Research Article
Austin J Endocrinol Diabetes. 2014;1(4): 1017.
The Effect on Bone Mineral Density in Patients with Osteoporosis and Obesity of Once-weekly Treatment with Risedronate/Vitamin D3 Combined in a Single Pill for 12 Months: A Post-marketing Study
Luis Beltrán-Lagunes1, Silvia Munguía-Lozano1 and Daniel López-Hernández1,2*
1Clinic of Family Medicine “Gustavo A Madero”, Institute of Security and Social Services for State Workers, Mexico
2Department of Biostatistical, Centro de Investigación y de Educación Continua, Mexico
*Corresponding author: Daniel López Hernández, Department of Biostatistical, Centro de Investigación y de Educación Continua (CENINVEC), Oyameles No. 30, Postal code 57820, Nezahualcóyotl, Mexico State, Mexico,
Received: June 24, 2014; Accepted: July 28, 2014; Published: July 29, 2014
Abstract
Several health authorities around the world propose conducting additional observational studies after controlled clinical trials as a part of the regulatory approval process of a drug for marketing. These studies, known as post-marketing surveillance studies, are intended give further insight into the safety, efficacy, and/or optimal use of the drug under the usual conditions of clinical practice. Limited post-marketing studies have been conducted on Risedronate. Therefore, the aim of this study was to evaluate and compare the effect of recent treatment with Risedronate (35 mg) and vitamin D3 (2800 IU) combined in a single pill on bone mineral density in female patients with postmenopausal osteoporosis. Patients were classified according to body mass index criteria. An open-label, observational, post-marketing surveillance study was conducted in a sample group of 345 osteoporosis patients. All patients were allocated according to CONSORT recommendations and treated for 12 months with the study drug. We considered overweight (144) and obese (116) patients as the intervention group and patients with normal weight (84) as the reference group. We excluded one underweight patient from the statistical analysis. Osteoporosis was detected using peripheral bone mineral densitometry. We calculated the number needed to treat for both improvement and no effect on bone mineral density, and we conducted the appropriate statistical analysis. In obese patients, the number needed to treat was 20 and 33, and for overweight patients, the corresponding number was 50 and 20. In conclusion, treatment with Risedronate (35 mg) and vitamin D3 (2800 IU) combined in a single pill showed the most favorable antiresorptive effect in obese osteoporotic patients. These data suggest that health authorities and clinicians should focus on implementing a clinical algorithm that includes screening and monitoring of osteoporotic patients with different body mass indices during treatment.
Introduction
Obesity and osteoporosis are considered to be public health problems worldwide [1], affecting approximately 130 or 500 million people according to the International Osteoporosis Foundation [2] and the World Health Organization [3] (WHO), respectively. Osteoporosis leads to more than 8.9 million fractures annually, of which over 4.5 million occur in Europe and America [4], and this disease is the most common metabolic bone disease in the United States and Mexico. A significant economic cost is associated with osteoporosis [5].
Obesity is the result of chronic excessive storage of body fat due to an imbalance between the intake and expenditure of energy [6]. Osteoporosis is characterized by decreased bone tissue per unit volume of bone [7] and the consequent microarchitectural deterioration of the bone [6-10]. This is associated with increased osteoclast activity, decreased osteoblast activity, or both [11]. Osteoporosis leads to excessive bone fragility and increased susceptibility to future bone fractures [6,8].
Bone remodeling is regulated locally by various hormonal factors, including estrogens, androgens and parathyroid hormone. This latter agent stimulates the hydroxylation of vitamin D in the kidneys and increases the absorption of calcium [11]. Some medical organizations have recommended that bone mineral density (BMD) should be determined in subjects with osteoporotic risk factors because the increase in bone fragility is asymptomatic [4,12]. Osteoporosis risk factors related to weight include a body mass index (BMI) between 20 and 25 kg/m2, body weight less than 40 kg, weight loss greater than 10% of usual body weight in young or adult subjects, or recent weight loss [12]. Bone densitometry (BD) is recognized by WHO as the reference method for the diagnosis of osteoporosis in postmenopausal women because it can be used to quantify BMD with acceptable accuracy and reproducibility [13]. Medical treatment for osteoporosis around the world recommends the use of bisphosphonates as the first-line agents [13]. Administration of Risedronate (5 mg) in postmenopausal women [13-16] every 24 hours for two or three years has been shown to reduce bone turnover [13-15] and increase bone mass in the lumbar spine, femoral neck and trochanter, and to increase diaphysis of the radius [16]. Compared to placebo, Risedronate reduces the risk of vertebral and non-vertebral bone fractures by 33- 65% [16,17]. Moreover, once-weekly doses of Risedronate at 35 mg once provide the same efficacy and safety [18]. All drugs used to treat postmenopausal osteoporosis must be accompanied by an adequate intake of calcium (1500 mg/day) and vitamin D (800 U/day) to prevent the accompanying increase in bone remodeling, bone loss and fractures [11].
The effectiveness of a medical drug is initially evaluated in controlled clinical trials, but these studies do not assess the drug under the usual conditions of clinical practice [19]. Several health authorities around the world propose conducting additional observational studies after controlled clinical trials as part of the regulatory approval process for a drug to be marketed. These studies, known as post-marketing surveillance studies, are intended to further understand the safety, efficacy, and/or optimal use of the drug [19]. Limited post-marketing studies of Risedronate have been conducted. Therefore, the aim of this study was to evaluate and compare the effect on BMD of recent, once-weekly treatment with Risedronate and vitamin D3 (35 mg/2800 IU) combined in a single pill in osteoporotic female patients from Mexico compared to BMI at the level of primary care.
Materials and Methods
Study design and patient enrollment
We conducted an open-label, observational, post-marketing surveillance study at the Clinic of Family Medicine (CFM) “Gustavo A. Madero” of the Institute of Security and Social Services for State Workers (ISSSTE: Instituto de Seguridad y Servicios Sociales de los Trabajadores del Estado, for its acronym in Spanish) in Mexico City. The study was conducted in accordance with Good Clinical Practices of our laws and the Helsinki Declaration. The protocol was approved by the appropriate ethics committee and an investigational review board (IRB). A total of 345 women (aged 40-99 years) were recruited between March 10, 2008 and April 2, 2009. All females were enrolled after osteoporosis was detected through outpatient services of family medical consultations. Patients were monitored through outpatient family medicine consultations. We also determined systolic and diastolic blood pressure (based on the 2007 recommendations of the European Society of Hypertension-European Society of Cardiology) and the Quetelet index (kg/m2) [20,21]. Serum concentration of glucose, total cholesterol (TC), low density lipoprotein cholesterol (LDL), high density lipoprotein cholesterol (HDL) and triglycerides (TG) were determined after 8-12 hours of fasting due to the higher prevalence of comorbidities. Exclusion criteria were: lacking a second BMD measurement or possible failure to complete one year of treatment.
Bone mineral density measurements and operational definitions
Bone mineral density was performed using the Omnisense 7000S device (Sunlight, Germany) according to the manufacturer’s recommendations. Baseline BMD was determined in the distal portion of the radius bone using an Omnisense 7000S bone densitometer. Subsequent BD was measured one year after starting treatment. Osteoporosis was defined according to the operational definition of WHO as a BMD value 2.5 standard deviations (SD) or more below that of the maximum density average value for a young normal adult (T score at or below 2.5 SD) [4]. Patients were considered to be overweight if they had BMI between 25 and 29.9 kg/m2 and were considered to be obese if they had BMI of 30 kg/m2 or higher [21]. We defined clinical improvement outcomes (increase in BMD) as T scores higher than the previous baseline densitometry values or changes in clinical diagnoses (from osteoporosis to osteopenia). Similarly, we considered worsening to be a clinical adverse outcome (decrease in BMD). Worsening was defined as a T score lower than the baseline densitometry value.
Drug information, dosage and administration
All patients received Risedronate (35 mg) and vitamin D3 (2800 IU) combined in a single pill and administered once-weekly for 12 months. Patients were instructed to take the study drug with at least 120mL of water, on an empty stomach, at least 30 minutes before breakfast. Patients were also to remain upright (sitting or standing) for at least 1 h, according to the recommendations of the data sheet [22]. Risedronate (35 mg) and vitamin D3 (2800 IU) combined in a single pill is a new drug approved and registered (Reg. Núm. 188 M2007, SSA IV) according to our laws with the Secretaria de Salud (SS; by its acronym in Spanish).
Analysis of clinical outcomes
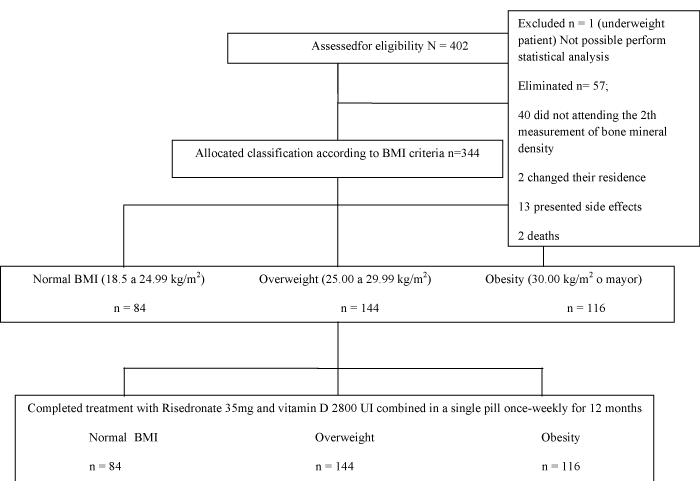
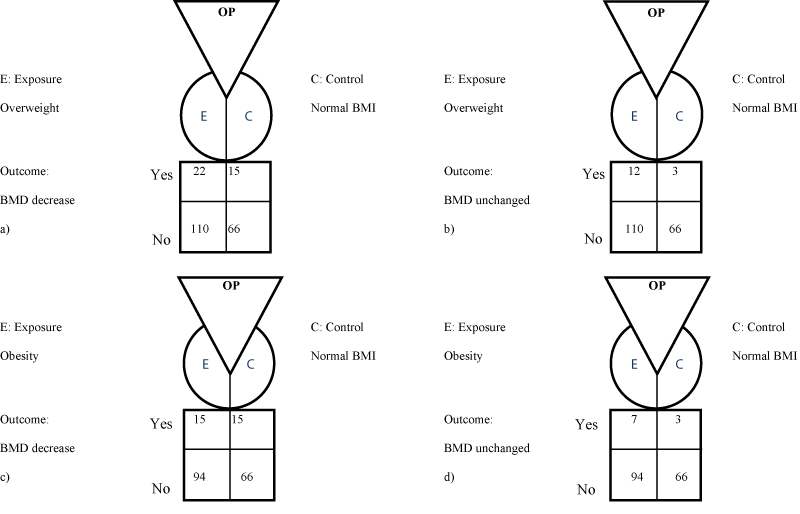
We used the Graphical Appraisal Tool for Epidemiology (GATE) frame to compare the effectiveness of treatment with Risedronate and vitamin D3 relative to BMI classification [23,24]. We then estimated: 1) absolute risk, 2) relative risk, 3) the number needed to treat (NNT), and 4) the number needed to harm (NNH). Therefore, we stratified patients based on the WHO criteria for BMI classification (Figure 1). To study the effect on bone mineral density of Risedronate and vitamin D combined in a single pill, we divided all patients into three subgroups (according to BMI) and two categories (depending clinical outcomes). Study subgroups included patients with osteoporosis and normal BMI as a reference group and osteoporotic females who were overweight or obese as study groups. Clinical outcomes were stratified to assess the effect of treatment on BMD in two categories. To prevent a decrease (worsening) in BMD, we evaluated the number needed to treat (NNT) to achieve improvement in patients. To determine the number of patients who showed no effect on BMD after one year of treatment, data were analyzed under the assumption that a persistent unchanged value of BMD was an adverse event (NNH). We thus analyzed four groups (Figure 2a-d).
Figure 1: Diagram of patient distribution for the study.
Figure 2: Graphical Appraisal Tool for Epidemiology (GATE) frame to compare the effectiveness of treatment with Risedronate 35mg and vitamin D3 2800 UI according to BMI. a) OP with overweight and decrease on BMD, b) OP with overweight and a BMD unchanged, c) OP with obesity and decrease on BMD, and d) OP with obesity and a BMD unchanged. BMI: Body Mass Index, BMD: Bone Mineral Density. OP: Osteoporotic Patients.
Statistical Analysis
Continuous variables are expressed as the mean (X) and standard deviation (SD) and were performed using the non-parametric Mann- Whitney U test. Comparisons between the first and second values of densitometry were performed using the Wilcoxon signed-rank test. Correlation between quantitative variables was analyzed by the Pearson correlation test. Categorical variables are described by absolute frequency and the corresponding 95% confidence interval and were compared using the Yates’s corrected chi square test. We used a logistic regression analysis to study the association between clinical outcomes of BMD with body mass index classification after treatment. The goodness-of-fit of the logistic regression model was assessed by the Hosmer-Lemeshow test. We calculated the absolute risk, relative risk, NNT and NNH. We considered a p value <0.05 to be statistically significant.
Results
General characteristics of study population and correlation analysis between bone mineral density and body mass index at baseline
A total of 345 females with osteoporosis were included in the study. Of these, 116 (34%, 29-39) were obese, 144 (42%, 37-47) were overweight, 84 (24%, 20-29) had a normal BMI and 1 (0.3%, 0.0 to 1) was underweight. The data from underweight patient were excluded from statistical analysis. The average patient age was 67 years (SD=9). More of 10% of the patients had other clinical comorbidities, including systolic arterial hypertension, type 2 diabetes mellitus, dyslipidemia, degenerative joint disease and peripheral vascular disease. A lower prevalence (1 to ≤ 10%) of patients had other co-morbidities such as hypothyroidism, peptic ulcer disease, chronic obstructive pulmonary disease, Parkinson’s disease, and previous traumatic fractures. The comorbidities with lowest frequency (<%) included cataracts, cholecystitis, congestive heart failure, epilepsy, cystocele, fibrocystic breast disease, rheumatoid arthritis, brain tumor, spastic colon, glaucoma, liver cirrhosis, hepatitis C, breast cancer, arrhythmia, carpal tunnel syndrome, systemic lupus erythematosus, ischemic heart disease, multiple myeloma, and mitral and aortic valve insufficiency.
No significant differences were observed for systolic and diastolic blood pressure, for TC, LDL and HDL serum concentrations, or for baseline BMD values among patients who had obese, overweight and normal BMI values. As shown in Table 1, only the mean TG and glucose concentrations were significantly lower for normal BMI subjects than for those patients who were obese or overweight. We found a non-significant correlation between BMD and BMI at baseline (r=-0.037, r2=0.0014, p=0.492).
Total population n=344
Mean (SD)
normal BMI n=84
Mean (SD)
Overweight n=144
Mean (SD)
Obesity n=116
Mean (SD)
Age
67 (9)
69 (10)
67 (8)
65 (9)
BMI
28.62 (4.92)
22.78 (1.61)
27.58 (1.38)
34.02 (3.47)
T score
-3.25 (0.56)
-3.26 (0.57)
-3.18 (0.52)
-3.31 (0.61)
Calcium
9.31 (0.58)
9.33 (0.37)
9.20 (0.63)
9.49 (0.15)
Total Cholesterol
189.88 (39.37)
188.85 (39.34)
190.55 (38.38)
189.53 (41.05)
LDLc
110.42 (37.98)
103.56 (42.87)
114.26 (37.92)
111.00 (34.43)
HDLc
41.95 (29.89)
49.76 (8.84)
39.45 (11.58)
39.05 (8.20)
Triglycerides
149.02 (74.20)
136.03 (84.62)*
152.45 (74.55)
153.22 (64.81)
SBP
124 (15)
123 (13)
124 (15)
124 (17)
DBP
77 (8)
76 (7)
77 (9)
78 (8)
Glucose
117.05 (43.43)
112.58 (49.82)**
116.32 (37.33)
121.37 (45.90)
Table 1: General characteristics of patients with osteoporosis at baseline, based on Body Mass Index.
Bone mass changes after 12 months of treatment with Risedronate (35 mg) and vitamin D (2800 UI)
After 12 months of treatment we observed significantly (Z=-12.648, p=1.15 x 10-36) higher bone mass density (Md=- 2.9, interquartile range=-3.2 to -2.8) compared to the baseline densitometry value (Md=-3.2, interquartile range=-3.6 to -2.8). Moreover, a greater percentage of patients (79%, 74-83) showed increased bone mass density than showed decreased (15%, 11-19) or unchanged BMD (6%, 4-9). As shown in Table 2, we observed that a higher percentage of patients improved independent of BMI; however, in all groups the percentage of patients with decreased bone mass density was greater than the percentage of patients who showed no change in BMD. We also found that a higher percentage of overweight patients showed decreased bone mass density compared to obese patients. We further observed more overweight and obese patients with unchanged BMD compared to patients with normal BMI. However, while a higher percentage of obese patients showed increased bone mass density compared to the other study groups, we did not find an association between baseline BMI and BMD outcomes at the end of treatment. This determination was made based on the likelihood models (Table 2).
Outcomes on bone densitometry; n, (%, 95%CI)
Univariate OR (95% CI)
BMI
Unchanged n=22
Decrease n=52
Improve n=271
unchanged
vs improve
Decrease
vs improve
Normal
(n=84)
3, (4%, 0-8)
15, (18%, 10-26)
66, (79%, 70-87)
1 (referencia)
1 (referencia)
Underweight
(n=1)
0
0
1 (100%)
NA
NA
Overweight
(n=144)
12, (8%, 4-13)
22, (15%, 9-21)
110, (76%, 69-83)
2.4 (0.7-9.0)
0.9 (0.4-1.8)
Obesity
(n=116)
7, (6%, 2-10)
15, (13%, 7-19)
94, (81%, 74-88)
1.7 (0.4-6.7)
0.7 (0.3-1.6)
Table 2: Comparison between different responses to treatment with Risedronate (35 mg) and vitamin D3 (2800 IU) combined in a single pill dosed orally, once weekly for 12 months, according to body mass index.
Clinical efficacy of treatment according to body mass index
As shown in Table 3, we observed a higher prevalence of decreased BMD in overweight patients (worsening) after treatment than in obese patients. We also found that 12 months of once-weekly Risedronate and vitamin D treatment must be administered to more overweight patients than obese patients in order for a clinician to expect to prevent one case of worsening bone mass. However, in obesity patients the number needed to treat to observe unchanged BMD was higher than for overweight patients (Table 4).
AR (95% CI)
RR (95% CI)
RRR (95% CI)
ARR (95% CI)
NNT
Control group
0.19 (0.10-0.27)
1 reference
1 reference
1 reference
1 reference
Overweight
0.17 (0.10-0.23)
0.90 (0.50-1.63)
0.10 (-0.63-0.50)
0.02 (-0.09-0.12)
50
Obesity
0.14 (0.07-0.20)
0.74 (0.39-1.43)
0.26 (-0.43-0.61)
0.05 (-0.06-0.15)
20
Table 3: Absolute Risk, Relative Risk, Relative Risk Reductions, Absolute Risk Reductions and values of Number needed to treat for patients with osteoporosis to prevent worsening of bone mineral density according to body mass index.
AR (95% CI)
RR (95% CI)
RRI (95% CI)
ARI (95% CI)
NNTh
Control group
0.04 (0.00-0.09)
1 reference
1 reference
1 reference
1 reference
Overweight
0.10 (0.05-0.15)
2.26 (0.66-7.74)
1.26 (-0.34-6.74)
0.05 (-0.02-0.13)
20
Obesity
0.07 (0.02-0.12)
1.59 (0.43-5.95)
0.59 (-0.57-4.95)
0.03 (-0.04-0.09)
33
Table 4: Absolute Risk, Relative Risk, Relative Risk Reductions, Absolute Risk Reductions and values of Number needed to treat for patients with osteoporosis and no effect on BMD after one year of treatment, according to body mass index.
Discussion
Osteoporosis is a global public health problem that mainly affects the female population: women experience a 40% risk of fractures compared to 13% in males [25]. The prevalence of osteoporosis in Mexico is 16% in women older than 50 years. This prevalence increases with age, affecting up to 80% of women over 80 years of age [25]. In 2005 the rates of osteoporotic fractures were 169 females and 98 males per 10,000 inhabitants [26].
Osteoporosis is the main factor leading to the increased incidence of hip fractures after the fifth decade of life [27]. Approximately 12 to 20% of females who suffer a hip fracture die during the first year of fracture evolution [25,28]. Osteoporotic fractures are responsible for most of the morbidity, mortality and cost of illness due to surgical complications and comorbid conditions [25,28]. Approximately 50% of patients lose their ability to walk independently and 30% are totally dependent on a family member or caregiver [28]. This renders many elderly patients disabled despite having their higher brain functions intact [25].
In the present study, we did not find a significant difference in baseline T-scores across the three groups despite substantial differences in body size. Furthermore, we did not find a correlation between BMD and baseline BMI. Our data on BMD in the distal radius and BMI are consistent with those from a previous study in females from Israel [29], but are different from data found in females from Japan [30] and Northern Thailand [31]. However, in these latter populations a low positive correlation has been reported between BMD in the distal radius with BMI [30, 31]. These results suggest that regions free of body weight as a load are only weakly influenced by tissue weight. This hypothesis is supported by the fact that regions such as the femoral neck or lumbar spine are directly influenced by tissue weight [29-33], which varies depending on the genetic background of each population. In addition to these results, several studies in Thai females [34] and Caucasian women from England [35], USA and Denmark [36,37] have described a significant correlation between BMD of the distal radius with BMD of the hip [34], lumbar spine, femoral neck [31] and Ward’s triangle [38]. The lowest mean BMD values are often observed at the distal forearm, as compared to BMD values in the hip and lumbar spine [37,39].
We did not observe correlations between baseline BMI and both clinical outcomes on BMD at the end of treatment, as determined by logistic regression analysis (Table 2). Moreover, no statistically significant difference was observed between the absolute and relative risks. Nevertheless, we observed higher bone mass densities after treatment compared to baseline densities. This effect of bisphosphonates on bone density has been widely studied by clinical trials. Several studies have shown that bisphosphonates are safe and effective for the treatment and prevention of postmenopausal and glucocorticoid-induced osteoporosis [13-18,40-52]. However, clinical trials do not evaluate the effectiveness of a therapeutic intervention under the usual conditions of clinical practice through outpatient consultations. The number needed to treat enables the measurement of the effectiveness of any given intervention. This statistical method could be used not only to describe the difference between the treatment and control groups but also to distinguish differences between several clinical outcomes. Number needed to treat can thus be used to describe any outcome where event rates are available for both a treatment and a control. On the other hand, post-marketing surveillance studies play important roles for the discovery of undesirable effects and can provide additional information on the benefits or risks of a drug. Such studies may also be used to identify whether treatment leads to a positive clinical effect or fails to induce the desired effect in different clinical conditions observed through outpatient consultation services. The primary objective of this analysis was to assess efficacy according to BMI.
Our results indicate that to prevent one case of worsening bone mass density, a clinician must treat 50 overweight patients once-weekly with Risedronate 35 mg and vitamin D 2800 UI for 12 months. However, 20 obese patients must be similarly treated to obtain the same outcome. We also observed that for every 20 overweight subjects treated with the study drug, one additional case of unchanged BMD will occur. A clinician would need to treat 33 patients, more than twice as many obese patients as compared to overweight subjects. These data suggest that females with osteoporosis and obesity respond better than overweight patients to treatment with Risedronate (35 mg) and vitamin D3 (2800UI), combined in a single pill, once-weekly for one year. Obese patients thus will have better prognoses. The prevalence of patients with improved BMD is greater in the obesity group as compared to the overweight group and even to those with normal BMI. These data are consistent with the observation that the incidence of osteoporosis and fracture is lower in obese patients [53].
Several factors can influence and increase osteoblast activity. These factors include physical activity, muscle, fat tissue level or simply increased weight and, therefore, increased bone mass. Additional weight can increase the mechanical load on the skeleton, particularly in the cortical elements. In response to this stress, the body promotes bone formation [1,5,25]. It has been suggested that leptin may directly promote the differentiation of osteoblasts and that it affects bone resorption [53].
Bisphosphonates are pyrophosphate analogue drugs that can bind to hydroxyapatite and inhibit bone resorption by inducing apoptosis in osteoclasts [13-16]. These drugs are considered to be highly potent antiresorptive agents and can bind to bone and remain there for many years [13]. Several studies have shown that Risedronate 5 mg, dosed once daily, can increase BMD after 2 to 3 years of treatment [14-16] and is effective in reducing the risk of vertebral and non-vertebral fractures [16,17,54].
In this study, we found that the changes generated by, or attributed to, Risedronate and vitamin D can be observed from the first year of treatment and that the response is different among patients with lower values of body mass index. Overall, we observed that a higher proportion of patients showed improvement (79%, 74-83), but we also observed a group of patients who did not show changes in BMD at the end of the treatment (6%, 4-9). However, the percentage of patients with decreased BMD (15%, 11-19) was higher than in the previous group. These findings are similar to the results observed by Chiodini I. et al., who reported 25.8 % of primary osteoporosis postmenopausal women inadequately responds to bisphosphonates, and showed that current smoking and baseline alkaline phosphatase total activity levels ≥ 66.5 U/L are associated with the inadequate response to bisphosphonates [55].
We thus suggest that health authorities and clinicians should focus on implementing a clinical algorithm to include screening and monitoring of osteoporotic patients with different body mass index values during treatment. Our data suggests that studies along this line of research will contribute to substantial changes in the way that patients with osteoporosis are managed. However, several studies report mono therapy administration of Risedronate (5 mg) every 24 hours or once-weekly doses increase bone mass in several parts of the body and reduce bone turnover and the risk of vertebral and non-vertebral bone fractures [13-17,54]. We can assume, by considering the high prevalence of patients with an increase on BMD after treatment it would have positive effects on reduction in the risk of fracture.
This study by its design includes information of several clinical co-morbidities that may associate and independently affects the response to the study drug. For instance, systolic arterial hypertension, type 2 diabetes mellitus, dyslipidemia as components of metabolic syndrome, and degenerative joint disease and peripheral vascular disease. Another factors may be affect the response of patients to risedronate, into these factors we may include hypothyroidism, peptic ulcer disease, systemic lupus erythematosus, but its prevalence was very low (≤ 10%). Vestergaard P demonstrated that the antiresorptive effect of alendronate, etidronate, and raloxifene do not seem associated with an increased risk of type 2 diabetes and postulated these three drugs may provide a protective effect related to the suppression of bone turnover [56]. By other hand, there are evidence that visceral adiposity and the metabolic syndrome have detrimental effects on bone health, such as a higher incidence of osteoporotic fractures and an impaired bone structure [57-59]. In addition Giustina A. et al., suggests that hyperglycemia is an important factor that has direct and indirect deleterious effects on osteoblast function and bone formation [59,60]. Even some animal’s models show that insulin resistance might associate with osteoporosis [61,62]. Moreover, the genetic background could to explain at least a part the response of patients to the study drug. Several studies have identified polymorphisms in some candidate genes that have been associated with bone mass or osteoporotic fracture, including the Vitamin D receptor, and the estrogen receptor or the collagen type I alpha-1 gene [63,64]. However, there are several genes and transcription factors involving with the bone formation and bone resorption in relation to the regulation of several cellular and molecular mechanisms that could to explain or elucidate the response of patients to the drugs, because they play an important role like regulators of biological process and gene expression, respectively.
Limits
The present study had the limitation than we did not include the comparative interventional group (placebo controlled group). In addition, we only described the changes on BMD before and after treatment with bone densitometer. Therefore, we could not ascertain the direct relationship between drug efficacy and fracture reduction. However, our results are similar of a post-marketing surveillance study in Korean postmenopausal women with osteoporosis [65].
In conclusion, our data suggest that under the usual conditions of outpatient family medicine consultation, a reasonable number of patients treated once-weekly with Risedronate (35 mg) and vitamin D3 (2800 IU) combined in a single pill will experience increased bone mineral density during the first year of treatment. We further conclude that a more favorable response can be expected in obese patients.
Acknowledgment
To Dr. Leticia Brito Aranda for reviewing the manuscript.
References
- Rosen CJ, Bouxsein ML. Mechanisms of disease: is osteoporosis the obesity of bone? Nat Clin Pract Rheumatol. 2006; 2: 35-43.
- International Osteoporosis Foundation. Osteoporosis Facts and Statistics. 2012.
- Organización Mundial de la Salud. Obesidad y sobrepeso. Centro de prensa, nota descriptiva No311; marzo. 2011.
- World Health Organization. WHO Scientific group of the assessment of osteoporosis at primary health care level. Summary Meeting Report Brussels, Belgium, 5-7 May 2004; Geneva. 2007.
- Riera-Espinoza G. Epidemiology of osteoporosis in Latin America 2008. Salud Publica Mex. 2009; 51 Suppl 1: S52-55.
- Zhao LJ, Jiang H, Papasian CJ, Maulik D, Drees B, Hamilton J, et al. Correlation of obesity and osteoporosis: effect of fat mass on the determination of osteoporosis. J Bone Miner Res. 2008; 23: 17-29.
- [No authors listed]. Consensus development conference: prophylaxis and treatment of osteoporosis. Br Med J (Clin Res Ed). 1987; 295: 914-915.
- Prentice A. Is nutrition important in osteoporosis? Proc Nutr Soc. 1997; 56: 357-367.
- Prentice A. Diet, nutrition and the prevention of osteoporosis. Public Health Nutr. 2004; 7: 227-243.
- Raisz LG. Pathogenesis of osteoporosis: concepts, conflicts, and prospects. J Clin Invest. 2005; 115: 3318-3325.
- Kasper DL, Braunwald E, Fauci AS. Harrison Principios de Medicina Interna 16ª ed. Editorial McGraw-Hill, 2005.
- Roig D, Valero C, Romera M, Rozadilla A, Mateo L, Juanola X, et al. [Prevalence of criteria indicating bone densitometry and risk factors for low bone mass and fracture in rheumatology outpatient units]. Reumatol Clin. 2005; 1: 12-19.
- Fernández-Ávila DG, Mora C, Reyes-Sanmiguel E. Tratamiento farmacológico de la osteoporosis postmenopáusica. Rev Colomb Reumatol. 2010; 17: 96-110.
- Mortensen L, Charles P, Bekker PJ, Digennaro J, Johnston CC Jr. Risedronate increases bone mass in an early postmenopausal population: two years of treatment plus one year of follow-up. J Clin Endocrinol Metab. 1998; 83: 396-402.
- Fogelman I, Ribot C, Smith R, Ethgen D, Sod E, Reginster JY, et al. Risedronate reverses bone loss in postmenopausal women with low bone mass: results from a multinational, double-blind, placebo-controlled trial. BMD-MN Study Group. J Clin Endocrinol Metab. 2000; 85: 1895-1900.
- Harris ST, Watts NB, Genant HK, McKeever CD, Hangartner T, Keller M, et al. Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. Vertebral Efficacy With Risedronate Therapy (VERT) Study Group. JAMA. 1999; 282: 1344-1352.
- Reginster J, Minne HW, Sorensen OH, Hooper M, Roux C, Brandi ML, et al. Randomized trial of the effects of risedronate on vertebral fractures in women with established postmenopausal osteoporosis. Vertebral Efficacy with Risedronate Therapy (VERT) Study Group. Osteoporos Int. 2000; 11: 83-91.
- Brown JP, Kendler DL, McClung MR, Emkey RD, Adachi JD, Bolognese MA, et al. The efficacy and tolerability of risedronate once a week for the treatment of postmenopausal osteoporosis. Calcif Tissue Int. 2002; 71: 103-111.
- Dirección General de Farmacia y productos Sanitarios. Estudios postautorización observacionales. Programa de Ensayos Clínicos de la Comunitat Valenciana, 1994-2008. Revista Estudios Clínicos y Observacionales de Productos Farmacéuticos de la Comunitat Valenciana. 2009; 1, no01: 1-9.
- Mancia G, De Backer G, Dominiczak A, Cifkova R, Fagard R, Germano G, et al. 2007 Guidelines for the Management of Arterial Hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2007; 25: 1105-1187.
- World Health Organization. Obesity: Preventing and managing the global epidemic. Report on a 16 WHO Consultation on Obesity, Geneva, 3-5 June, 1997. 1998.
- Diccionario de Especialidades Farmacéuticas, Risedronato grageas 5, 30 y 35 mg, Reg. Núm. 444 M2000, SSA IV; Thomson PLM. 2011.
- Jackson R, Ameratunga S, Broad J, Connor J, Lethaby A, Robb G, et al. The GATE frame: critical appraisal with pictures. Evid Based Med. 2006; 11: 35-38.
- Martin A, Srihari V. Geometrically evident: framing studies using the Graphic Appraisal Tool for Epidemiology (GATE). J Am Acad Child Adolesc Psychiatry. 2006; 45: 1521-1526.
- Padilla-Vázquez AV, Lamadrid-Figueroa H, Cruz-Valdez A. El peso, el porcentaje de grasa y la densidad mineral ósea materna son determinantes de la densidad mineral ósea en mujeres adolescentes y adultas jóvenes. Bol Med Hosp Infant Mex. 2007; 64: 72-82.
- Riera-Espinoza G. Epidemiology of osteoporosis in Latin America 2008. Salud Publica Mex. 2009; 51 Suppl 1: S52-55.
- Melton LJ 3rd. How many women have osteoporosis now? J Bone Miner Res. 1995; 10: 175-177.
- Padierna-Luna JL. Factores de riesgo y prevalencia de osteoporosis. Estudio por ultrasonometría del calcaneo. Med Int Mex. 2008; 24: 278-283.
- Steinschneider M, Hagag P, Rapoport MJ, Weiss M. Discordant effect of body mass index on bone mineral density and speed of sound. BMC Musculoskelet Disord. 2003; 4: 15.
- Arimatsu M, Kitano T, Kitano N, Inomoto T, Shono M, Futatsuka M. Correlation between forearm bone mineral density and body composition in Japanese females aged 18-40 years. Environ Health Prev Med. 2005; 10: 144-149.
- Namwongprom S, Ekmahachai M, Vilasdechanon N, Klaipetch A, Wongboontan C, Boonyaprapa S, et al. Bone mineral density: correlation between the lumbar spine, proximal femur and Radius in northern Thai women. J Med Assoc Thai. 2011; 94: 725-731.
- Baheiraei A, Pocock NA, Eisman JA, Nguyen ND, Nguyen TV. Bone mineral density, body mass index and cigarette smoking among Iranian women: implications for prevention. BMC Musculoskelet Disord. 2005; 6: 34.
- Murillo-Uribe A, Aranda-Gallegos JE, Río de la Loza-Cava MF, Ortíz-Luna G, Mendoza-Torres LJ, Santos-González J, et al. [Relation between body mass index and bone mineral density in a sample population of Mexican women]. Ginecol Obstet Mex. 1998; 66: 267-271.
- Limpaphayom K, Bunyavejchevin S, Taechakraichana N. Similarity of bone mass measurement among hip, spines and distal forearm. J Med Assoc Thai. 1998; 81: 94-97.
- Jones T, Davie MW. Bone mineral density at distal forearm can identify patients with osteoporosis at spine or femoral neck. Br J Rheumatol. 1998; 37: 539-543.
- Ravn P, Overgaard K, Huang C, Ross PD, Green D, McClung M, et al. Comparison of bone densitometry of the phalanges, distal forearm and axial skeleton in early postmenopausal women participating in the EPIC Study. Osteoporos Int. 1996; 6: 308-313.
- Nieves JW, Cosman F, Mars C, Lindsay R. Comparative assessment of bone mineral density of the forearm using single photon and dual X-ray absorptiometry. Calcif Tissue Int. 1992; 51: 352-355.
- Trivitayaratana W, Trivitayaratana P, Kongkiatikul S. Prediction of bone mineral density of lumbar spine, hip, femoral neck and Ward's triangle by forearm bone mineral density. J Med Assoc Thai. 2001; 84: 390-396.
- Iki M, Kagamimori S, Kagawa Y, Matsuzaki T, Yoneshima H, Marumo F, et al. Bone mineral density of the spine, hip and distal forearm in representative samples of the Japanese female population: Japanese Population-Based Osteoporosis (JPOS) Study. Osteoporos Int. 2001; 12: 529-537.
- Miller PD, Roux C, Boonen S, Barton IP, Dunlap LE, Burgio DE, et al. Safety and efficacy of risedronate in patients with age-related reduced renal function as estimated by the Cockcroft and Gault method: a pooled analysis of nine clinical trials. J Bone Miner Res. 2005; 20: 2105-2115.
- Rosen CJ, Hochberg MC, Bonnick SL, McClung M, Miller P, Broy S, et al. Treatment with once-weekly alendronate 70 mg compared with once-weekly risedronate 35 mg in women with postmenopausal osteoporosis: a randomized double-blind study. J Bone Miner Res. 2005; 20: 141-151.
- Boonen S, Orwoll ES, Wenderoth D, Stoner KJ, Eusebio R, Delmas PD, et al. Once-weekly risedronate in men with osteoporosis: results of a 2-year, placebo-controlled, double-blind, multicenter study. J Bone Miner Res. 2009; 24: 719-725.
- Chung HY, Chin SO, Kang MI, Koh JM, Moon SH, Yoon BK, et al. Efficacy of risedronate with cholecalciferol on 25-hydroxyvitamin D level and bone turnover in Korean patients with osteoporosis. Clin Endocrinol (Oxf). 2011; 74: 699-704.
- Reid DM, Hughes RA, Laan RF, Sacco-Gibson NA, Wenderoth DH, Adami S, et al. Efficacy and safety of daily risedronate in the treatment of corticosteroid-induced osteoporosis in men and women: a randomized trial. European Corticosteroid-Induced Osteoporosis Treatment Study. J Bone Miner Res. 2000; 15: 1006-1013.
- McClung MR, Miller PD, Brown JP, Zanchetta J, Bolognese MA, Benhamou CL, et al. Efficacy and safety of a novel delayed-release risedronate 35 mg once-a-week tablet. Osteoporos Int. 2012; 23: 267-276.
- Kishimoto H. [Efficacy and tolerability of risedronate for the treatment of osteoporosis]. Clin Calcium. 2008; 18: 1417-1426.
- Eastell R, Barton I, Hannon RA, Chines A, Garnero P, Delmas PD, et al. Relationship of early changes in bone resorption to the reduction in fracture risk with risedronate. J Bone Miner Res. 2003; 18: 1051-1056.
- Seibel MJ, Naganathan V, Barton I, Grauer A. Relationship between pretreatment bone resorption and vertebral fracture incidence in postmenopausal osteoporotic women treated with risedronate. J Bone Miner Res. 2004; 19: 323-329.
- Watts NB, Geusens P, Barton IP. Relationship between changes in BMD and nonvertebral fracture incidence associated with risedronate: reduction in risk of nonvertebral fracture is not related to change in BMD. J Bone Miner Res. 2005; 20: 2097-2104.
- Zoehrer R, Roschger P, Paschalis EP, Hofstaetter JG, Durchschlag E, Fratzl P, et al. Effects of 3- and 5-year treatment with risedronate on bone mineralization density distribution in triple biopsies of the iliac crest in postmenopausal women. J Bone Miner Res. 2006; 21: 1106-1112.
- Casado A, Ribas S, Pérez-Edo L. Metaanálisis de fracturas de cadera, vertebrales y de muñeca en mujeres posmenopáusicas con indicaciones preventivas o de tratamiento para osteoporosis con Risedronato o raloxifeno. Rev Esp Reumatol. 2004; 31: 462-474.
- Bahlous A, Bouzid K, Sahli H. Effet du risédronate sur les marqueurs du remodelage osseux chez les femmes ostéoporotiques ménopausées: Comparaison de deux protocoles de traitement. La tunisie Medicale. 2009; 87: 380-381.
- Hinojosa-Andia LJ, Berrocal-Kasay A. Relación entre obesidad y osteoporosis, en mujeres posmenopáusicas del Hospital Nacional Arzobispo Loayza. Acta méd. Peruana. 2007; 24: 172-176.
- Ellis AG, Reginster JY, Luo X, Cappelleri JC, Chines A, Sutradhar S, et al. Bazedoxifene versus Oral Bisphosphonates for the Prevention of Nonvertebral Fractures in Postmenopausal Women with Osteoporosis at Higher Risk of Fracture: A Network Meta-Analysis. Value Health. 2014; 17: 424-432.
- Cairoli E, Eller-Vainicher C, Ulivieri FM, Zhukouskaya VV, Palmieri S, Morelli V, et al. Factors associated with bisphosphonate treatment failure in postmenopausal women with primary osteoporosis. Osteoporos Int. 2014; 25: 1401-1410.
- Vestergaard P. Risk of newly diagnosed type 2 diabetes is reduced in users of alendronate. Calcif Tissue Int. 2011; 89: 265-270.
- Gilsanz V, Chalfant J, Mo AO, Lee DC, Dorey FJ, Mittelman SD, et al. Reciprocal relations of subcutaneous and visceral fat to bone structure and strength. J Clin Endocrinol Metab. 2009; 94: 3387-3393.
- von Muhlen D, Safii S, Jassal SK, Svartberg J, Barrett-Connor E. Associations between the metabolic syndrome and bone health in older men and women: the Rancho Bernardo Study. Osteoporos Int. 2007; 18: 1337-1344.
- Tamura Y, Kawao N, Yano M, Okada K, Matsuo O, Kaji H, et al. Plasminogen activator inhibitor-1 deficiency ameliorates insulin resistance and hyperlipidemia but not bone loss in obese female mice. Endocrinology. 2014; 155: 1708-1717.
- Mazziotti G, Bilezikian J, Canalis E, Cocchi D, Giustina A. New understanding and treatments for osteoporosis. Endocrine. 2012; 41: 58-69.
- Ogata N, Chikazu D, Kubota N, Terauchi Y, Tobe K, Azuma Y, et al. Insulin receptor substrate-1 in osteoblast is indispensable for maintaining bone turnover. J Clin Invest. 2000; 105: 935-943.
- Cao JJ, Sun L, Gao H. Diet-induced obesity alters bone remodeling leading to decreased femoral trabecular bone mass in mice. Ann N Y Acad Sci. 2010; 1192: 292-297.
- Obermayer-Pietsch B, Chararas C, Kotschan S, Walter D, Leb G. Genetic background of osteoporosis. Acta Med Austriaca. 2000; 27: 18-22.
- Stewart TL, Ralston SH. Role of genetic factors in the pathogenesis of osteoporosis. J Endocrinol. 2000; 166: 235-245.
- Suh HW, Kim HO, Kim YS, Sunwoo S, Lee JA; Korea Post-Marketing Surveillance Research Group, et al. The efficacy and safety of a combined alendronate and calcitriol agent (maxmarvil): a postmarketing surveillance study in korean postmenopausal women with osteoporosis. Korean J Fam Med. 2012; 33: 346-355.
Citation: Beltrán-Lagunes L, Munguía-Lozano S and López-Hernández D. The Effect on Bone Mineral Density in Patients with Osteoporosis and Obesity of Once-weekly Treatment with Risedronate/Vitamin D3 Combined in a Single Pill for 12 Months: A Post-marketing Study. Austin J Endocrinol Diabetes. 2014;1(4): 1017. ISSN: 2381-9200.