Review Article
Austin J Endocrinol Diabetes. 2014;1(2): 1007.
Sympathetic nerve activity in type 2 diabetes mellitus; a promising potential therapeutic target
Daisuke Kobayashi*, Hisayoshi Murai, Soichirou Usui, Shuichi Kaneko and Masayuki Takamura
Department of Disease Control and Homeostasis, Graduate School of Medical Science Kanazawa University, Kanazawa, Japan
*Corresponding author: Daisuke Kobayashi, assistant professor, Disease Control and Homeostasis, Graduate School of Medical Science, Kanazawa University, 13-1 Takara-machi, Kanazawa 920-8641, Japan,
Received: February 05, 2014; Accepted: February 21, 2014; Published: February 26, 2014;
Abstract
Augmented sympathetic nerve activity (SNA) has an important effect on various diseases. SNA is significantly related to glucose metabolism in type 2 diabetes mellitus (DM). Type 2 DM causes cardiovascular complications such as heart failure, arrhythmia, and myocardial infarction. These complications are reported to be associated with increasing SNA. Previous studies have not shown a beneficial effect of insulin and conventional sulfonylurea therapy on cardiovascular mortality and morbidity. In previous studies, the principal therapeutic target in type 2 DM is recognized as lowering glycemic control. However, there is a lack of evidence for improvement of SNA and glycemic control in type 2 DM. Therefore, additional therapeutic strategy is required to prevent major cardiovascular complications in type 2 DM. In this review, we reconsidered effect of conventional anti-diabetic drugs on SNA and also discuss the risk and benefit of using beta blockade in the treatment of type 2 DM patients.
Introduction
Augmented sympathetic nerve activity (SNA) has an important effect on various diseases such as heart failure [1,2], hypertension [3], metabolic syndrome [4], and type 2 diabetes mellitus (DM) [5]. In heart failure patients, the sympathetic activation initially plays a compensatory role in acute decompensate state but increased SNA in chronic state is associated with adverse consequences at both cardiac and vascular levels which may aggravate the clinical status and negatively affect prognosis [6]. Sympathoexcitation induces also fatal arrhythmia [2,7]. It is well known that reducing SNA with beta blockade improve prognosis in heart failure. Beneficial effects of beta blockade have been reported. In CIBIS–II trial, bisoprolol showed 44 percent risk reduction of death [8]. In COPERNICUS trial, 35 percent decrease in the risk of death with carvedilol compared with placebo [9]. Likewise in type 2 DM, it is considered to be crucial to improve augmented SNA, which might contribute to better prognosis.
Type 2 DM causes cardiovascular complications that are related to mortality and morbidity. Previous studies have not shown a beneficial effect of insulin and conventional sulfonylurea therapy on cardiovascular mortality and morbidity [10,11]. Therefore, additional therapeutic strategy is required to prevent major cardiovascular events in type 2 DM, and one of the targets might be sympathetic nerve activity.
In this review, we reconsidered effect of conventional antidiabetic drugs on SNA and also discuss the risk and benefit of using beta blockade in the treatment of type 2 DM patients.
Insulin Activates Sympathetic Nerve Activity
In type 2 DM patients sympathetic nerve activity is higher than normal subjects [12]. Anderson et al. reported that acute increase of plasma insulin elevated muscle sympathetic nerve activity (MSNA) in healthy young control [13]. There are three pathways by which insulin activates sympathetic nerve activity; one is a direct effect oncentral nervous system [14], the others is hypoglycemia by insulin [15], and finally, feedback mechanism against vasodilatation induced by insulin [13]. Increased insulin resistance observed in the patient, such as obese, requires more insulin which activates sympathetic nerve activity.
Many types of anti–diabetic drugs are available to lower blood glucose. However, little is known about anti–diabetic drugs affect sympathetic nerve activity for treatment of type 2 DM patients.
Relationship Anti–Diabetic Drugs With Sympathetic Nerve Activity
Sulfonylurea
It was shown that sulfonylurea stimulate beta cell in pancreas, leading to lowering blood glucose. Sulfonylurea developed positive inotropic effect and increasing blood pressure without the mediation of glucagon, insulin, or adrenaline in dogs [16]. In human study, glibenclamide therapy is associated with greater responses of blood pressure and higher nocturnal blood pressures [17]. In the study, plasma insulin levels were significantly higher during glibenclamide treatment. The relationship blood pressure and plasma insulin was unclear, but hypoglycemia might induce the response. There is no report about the direct effect on central nervous system. However, sulfonylurea might increase the secretion of insulin, which might contribute to sympathoexcitation.
Biguanide
Metformin significantly increased insulin–stimulated glucose transport by 2.6 fold in rats, resulting in improvement in insulin resistance [18]. Intravenous administration of metformin decreased arterial pressure and sympathetic nerve activity [19]. With improvement in insulin resistance, metformin decreased blood pressure in diabetic hypertension patients [20]. Resting MSNA, total body and right renal norepinephrine spillover did not differ significantly after placebo and metformin treatment [21]. Inmeta–analysis, metformin reduced systolic and diastolic pressure. Metformin treatment was associated with a significant improvement in cardiac sympathovagal balance [22].
Its effectiveness was seen especially in type 2DM patients with obesity. UKPDS study was performed in obese type 2 DM, cardiovascular event decreased in metformin group [11]. These results suggest that metformin affect not only diabetic profiles but also improvement in sympathetic activity, which contribute to favorable outcomes
Alpha–glucosidase inhibitor
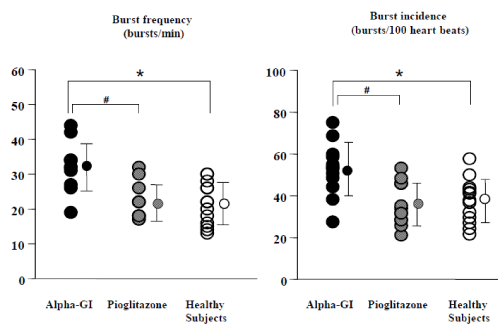
STOP–NIDDM trial showed that acarbose treatment in patients with impaired glucose tolerance (IGT) is associated with a significant reduction in the risk of developing diabetes, hypertension, and cardiovascular complications [23]. Acarbose acts as an alphaglucosidase inhibitor (GI) and slows the digestion of starch in the small intestine without reducing insulin release [24] or insulin sensitivity [25]. This study was performed in IGT subjects, so early intervention is associated with preventing progression of macro vascular disease. From our previous study, MSNA was significant higher in type 2 DM patient even treated with alpha–GI group compared to healthy control (Figure) [5].
Figure 1: Comparison of the burst frequency and burst incidence between DM treated with before and after pioglitazone, and healthy subjects. Both the burst frequency and burst incidence in DM patients treated with pioglitazone were significantly lower than those in alpha–GI. In addition, both MSNA in pioglitazone were similar to those in healthy subjects. The results are expressed as means ± SD. # p < 0.01 compared between alpha–GI and pioglitazone. ∗ p < 0.01 compared between alpha–GI and healthy subjects [3].
Thiazolidinedion
Proactive study showed that pioglitazone, which acts on peroxisome proliferator responsive elements, reduced the composite of all–cause mortality, non–fatal myocardial infarction, and stroke in patients with type 2 DM at a high risk of macrovascular events [26]. One possible explanation for the reduction in cardiovascular events is thought to be the improved serum insulin level and insulin resistance. In our previous study, pioglitazone significantly decreased MSNA andhomeostasis model assessment of insulin resistance index (HOMAIR) in DM patients compared with alpha–GI (Figure) [5]. In addition, a significant relationship was found between the absolute change in MSNA and HOMA–IR. These results indicate that improvement in insulin resistance is associated with reduction in sympathetic nerve activity.
Incretin related drugs
Glucagon like polypeptide–1 (GLP–1) is one of the incretin hormones, which act on pancreas to secrete insulin. In human, GLP–1 was reported to increase MSNA but did not affect cardiac sympathetic and parasympathetic indices, as assessed by spectral analysis [27]. Two types of incretin related drugs are developed; one is GLP–1 mimetic, the other is dipeptidyl peptidase–4 (DPP–4) inhibitor. Recently, Saxagliptin, one of DPP–4 inhibitors, did not improve the rate of cardiovascular events [28]. There is no study about how these drugs effect on sympathetic nerve activity. However, insulin secretion is promoted by these drugs, so that it is likely that SNA might be increased.
In terms of insulin secretion, these drugs are assumed to be divided into two groups. Sulfonylurea and incretin related agents are categorized as the drugs which stimulate insulin secretion. They might have a potential ability of sympathoexcitation via elevation of serum insulin. Metformin and pioglitazone are included in the drugs that improve the insulin resistance and the reducing requirement of insulin. Alpha–GI inhibits the overshoot of postprandil hyperglycemia, and does not affect insulin secretion or insulin sensitivity. So far, there is no report that former group improve cardiovascular event in the treatment of type 2 DM. Thus, it is important to consider the interaction of SNA and insulin.
The role of beta blockade in DM
The augmentation of sympathetic nerve activity in type 2 DM patients is mainly caused by elevated serum insulin induced by insulin resistance. From the point of view, thiazolidinedion or biguanide that improve insulin resistance are thought to be superior to the drugs which secrete insulin. Taken together, the role of beta blockade in treatment with DM is warranted to be reconsidered as potential therapeutic agents as well as diabetic agent. In fact, beta blockade negatively affects glucose metabolism. Insulin release is suppressed by blocking of beta 2 receptor. Blood flow of skeletal muscles is decreased by vasoconstriction with relative alpha effect. Insulin resistance is increased by decline of metabolism in the tissues in association with reduced blood flow to peripheral muscles. Kjekshus et al reported hat beneficial effect of beta blockade on one–year mortality was greater in MI patients with diabetes than without diabetes [29]. This fact indicated that the treatment with beta blockade provide an additional favorable effect in DM patients complicated with heart failure or ischemic heart disease. Insulin resistance was elevated by almost types of beta blockade. For avoiding these adverse effects to glucose metabolism, beta 1 selective type blockade was reported to be promising. Agent with alpha blockade effect is also thought to be better. Actually, in Glycemic Effects in Diabetes Mellitus; Carvedilol–Metoprolol Comparison in Hypertensives (GEMINI) trial, haemoglobin A1c elevated in metoprolol time dependently [30]. However, in carvedilol haemoglobin A1c did not change and improved insulin resistance. In COMET, new onset diabetes was lesser in carvedilol group than metoprolol group [31].
Conclusions
It is important to recognize that we should treat type 2 DM for the purpose of not only reducing blood glucose but also improving SNA. The anti–diabetic agent should be selected with considering insulin resistance related with sympathoexcitation. We also emphasized that beta blockade provide favorable outcomes to treat type 2 DM patients complicated with cardiovascular diseases.
References
- Murai H, Takamura M, Maruyama M, Nakano M, Ikeda T. Altered firing pattern of single-unit muscle sympathetic nerve activity during handgrip exercise in chronic heart failure. J Physiol. 2009; 587: 2613-2622.
- Ikeda T, Murai H, Kaneko S, Usui S, Kobayashi D. Augmented single-unit muscle sympathetic nerve activity in heart failure with chronic atrial fibrillation. J Physiol. 2012; 590: 509-518.
- Krum H, Lambert E, Windebank E, Campbell DJ, Esler M. Effect of angiotensin II receptor blockade on autonomic nervous system function in patients with essential hypertension. Am J Physiol Heart Circ Physiol. 2006; 290: H1706-1712.
- Lambert E, Dawood T, Straznicky N, Sari C, Schlaich M. Association between the sympathetic firing pattern and anxiety level in patients with the metabolic syndrome and elevated blood pressure. J Hypertens. 2010; 28: 543-550.
- Kobayashi D, Takamura M, Murai H, Usui S, Ikeda T, et al. Effect of pioglitazone on muscle sympathetic nerve activity in type 2 diabetes mellitus with α-glucosidase inhibitor. Auton Neurosci. 2010; 158: 86-91.
- Mancia G. Sympathetic activation in congestive heart failure. Eur Heart J. 1990; 11 Suppl A: 3-11.
- Grassi G, Seravalle G, Dell'Oro R, Facchini A, Ilardo V. Sympathetic and baroreflex function in hypertensive or heart failure patients with ventricular arrhythmias. J Hypertens. 2004; 22: 1747-1753.
- [No authors listed] . The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): a randomised trial. Lancet. 1999; 353: 9-13.
- Packer M, Coats AJS, Fowler MB, Katus HA, Krum H, et al. Carvedilol Prospective Randomized Cumulative Survival Study Group. Effect of Carvedilol on Survival in Severe Chronic Heart Failure. N Eng J Med. 2001; 344: 1651-1658
- NICE-SUGAR Study Investigators, Finfer S, Chittock DR, Su SY, Blair D. Intensive versus conventional glucose control in critically ill patients. N Engl J Med. 2009; 360: 1283-1297.
- [No authors listed]. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998; 352: 837-853.
- Huggett RJ, Scott EM, Gilbey SG, Stoker JB, Mackintosh AF. Impact of type 2 diabetes mellitus on sympathetic neural mechanisms in hypertension. Circulation. 2003; 108: 3097-3101.
- Anderson EA, Hoffman RP, Balon TW, Sinkey CA, Mark AL. Hyperinsulinemia produces both sympathetic neural activation and vasodilation in normal humans. J Clin Invest. 1991; 87: 2246-2252.
- Muntzel MS, Morgan DA, Mark AL, Johnson AK. Intracerebroventricular insulin produces nonuniform regional increases in sympathetic nerve activity. Am J Physiol. 1994; 267: R1350-1355.
- Hoffman RP, Sinkey CA, Anderson EA. Hypoglycemia increases muscle sympathetic nerve activity in IDDM and control subjects. Diabetes Care. 1994; 17: 673-680.
- Pogátsa G, Dubecz E. The direct effect of hypoglycaemic sulphonylureas on myocardial contractile force and arterial blood pressure. Diabetologia. 1977; 13: 515-519.
- Williams S, Abbott D, Morfis L, Manwaring P, Diamond T. Effects of glibenclamide on blood pressure and cardiovascular responsiveness in non-insulin dependent diabetes mellitus. J Hypertens. 1998; 16: 705-711.
- Matthaei S, Reibold JP, Hamann A, Benecke H, Haring HU, et al. In vivo metformin treatment ameliorates insulin resistance: evidence for potentiation of insulin-induced translocation and increased functional activity of glucosetransporters in obese (fa/fa) Zucker rat adipocytes. Endocrinology. 1993; 133: 04-311 .
- Petersen JS, Liu W, Kapusta DR, Varner KJ. Metformin inhibits ganglionic neurotransmission in renal nerves. Hypertension. 1997; 29: 1173-1177.
- Landin K, Tengborn L, Smith U. Treating insulin resistance in hypertension with metformin reduces both blood pressure and metabolic risk factors. J Intern Med. 1991; 229: 181-187.
- Gudbjornsdottir S, Friberg P, Elam M, Attvall S, Lonnroth P, et al. The effect of metformin and insulin on sympathetic nerve activity, norepinephrine spillover and blood pressure in obese, insulin resistant, normoglycemic, hypertensive men. Blood Press. 1994; 3: 394-403.
- Wulffelé MG, Kooy A, de Zeeuw D, Stehouwer CD, Gansevoort RT. The effect of metformin on blood pressure, plasma cholesterol and triglycerides in type 2 diabetes mellitus: a systematic review. J Intern Med. 2004; 256: 1-14.
- Chiasson JL, Josse RG, Gomis R, Hanefeld M, Karasik A. Acarbose treatment and the risk of cardiovascular disease and hypertension in patients with impaired glucose tolerance: the STOP-NIDDM trial. JAMA. 2003; 290: 486-494.
- Meneilly GS, Ryan EA, Radziuk J, Lau DC, Yale JF. Effect of acarbose on insulin sensitivity in elderly patients with diabetes. Diabetes Care. 2000; 23: 1162-1167.
- Matsumoto K, Yano M, Miyake S, Ueki Y, Yamaguchi Y. Effects of voglibose on glycemic excursions, insulin secretion, and insulin sensitivity in non-insulin-treated NIDDM patients. Diabetes Care. 1998; 21: 256-260.
- Dormandy JA, Charbonnel B, Eckland DJ, Erdmann E, Massi-Benedetti M, et al. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet. 2005; 366: 1279-1289.
- Bharucha AE, Charkoudian N, Andrews CN, Camilleri M, Sletten D. Effects of glucagon-like peptide-1, yohimbine, and nitrergic modulation on sympathetic and parasympathetic activity in humans. Am J Physiol Regul Integr Comp Physiol. 2008; 295: R874-880.
- Scirica BM, Bhatt DL, Braunwald E, Steg PG, Davidson J. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013; 369: 1317-1326.
- Kjekshus J, Gilpin E, Cali G, Blackey AR, Henning H. Diabetic patients and beta-blockers after acute myocardial infarction. Eur Heart J. 1990; 11: 43-50.
- Bakris GL, Fonseca V, Katholi RE, McGill JB, Messerli FH, et al. Metabolic effects of carvedilol vs metoprolol in patients with type 2 diabetes mellitus and hypertension: a randomized controlled trial. JAMA. 2004; 292: 2227-2236.
- Torp-Pedersen C, Metra M, Charlesworth A, Spark P, Lukas MA. Effects of metoprolol and carvedilol on pre-existing and new onset diabetes in patients with chronic heart failure: data from the Carvedilol Or Metoprolol European Trial (COMET). Heart. 2007; 93: 968-973.