Research Article
Austin J Endocrinol Diabetes. 2014;1(5): 1023.
Legacy of Maternal Obesity
Fawwad A1*, Sabir R2, Riaz M3 and Basit A3
1Research Department, Baqai Medical University, Pakistan
2Laboratory Manager, Baqai Medical University, Pakistan
3Department of Medicine, Baqai Medical University, Pakis
*Corresponding author: Asher Fawwad, Baqai Medical University, Research Department, Baqai Institute of Diabetology and Endocrinology, Plot No. 1-2, II-B, Nazimabad No2, Karachi-74600, Pakistan,
Received: August 15 2014; Accepted: September 11, 2014; Published: September 12, 2014
Abstract
Purpose: To determine the frequency of different Uropathogens and their susceptibility pattern in patients with type 2 diabetes mellitus.
Method: All urine specimens of patients with type 2 diabetes mellitus received in Microbiology section of Clinical and Research Laboratory of Baqai Institute of Diabetology and Endocrinology during January to December 2012 were included in the study. Samples were cultured by standard methods by 1 μL loop on Cled medium with Andrade indicator and blood agar plates. Samples with more than 105 CFU/mL bacteria were considered as positive. Identification and susceptibility testing was determined by using the Clinical and Laboratory Standards Institute (CLSI) guidelines.
Results: Out of 67 urine specimens 33 (49.25%) were positive culture. The magnitude of positivity was differed significantly between females and males (20 male and 47 female). Uropathogens was more frequent in females 88% as compare to male 12% in patients with diabetes. Escherichia coli was the most frequent pathogen 72%, followed by Klebsiella pneumoniae 14%, Acinetobacter baumannii 6.9% and Proteus vulgaris 3.90%. Majority of gram negative uropathogens were highly resistant against Cefuroxime, nalidixic acid, norfloxacin, sulphamethoxazole, ciprofloxacin and cefotaxime. 90.48% of E. coli strains showed the high resistance towards cefuroxime, nalidixic acid and sulphamethoxazole and 85.71% towards norfloxacin and ciprofloxacin.
Conclusion: The most frequent isolate was Escherichia coli while Acinetobacter baumannii was less frequent but highly resistant. Imipenem, piperacillin/tazobactam, sulbactam / cefoperazone and ertapenem were most susceptible drugs against uropathogens in patients with diabetes.
Introduction
Diabetes mellitus is a metabolic disorder associated with long-term vascular complications leading to morbidity and mortality [1]. It is the fastest growing non-communicable disease throughout the world [1,2] and the fourth leading cause of death in most developed countries. Pakistan belongs to high prevalence area, having 6.6 million affected people, with projected estimates expected to 11.4 million by the year 2030 [3].
Urinary tract infections (UTIs) are one of the most common and the most frequently occurring infections in humans [4-7], counting for 7 million office visits, more than 1 million emergency room visits, and 100,000 hospitalizations each year [6]. Diabetes mellitus is associated with many complications and in the long run it has some major effects on the genitourinary system which makes diabetic patients more liable to Urinary tract infections (UTIs) and particularly to upper UTIs [8] Urinary tract infection is a significant problem both in community and Patients with diabetes. Patients with diabetes are at high risk to develop Urinary tract infections. The longer duration of diabetes with uncontrolled hyperglycemia is associated with the prevalence of bacteriuria [9]. According to a study from Netherlands 10% of these UTI occurs in patients with diabetes [10].
Escherichia coli (E. coli) is the most common isolates in 75% to 90% of UTI [5,11]. Other commonly reported organisms in urine cultures are Proteus, Klebsiella, Enterobacter, Pseudomonas species, Enterococcus species, streptococci, staphylococci and Candida albicans [11]. There are controversies on incidence, prevalence and microbiological features of UTI between diabetic and non-diabetic patients but various studies showed Escherichia coli as the most frequent causative agent of UTIs in patients with diabetes [5,9,12].
The knowledge regarding the prevalence of different microorganisms and antibiotics susceptibility is important for the treating physician so that the proper antibiotics can be prescribed.
Therefore the aim of the present study was to determine the frequency of different uropathogens and there antibiotics susceptibility pattern in patients with diabetes attending a tertiary care diabetes unit.
Material and Methods
Urine specimens of patients with type 2 diabetes from outpatient department and admitted patients at Baqai Institute of Diabetology and Endocrinology (BIDE) received in Microbiology section of Clinical and Research Laboratory of BIDE from January to December 2012 were included in the study. Clean-catch midstream urine specimens were received from 67 patients with diabetes (47 female and 20 male). Urine specimens were cultured by standard methods by 1 μL loop on Cled medium (Oxoid) with Andrade indicator and blood agar (Oxoid) plates [7,13-16] and were incubated at 37°C for 24 hours or overnight. Samples with more than 105 CFU/mL bacteria were considered as positive. Growth of Normal Skin Flora (NSF) was considered as insignificant [7]. Gram negative organisms were identified using Triple Sugar Iron (TSI), citrate utilization, Sulphide Indole Motility media (SIM) and urea hydrolysis [7,17]. Mueller- Hinton agar was used for antibiogram test [13,17]. Susceptibility testing was determined by using the Clinical and Laboratory Standards Institute (CLSI) guidelines recommended modified Kirby-Bauer disc diffusion method with commercial antibiotic discs (Oxoid).
The antimicrobial discs used for susceptibility testing were clavulanic acid (30 μg), piperacillin/tazobactam (110 μg), cefuroxime (30 μg), cefotaxime (30 μg), cefpirome (30 μg), sulbactam / cefoperazone(105 μg), aztereonam (30 μg), imipenem (10 μg), ertapenem (10 μg), amikacin (30 μg), nalidixic acid (30 μg), norfloxacin (10 μg), ofloxacin (5 μg), ciprofloxacin (5 μg) and sulphamethoxazole (25 μg) (Table 1).
Escherichia coli
Klebsiella pneumoniae
ANBIOTIC
S (%)
R (%)
S (%)
R (%)
CXM (30 μg)
9.52
90.48
0.00
100
NA (30 μg)
9.52
90.48
0.00
100
SXT (25 μg)
9.52
90.48
25
75
CIP (5 μg)
14.29
85.71
0.00
100
NOR (10 μg)
14.29
85.71
0.00
100
CTX (30 μg)
28.57
71.43
0.00
100
AMC (30 μg)
33.33
66.67
25
75
CPO (30 μg)
38.10
61.90
0.00
100
ATM (30 μg)
61.90
38.10
25
75
AK (30 μg)
76.19
23.81
75
25
SCF (105 μg)
90.48
9.52
100
0.00
TZP (110 μg)
95.24
4.76
75
25
ETP (10 μg)
95.24
4.76
100
0.00
IPM (10 μg)
100
0.00
100
0.00
Table 1: Antimicrobial susceptibility pattern of Escherichia coli and Klebsiella pneumonia.
Zone of inhibition ≥ 26 mm for cefotaxime and cefuroxime, ≥ 22 mm for aztereonam, ≥ 21 mm for piperacillin / tazobactam, cefpirome, sulbactam / cefoperazone and ciprofloxacin, ≥ 19 mm for norfloxacin, ≥ 18 mm for clavulanic Acid, ≥ 17 mm for amikacin and nalidixic acid, ≥ 16 mm for Imipenem, ofloxacin and sulphamethoxazole considered sensitive.
Statistical analysis
Analysis of data was done on Statistical Package for Social Sciences (SPSS), version 13.0. Data presented in the form of frequency and percentage.
Results
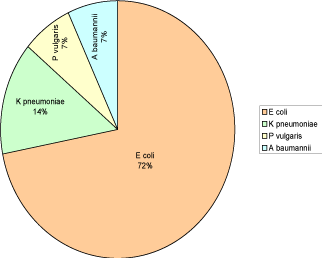
A total of 67 urine specimens (20 male and 47 female) were analyzed during the study period. Thirty three (49.25%) uropathogens were isolated from 67 urine specimens of patients with diabetes. High frequency of uropathogens was observed in female 88% than in male 12%. Gram negative bacilli were 88% and Candida was 12%. Escherichia coli was the most common pathogen isolated in 72% followed by Klebsiella pneumoniae 14%, Acinetobacter baumannii 6.9% and Proteus vulgaris 3.90% as shown in Figure 1.
Figure 1: Frequency of different uropathogens in patients with diabetes.
E. coli strains were mostly susceptible to imipenem (100%) followed by ertapenem and piperacillin/tazobactam (95.24%), sulbactam / cefoperazone (90.48%), amikacin (76.09%) and aztereonam (61.90%) as shown in Table 1.
K. pneumoniae strains showed high susceptibility towards imipenem, sulbactam / cefoperazone and ertapenem (100%) followed by piperacillin/tazobactam and amikacin (75%) as shown in Table 1.
Proteus vulgaris showed the highest susceptibility towards imipenem and aztereonam (100%) followed by Cefpirome and amikacin (50%). Acinetobacter baumannii strains were susceptible to sulbactam / cefoperazone only.
Discussion
E. coli was the most frequent uropathogen in patients with type 2 diabetes in our study, an observation supported by some other studies [8,9,11,18,19]. We found 72% of E.coli in urine specimens of patient with type 2 diabetes whereas it was 13% [11] in a Libyan and 28% in a study from Ethiopia [18]. K. pneumoniae was found to be the second frequent uropathogen in our study. The frequency of K. pneumoniae found in urine specimens of patient with type 2 diabetes vary in different reports. 6% in an Ethiopian study [18], 3.8% in a local study [9] and it was 14% in our study. Proteus vulgaris (non fermenting gram negative bacilli) was found in only 0.4% in an Indian study [4] where as our study results showed 3.90% Proteus vulgaris in urine specimens of patients with diabetes.
In our study majority of E.coli strains showed susceptibility toward imipenem, same findings obtained in a study from Bangladesh [20]. 95.24% and 90.48% of E.coli strains showed susceptibility toward Piperacillin/tazobactam and sulbactam / cefoperazone while it was 68.5% and 85% respectively in an Indian study [21].
We found 76.09% E.coli strains susceptible to amikacin. Whereas studies in Bangladesh 97% [4], 84.7% [20] of E coli strains were susceptible to Amikacin. Results of an Indian study showed 80.7% E coli susceptible to Amikacin [21]. 61.90% of E. coli strains susceptible to aztereonam in our study. It was as low as 22.2% in an Indian study [21]. 58% E. coli strains susceptible to clavulanic acid in a Libyan [5] and 42.6% in an Indian study [21]. It was only 33.33% in our study. E. coli strains showed susceptibility to cefotaxime 44.4% in an Indian study [21] and 28.57% in our study. Results of studies from Bangladesh showed 21% [4] and 25.0% [20], from Libya 68% [5], from Italy 91.6% [12] and from Africa 100% [22] of E coli strains susceptible to ciprofloxacin whereas 14.29% in our study. E. coli strains showed less susceptibility to cefuroxime in our study 9.52%. While it was high in two studies 86.7% [22] and 30.0% [20]. E. coli strains showed susceptibility to Nalidixic acid as low as 9.52% in our study, while 12.6% in a study from Bangladesh [20], 86.7% in a study from Africa [22] and 68% in a Libyan study [5]. Only 9.52% of E. coli strains showed susceptibility to sulphamethoxazole. It was 38.9% [21], 41.6%, [20], 68% [5] and 80.8% [12] in Indian, Bangladesh, Libyan and Italian studies respectively.
Majority of K. pneumoniae strains showed high susceptibility towards imipenem and sulbactam / cefoperazone in our study. Whereas it was 98.5% towards imipenem in a study from Cameron [23] and 87.9% towards sulbactam/cefoperazone in an Indian study [21]. Susceptibility of K. pneumoniae strains towards amikacin was 100% in a study from Bangladesh [4] 95.4% in a study from Cameron [23], 88.9% in Libyan study [5] and 75% in our study.
88.9% K. strains showed susceptibility towards piperacillin / tazobactam in an Indian study [21] while our study results showed 75% of K. pneumoniae strains susceptibility towards piperacillin / tazobactam.
25%of K. pneumoniae strains showed susceptibility towards Sulphamethethoxazole in our study while it was high in a Libyan study 83% [5].
K. pneumoniae strains showed susceptibility towards clavulanic acid 25% in our study whereas it was 56% [5] and 55.6% [21] in Libyan and Indian studies respectively. 38.2% K. pneumoniae sensitive to Aztereonam in an Indian study [21] and 25% in our study.
Conclusion
The most frequent isolate was Escherichia coli while Acinetobacter baumannii was less frequent but highly resistant. Imipenem, piperacillin/tazobactam, sulbactam / cefoperazone and ertapenem were most susceptible drugs against uropathogens in patients with diabetes.
Limitations
Our study has limitation, a relatively small number of specimens because it was a laboratory data base study.
References
- Imam K. Clinical features, diagnostic criteria and pathogenesis of diabetes mellitus. Adv Exp Med Biol. 2012; 771: 340-355.
- Akash MS, Rehman K, Chen S. An overview of valuable scientific models for diabetes mellitus. Curr Diabetes Rev. 2013; 9: 286-293.
- IDF diabetes atlas 5th edn, update 2012.
- Shill MC, Huda NH, Moain FB, Karmakar UK. Prevalence of uropathogens in diabetic patients and their corresponding resistance pattern: results of a survey conducted at diagnostic centers in dhaka, bangladesh. Oman Med J. 2010; 25: 282-285.
- Ghenghesh KS, Elkateb E, Berbash N, Abdel Nada R, Ahmed SF, Rahouma A, et al. Uropathogens from diabetic patients in Libya: virulence factors and phylogenetic groups of Escherichia coli isolates. J Med Microbiol. 2009; 58: 1006-1014.
- Gupta N, Kundra S, Sharma A, Gautam V, Arora DR. Antimicrobial susceptibility of uropathogens. J Infect Dis Antimicrob Agents. 2007; 24: 13-18.
- Kashef N, Djavid GE, Shahbazi S. Antimicrobial susceptibility patterns of community-acquired uropathogens in Tehran, Iran. J Infect Dev Ctries. 2010; 4: 202-206.
- Ivancic V, Mastali M, Percy N, Gornbein J, Babbitt JT, Li Y, et al. Rapid antimicrobial susceptibility determination of uropathogens in clinical urine specimens by use of ATP bioluminescence. J Clin Microbiol. 2008; 46: 1213-1219.
- Janifer J, Geethalakshmi S, Satyavani K, Viswanathan V. Prevalence of lower urinary tract infection in South Indian type 2 diabetic subjects. Indian J Nephrol. 2009; 19: 107-111.
- Janifer J, Geethalakshmi S, Satyavani K, Viswanathan V. Prevalence of lower urinary tract infection in South Indian type 2 diabetic subjects. Indian J Nephrol. 2009; 19: 107-111. Gorter KJ, Hak E, Zuithoff NP, Hoepelman AI, Rutten GE. Risk of recurrent acute lower urinary tract infections and prescription pattern of antibiotics in women with and without diabetes in primary care. Fam Pract. 2010; 27: 379-385.
- Qaiser S, Zeeshan M, Jabeen K, Ahsan T, Zafar A. Comparison of chromogenic urinary tract infection medium with cysteine lactose electrolyte deficient media in a resource limited setting. J Pak Med Assoc. 2011; 61: 632-635.
- Bonadio M, Costarelli S, Morelli G, Tartaglia T. The influence of diabetes mellitus on the spectrum of uropathogens and the antimicrobial resistance in elderly adult patients with urinary tract infection. BMC Infect Dis. 2006; 6: 54.
- Zia Sheikholeslami N, Hassanshahi G. The Frequency of Coagulase Negative Staphylococci Urinary Infections With Antimicrobial Resistance Pattern In Rafsanjan. PJMS. 2010; 26: 107-110.
- Linhares I, Raposo T, Rodrigues A, Almeida A. Frequency and antimicrobial resistance patterns of bacteria implicated in community urinary tract infections: a ten-year surveillance study (2000-2009). BMC Infect Dis. 2013; 13: 19.
- Abdullah FE, Memon AA, Bandukda MY, Jamil M. Increasing ciprofloxacin resistance of isolates from infected urines of a cross-section of patients in Karachi. BMC Res Notes. 2012; 5: 696.
- Khan BA, Saeed S, Akram A, Khan FB, Nasim A. Nosocomial uropathogens and their antibiotic sensitivity patterns in a tertiary referral teaching hospital in Rawalpindi, Pakistan. J Ayub Med Coll Abbottabad. 2010; 22: 11-12.
- Baral P, Neupane S, Marasini BP, Ghimire KR, Lekhak B, Shrestha B. High prevalence of multidrug resistance in bacterial uropathogens from Kathmandu, Nepal. BMC Res Notes. 2012; 5: 38.
- Yeshitela B, Gebre-Selassie S, Feleke Y. Asymptomatic bacteriuria and symptomatic urinary tract infections (UTI) in patients with diabetes mellitus in Tikur Anbessa Specialized University Hospital, Addis Ababa, Ethiopia. Ethiop Med J. 2012; 50: 239-249.
- Niveditha S, Pramodhini S, Umadevi S, Kumar S, Stephen S. The Isolation and the Biofilm Formation of Uropathogens in the Patients with Catheter Associated Urinary Tract Infections (UTIs). J Clin Diagn Res. 2012; 6: 1478-1482.
- Md Hamza Saber, Lovely Barai, J Ashraful Haq, Md Shariful Alam Jilani, Mrs Jaheda Begum. The Pattern of Organism Causing Urinary Tract Infection in Diabetic and Non Diabetic Patients in Bangladesh. Bangladesh J Med Microbiol. 2010; 4: 6-8.
- Aswani SM, Chandrashekar U, Shivashankara K, Pruthvi B. Clinical profile of urinary tract infections in diabetics and non-diabetics. Australas Med J. 2014; 7: 29-34.
- Bissong ME, Fon PN, Tabe-Besong FO, Akenji TN. Asymptomatic bacteriuria in diabetes mellitus patients in Southwest Cameroon. Afr Health Sci. 2013; 13: 661-666.
- Njunda Anna Longdoh, Jules Clement Nguedia Assob, Shey Dickson Nsagha, Peter Fon Nde, Henry Lucien Fouamo Kamga, Ajebe Franklin Nkume, et al. Uropathogens From Diabetic Patients With Asymptomatic Bacteriuria And Urinary Tract Infections. The West London Medical Journal 2013; 5: 7-14.