean follow-up period of 34.4±5.2 weeks and 33.8±6weeks, respectively. The two groups of septic children were comparable in terms of the following baseline parameters: gender, age, body weight, PRISMIII scores, distribution of primary diseases, percentage receiving mechanical ventilation, duration of mechanical ventilation, length of PICU stay, family history of asthma relevant diseases (bronchial asthma, chronic bronchitis, eczema, rhinallergosis, and chronic obstructive pulmonary disease in first- and second-degree relatives), history of allergy, and living environment (with or without unpleasant odor) (P>0.05) (Table 1) (Figure 1).
Treatment groupn=48
Control groupn=47
Gender(male/female)
26/22
20/27
Age(months)
59.7±10.7
58.9±10
Weight(kg)
23.2±6.3
23±5.6
PRISMscore
9.55±2.7
9.94±3.7
Etiology of sepsis(n, %)
Respiratory system infection
22,45.8%
23,48.9%
Central nervous system infection
8,16.7%
10,21.3%
Gastrointestinal system infection
6,12.5%
5,10.6%
Others
12,25%
9,19.1%
Mechanical ventilation(n,%)
14,29.2%
13,27.7%
Duration of ventilation(h)
80±12.6
84.5±10.5
Length of PICU stay(d)
6.5±2.7
7.4±2.7
Allergic history(n, %)
9,18.8%
7,14.9%
Family history(n, %)
4,8.3%
5,10.6%
Residential environment(n, %)
6,12.5%
8,17%
Table 1: Baseline characteristics of two septic groups (mean±SD).
Figure 1: Study flow diagram for children with sepsis.
Pulmonary Function Assessment
The PEF% pred of the treatment group was significantly increased compared with the control group (P<0.05) but remained lower compared with healthy children (P<0.05). The FEV1/FVC of the treatment group was considerably higher than that of the control group (P<0.05) and similar to the level of healthy children. There were no significant differences among the three groups in FEV1% pred (P>0.05) (Table 2).
PEF % pred
FEV1 % pred
FEV1/FVC (%)
Treatment group
91.3±4.9
92.6±5.4
92.1±4.4
Control group
86.1±8.0
90.9±6
87.7±6.9
Healthy group
94.9±2.9
93.6±3.6
94.2±3.2
p-value
0.0001△
0.0509
0.0001☆
Note: △☆Significant differences were observed between three groups, p<0.05;PEF % pred:percentage of peak expiratory flow to predicted value; FEV1 % pred: percentage of forced expiratory volume in one second to predicted value; FEV1/FVC: percentage of forced expiratory volume in one second to forced vital capacity
Table 2: Pulmonary function variables among three groups (mean±SD).
Percentages of NKT Cells in the Peripheral Blood and Induced Sputum
Initially, the percentages of NKT cells in the peripheral blood were comparable between two septic groups before intervention. Then the indicators were on downward trends throughout the observation. However, the proportion in control sgot significantly higher than treatment group on Day 8 and in follow-up (P<0.05). What’s more, the treatment group and healthy control group had similar percentages of NKT cells during follow-up (P>0.05). The percentages of NKT cells in induced sputum were also decreased dramatically in treatment group compared with control group, but they did not reduce into the levels of healthy group (P<0.05) (Table 3) (Figure 2).
CD3+CD56+ T cell(peripheral blood)
CD3+CD56+ T cell(induced sputum)
Treatment group
2.38±0.9
2.51±0.88
Control group
2.87±0.84
3.53±1.0
Healthy group
1.95±0.68
1.73±0.52
p-value
0.0003§
0.0001●
Note: §●Significant differences were observed between three groups, p<0.05.
Table 3: The percentage of NKT cells in blood and induce sputum (%).
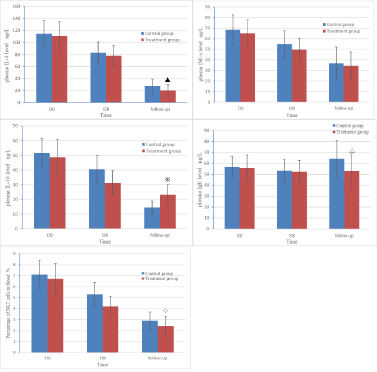
Figure 2: The changes of levels of inflammatory factors and percentage of NKT cells in blood among two septic groups.
Serum Levels of Inflammatory Factors
The concentrations of IL-4, IL-10 and IFN-γ in septic children were all in high levels at admission. Subsequently these indicators decreased by 20 percent or more on day 8 and no detectable differences in IL-4and IFN-γ levels were observed between groups (P>0.05). During follow-up, these three inflammatory factors continued to decline, but serum IL-4 levels in the treatment group became considerably lower compared with controls (P<0.05). Contrary to it, serum levels of IL-10 in treatment group were significantly higher than the control, but lower than healthy children (P<0.05).There were no significant differences among the three groups in serum levels of IFN-γ (P>0.05). Though IgE levels increased gradually in all the septic children after discharge, the concentrations for treatment group were lower than controls (P<0.05)and close to healthy children. (P>0.05) (Table 4) (Figure 2).
IL-4 (ng/L)
INF-γ(ng/L)
IL-10(ng/L)
IgE (µg/L)
Control group
26.8±11.1
37.4±12.9
14.5±4.1
63.4±15.1
Treatment group
19.9±8.6
32.9±14.5
23.7±6.4
52±16.2
Healthy group
15.3±5.9
33.8±13.4
35.3±7.1
50.7±16
p-value
0.0004♢
0.4064
0.0001#
0.0041*
Note: ♢#* Significant differences were observed between three groups, p<0.05
Table 4: The comparison of plasma inflammators among three groups (mean±SD).
Cumulative Incidence Analyses of CVA and Survival Analysis in Septic Children
During the follow-up period, children with CVA in the treatment and control groups had cumulative incidences of 4.6% (95% CI, 0.012-0.184) and 7% (95% CI, 0.023-0.217), respectively. Although a downward trend was observed for treatment group compared to the control, there was no significant difference between two groups (p=0.6130). In the Kaplan–Meier analysis, we did not find a better long-term survival rate in the study group with a log-rank test of p>0.05 (Figure 3,4).
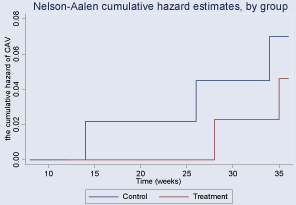
Figure 3: Cumulative Incidence by weeks after discharge of Cough Variant
Asthma (CVA) in two septic groups.
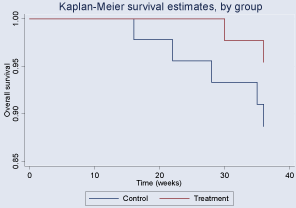
Figure 4: Kaplan–Meier survival analysis by weeks after discharge in two
septic groups.
Multivariate Regression Analysis of Percentages of NKT Cells in Induced Sputum
Univariate regression analysis showed that age, primary pulmonary infection, probiotic use, mechanical ventilation, duration of mechanical ventilation, family history of asthma relevant diseases, history of allergy, and living environment were important factors influencing the percentage of NKT cells in induced sputum from septic children (P<0.2). Multivariate linear regression analysis showed that the percentage of NKT cells in induced sputum was positively correlated with mechanical ventilation and history of allergy but negatively correlated with probiotic use p<0.05, (Table 5). Thus, probiotic use was an independent protective factor against increased levels of NKT cells (95% CI -1.3921~-0.6075).
Characteristic
Coefficient
Std. Err
t value
pvalue
95% CI
Age
-0.0142
0.0107
-1.33
0.189
-0.0356~0.0072
Pulmonary primary infection
0.3835
0.2082
1.84
0.071
-0.0338~0.8008
Mechanical ventilation
0.381
0.2645
1.44
0.155
-0.1490~0.9110
Duration of ventilation
0.0199
0.0094
2.13
0.038*
0.0012~0.0387
Use of probiotics
-0.9998
0.1958
-5.11
0.001*
-1.3921~-0.6075
Family history
0.3695
0.3691
1.00
0.321
-0.3702~1.1092
Allergic history
0.6306
0.3139
2.01
0.0015~ 1.2597
Living environment
0.3841
0.3088
1.24
0.219
-0.2348~1.0030
Costant term
0.8595
1.5860
0.54
0.590
-2.3189~4.0380
Note: *Significant differences, p<0.05.
Table 5: Multivariate regression analysis for percentage of NKT cells in induced sputum among septic children.
Discussion
Sepsis is one of the most common pediatric critical illnesses. Although more children with sepsis now survive because of advances in anti-infective and supportive treatments, they may suffer from long term complications. Approximately 23.8% of pediatric sepsis survivors do not fully recover [1]. Abnormal immune status during sepsis is considered an important factor influencing long-term outcome. The study in adult septic patients indicated that risk of ischemic stroke, intracranial hemorrhage and cardiovascular events remarkably increased during post-sepsis follow-up [11-13]. However, we discovered some survivors of pediatric sepsis usually developed recurrent or chronic cough after discharge. And they possessed varying degrees of abnormal lung functions, non-specific inflammatory airway manifestations and abnormal immunological indicators. Unfortunately, little information regarding long-term outcomes of sepsis has been reported by now. Therefore, we performed this study and found that probiotics could restore the continuous active NKT cells and inflammatory factors in long term, and then avoid the impairment of lung function during follow-up, which may be helpful for the improvement of prognosis in septic children.
Immune dysfunction during sepsis is induced by inflammatory and non-inflammatory factors [13]. Disruption of intestinal flora can perturb the immune system seriously. In children, the immune system is still at a developmental stage and the intestinal flora plays a vital role in regulating the numbers and functions of immune cells. Sometimes, imbalance of intestinal flora may cause more severe consequences in young children than sepsis itself [14,15]. According to a recent study [3], there exists a resident bacterium named Bacteroides fragilis in the normal gut. B. fragilis can generate a unique inhibitory sphingolipid to enhance the endogenous lipid antigen environment in the body and maintain numbers of NKT cells at low levels, thereby preventing autoimmune diseases. When severe infection occurs, the number of intestinal symbiotic bacteria may decline sharply. If intestinal symbiotic bacteria do not recover in the long term, survivors of pediatric sepsis may be at higher risk of allergic asthma and inflammatory bowel disease [16].
NKT cells are a special subset of immune cells unlike classical natural killer cells and T cells. NKT cells are not only involved in anti-tumor, anti-viral, and immunoregulatory responses but also play important roles in hypersensitivity and autoimmune diseases. NKT cells have been implicated in bronchial asthma in recent years. Once activated abnormally, NKT cells are rapidly recruited to the lungs, where they regulate the generation of inflammatory factors including IL-4, IL-10, and IFN-γ. Subsequently, the IL-4/IFN-γ ratio increases and IgE synthesis is accelerated. The differentiation and proliferation of eosinophils is subsequently enhanced. These cells migrate to the respiratory tract, accompanied by reduced secretion of IL-10 and impaired inflammatory responses. As a result, the Th1/Th2 balance is disrupted and the respiratory tract may be affected by non-specific inflammation or even episodes of asthma [5].
As non-pathogenic microbes, probiotics may help restore the number of symbiotic bacteria such as Bacteroidetes species and maintain intestinal homeostasis by inhibiting growth of harmful bacteria, thereby promoting immune recovery. At present, probiotics are used to treat a variety of autoimmune diseases [17]. Its efficacy has been demonstrated in improving prognosis for critically ill children at acute stage [18,19]. Now, this study showed us that abnormal increase of NKT cells during follow-up in septic children could also be mitigated by early probiotic administration. And IL-4 levels decreased dramatically in the probiotic-treated group compared with the control group, accompanied by an increase in IL-10 levels. Although probiotics did not reduce CVA incidence and mortality in septic children after discharge, it protected their lung functions effectively. It suggested probiotics could improve infection-related immunology dysfunction from hospitalization to post-discharge. Particularly, probiotics have the potential to help patients recover from sepsis fully and enhance post-discharge outcomes.
In clinical practice, the number of NKT cells may be affected by a variety of factors. Our univariate linear regression analysis showed that primary pulmonary diseases, probiotic use, mechanical ventilation, duration of mechanical ventilation, family history of relevant diseases, history of allergy, and living environment influenced the percentage of NKT cells in pediatric patients with sepsis. In addition to the common risk factors for asthma, local pulmonary diseases and treatment can both influence NKT cells. Multivariate regression analysis indicated that except for the history of allergy and mechanical ventilation, probiotic use was negatively correlated with the percentage of NKT cells in pediatric patients with sepsis. Thus, probiotic use was an independent protective factor against non-specific inflammation of the airway in pediatric patients with sepsis. While history of allergy and use of mechanical ventilation are not modifiable, probiotics become one of the tools available to regulate NKT cells.
There were several limitations to our study. Only a subset of in patients was included in the study. The cohort had few comorbidities and patients were admitted to our institution for new-onset sepsis. Another limitation was that follow-up was only for 9 months. Although our data suggest an improvement in the recurrence of CVA over time, the difference was not statistically significant. Further research is needed to better evaluate how CVA varies over longer time periods to determine whether probiotics contribute to this decline.
Despite these limitations, this study contributes to our understanding of various aspects of post sepsis syndromes [20]. For children with sepsis, efforts should focus both on life-saving treatments at acute stage as well as preventing the occurrence of anaphylactic diseases later in life. Probiotic use may improve longterm quality of life for children surviving sepsis.
References
- Killien EY, Farris RWD, Watson RS, Dervan LA, Zimmerman JJ. Health- Related Quality of Life Among Survivors of Pediatric Sepsis. Pediatric Critical Care Medicine. 2019; 20: 501-509.
- Delano MJ, Ward PA. The immune system’s role in sepsis progression, resolution, and long-term outcome. Immunological Reviews. 2016; 274: 330- 353.
- An D, Oh SF, Olszak T, Neves JF, Avci FY, Erturk-Hasdemir D, et al. Sphingolipids from a Symbiotic Microbe Regulate Homeostasis of Host Intestinal Natural Killer T Cells. Cell. 2014; 156: 123-133.
- Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, et a1. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 2013; 39: 165- 228.
- Koh Y, Shim J. Association between Sputum Natural Killer T Cells and Eosinophilic Airway Inflammation in Human Asthma. International Archives of Allergy and Immunology. 2010; 153: 239-248.
- Kaur A, Kaur G, Dhir SK, Rai S, Sethi A, Brar A, et al. Pediatric Risk of Mortality III Score – Predictor of Mortality and Hospital Stay in Pediatric Intensive Care Unit. Journal of Emergencies, Trauma, and Shock. 2020; 13: 146.
- Global Initiative for Asthma. Global strategy for asthma management and prevention main report 2019. https://ginasthma.org/reports/.2020 [accessed 15 MAY 2020].
- Chinese Medical Association for Pediatric Respiratory Group. The diagnosis for bronchial asthma in children and guideline. Chin J Pediatr. 2016; 54: 167- 81.
- Hui Y, Wang XM, Zhou YJ. Study on status of induced sputum T lymphocytes and NKT cells among bronchial asthma children during acute attack. Chin J Asthma. 2010; 4: 30-3.
- Boehme AK, Ranawat P, Luna J, Kamel H, Elkind MSV. Risk of Acute Stroke After Hospitalization for Sepsis: A Case-Crossover Study. Stroke. 2017; 48: 574-580.
- Yende S, Linde-Zwirble W, Mayr F, Weissfeld LA, Reis S, Angus DC. Risk of cardiovascular events in survivors of severe sepsis. American journal of respiratory and critical care medicine. 2014; 189: 1065-1074.
- Ou S, Chu H, Chao P, Lee Y, Kuo S, Chen T, et al. Long-Term Mortality and Major Adverse Cardiovascular Events in Sepsis Survivors. A Nationwide Population-based Study. American journal of respiratory and critical care medicine. 2016; 194: 209-217.
- Mostel Z, Perl A, Marck M, Mehdi SF, Lowell B,Bathija S,et al. Post-sepsis syndrome–an evolving entity that afflicts survivors of sepsis. Mol Med. 2019; 26: 6.
- Olszak T, An D, Zeissig S, Vera MP, Richter J, Franke A, et al. Microbial Exposure During Early Life Has Persistent Effects on Natural Killer T Cell Function. Science. 2012; 336: 489-493.
- Yoshida S, Ide K, Takeuchi M, Kawakami K. Prenatal and early-life antibiotic use and risk of childhood asthma: A retrospective cohort study. Pediatric Allergy and Immunology. 2018; 29: 490-495.
- Thomas DW, Greer FR. Probiotics and Prebiotics in Pediatrics. Pediatrics. 2010; 126: 1217-1231.
- Elazab N, Mendy A, Gasana J, Vieira ER, Quizon A, Forno E. Probiotic Administration in Early Life, Atopy, and Asthma: A Meta-analysis of Clinical Trials. Pediatrics. 2013; 132: e666-e676.
- Wang Y, Gao L, Zhang YH. Efficacy of probiotic therapy in full-term infants with critical illness, Asia Pac J Clin Nutr. 2014; 23: 575-80.
- Wang Y, Gao L, Yang Z, Chen F, Zhang Y. Effects of probiotics on ghrelin and lungs in children with acute lung injury: A double-blind randomized, controlled trial. Pediatric Pulmonology. 2018; 53: 197-203.
- Gritte RB, Souza-Siqueira T, Curi R, Machado MCC, Soriano FG. Why Septic Patients Remain Sick After Hospital Discharge?. Frontiers in Immunology. 2020; 11.