
Research Article
Austin J Endocrinol Diabetes. 2023; 10(1): 1101.
Obesity May Impairs Mice Testicular Structure by STAT3/CYP19A1/Testosterone
Mengnan Lu¹; Ruoyang Feng²; Yanfeng Xiao¹*; Chunyan Yin¹*
¹Department of Pediatrics, The Second Affiliated Hospital of Xi’an Jiao tong University, China
²Department of Joint Surgery, HongHui Hospital, Xi’an Jiao tong University, China
*Corresponding author: Yanfeng Xiao Department of Pediatrics, The Second Affiliated Hospital of Xi’an Jiao tong University, China.
Chunyan Yin Department of Pediatrics, The Second Affiliated Hospital of Xi’an Jiao tong University, China Email: xiaoyanfenggroup@sina.com
Received: July 10, 2023 Accepted: August 16, 2023 Published: August 23, 2023
Abstract
Obesity is an increasing serious global public health issue and a risk factor for male infertility. Aromatase expression level is elevated in obese men, and this high level is as-sociated with diminished serum testosterone levels. We explored the underlying mechanism of infertility in obese men. Genome-wide association study datasets were used to predict the causal relationship between obesity and male infertility. An obese male mouse model was generated by feeding mice a high-fat diet while control model was generated by feeding mice a normal chow diet. Serum indicators including free fatty acids, insulin, Follicle-stimulating hormone, and testosterone were measured using ELISA, and impaired fertility in mice was demonstrated using immunohistochemistry. The expression levels of STAT3 and CYP19A1 were measured by RT-qPCR and western blotting, respectively. Simultaneous bioinformatics analysis of publicly available data was performed using the R programming language and JASPAR database. Obesity might lead to male infertility from a genomic perspective. Obese mice displayed reduced serum testosterone and follicle stimulating hormone levels along with structural abnormalities, ectopic lipid deposition, and chronic inflammation in the testes. Upregulation of STAT3/CYP19A1 expression in obese mice was linked to reduced serum testosterone levels and impaired fertility. Our observation may support the hypothesis that since the elevation of STAT3 expression in obesity, the promoted expressed CYP19A1 could increase a shift from testosterone to estrogens. The persistently elevated level of estrogen will further stimulate STAT3, which may constitute a positive feedback loop. These findings provide new insights into the mechanisms of obesity-mediated male fertility impairment.
Keywords: STAT3; CYP19A1; Testosterone; Obesity; Male fertility impairment
Introduction
Obesity is an increasingly serious global public health issue [1]. The prevalence of obesity in children has increased rapidly [2]. Rising global obesity in childhood has profound implications, including obesity in adulthood, nonalcoholic fatty liver disease, type 2 diabetes, cardiometabolic disease, sleep apnea syndrome, and other conditions [3]. Childhood and adolescence are critical periods of growth and development, and childhood obesity is associated with penile and testicular dysplasia [4]. Compared to healthy controls, obese adolescents display significantly higher body mass index (BMI; 27.1±2.2 kg/m2, P<0.05), shorter natural penile length (5.6±1.7 cm, P<0.05), smaller testicular volume (7.6±2.3 cm3, P<0.05), and lower level of spermatogenesis (chi2 = 17.335, P<0.05) [5]. Epidemiological studies have provided some clear evidence that obesity impacts negatively on male fertility [6]. A cross-sectional study of 4400 infertile men in the United States has reported a significant negative association between obesity and semen parameters. The authors also noted that the occurrence of azoospermia and oligospermia was more prevalent in obese men [7].
Whether obesity affects male fertility potential is unclear, with some studies suggesting that BMI is not related to the quality of sperm parameters [8], while others suggest that obesity affects semen quality to some extent [9]. Obesity may diminish male fertility and reproductive potential, and it is associated with erectile dysfunction, poor semen quality, subclinical prostatitis, and other deleterious conditions [10]. High circulating levels of leptin is one of characterizing feature of obesity, which is associated with low testosterone in men [11]. Although it might be explained that the imbalanced leptin levels increase the aromatase activity, studies are needed to establish the molecular mechanism between leptin and testosterone in obese men. Male infertility is a major global health problem. However, the mechanisms by which obesity affects male fertility remain unclear.
Lifestyle can influence male spermatogenesis and fertility [12]. Western diet has been regarded as a risk factor for male infertility due to the increased oxidative stress and lower testosterone levels via saturated fatty acids intake [13]. Another study has demonstrated that dietary polyunsaturated fatty acids can be useful in male infertility [14]. Animal experiments show that High-Fat Diets (HFD) during early life correlates with testicular ectopic lipid deposition and low sperm quality [15], and HFD even damage sperm counts decrease in grandsons [16] via Transgenerational effects [17]. In addition, existing research has recognized that more moderate exercise improves spermatogenesis and semen quality by increasing body antioxidant defence and reducing pro-inflammatory cytokines level [18]. These results support a rationale for the under-standing of the hormonal and inflammation axis. Recent studies on obesity-mediated male infertility [19] have focused on alterations in the Hypothalamus–Pituitary–Gonad (HPG) axis, disruption of testicular steroidogenesis, metabolic abnormalities (disruption of insulin, cytokine, and adipokine homeostasis), and epigenetic aspects. In different studies, the prevalence of hypogonadism in obese men have been determined to be high, ranging from 40% to 79%. The complex interactions among excess body weight, body composition, sex steroids, and hypogonadism have been widely recognized [20]. Testosterone is a steroid hormone secreted by the testes in men. The hormone is important for the maintenance of male secondary sexual characteristics [21]. In men, a low androgenic status reduces total testosterone level, which is a frequent feature of visceral obesity [22]. Obese individuals display increased estrogen concentrations due to the overexpression of aromatase in adipose tissue. Such men present with symptoms of hypogonadotropic hypogonadism [23]. The use of Aromatase Inhibitors (AIs) can restore the balance between testosterone and estradiol levels and optimize the HPG axis to support spermatogenesis. AIs, including letrozole and anastrozole, have been used to treat male infertility for decades [24]. Excessive accumulation of adipose tissue around male gonads may lead to insufficient local androgen concentration, in turn leading to dysfunctional testicular development and penile dysplasia.
In this study, we predicted the causal relationship between obesity and male infertility using a Mendelian Randomization (MR) analysis based on data from large Genome-Wide Association Study (GWAS) datasets and bioinformatic analyses to predict the pathways that may play a role. A mouse model of obesity was developed to validate the role of aromatases in the process of obesity-mediated male fertility impairment.
Materials and Methods
GWAS Summary Data of Obesity and Male Infertility
All GWAS summary data can be found in the GWAS catalog (https://www.ebi.ac.uk/gwas/). The GWAS catalog provides a consistent, searchable, visualizable, and freely available database of Single Nucleotide Polymorphism (SNP)-trait associations, which can be easily integrated with other resources [25]. We used published GWAS summary data for childhood obesity [26]. The analyzed data included 5,530 obesity cases (=95th percentile of BMI achieved before 18 years of age, representing 5-30% of any given cohort) and 8,318 controls (relatively conservatively defined as <50th percentile of BMI consistent throughout all measures during childhood) of European ancestry, with data from 14 discovery cohorts including the Avon Longitudinal Study of Parents and Children (ALSPAC), Northern Finland 1966 Birth Cohort (NFBC1966), British 1958 Birth Cohort - Type 1 Diabetes Genetics Consortium subset (B58C-T1DGC), British 1958 Birth Cohort - Wellcome Trust Case Control Consortium Subset (B58C-WTCCC), French Young study (FRENCH YOUNG), Lifestyle Immune System Allergy Study (LISA), Western Australian Pregnancy Cohort study (RAINE), Children’s Hospital of Philadelphia (CHOP), Essen Obesity Study (ESSEN), Helsinki Birth Cohort Study (HBCS), Cardiovascular Risk in Young Finns Study (YF), Copenhagen Study on Asthma in Childhood (COPSAC), CM-GOYA study (CM-GOYA), and Generation R Study (GENERATIONR).
GWAS summary data for three clinical classes of obesity have been published [27]. The analyzed data for obesity class1 included 32,858 obesity cases (BMI =30 kg/m²) and 65,839 controls (BMI <25 kg/m²). The obesity class 2 data included 9,889 obesity cases (BMI =35 kg/m²) and 62,657 controls. The analyzed data for obesity class 3 included 2,896 obese patients (BMI =40 kg/m2) and 47,468 controls.
MR
MR using summary data from GWAS is an increasingly important tool for appraising causality in hypothesized exposure-outcome pathways. For each direction of potential influence, we combined MR estimates using inverse variance weighted (IVW) meta-analysis. This analysis essentially translates to a weighted regression of SNP-outcome effects on SNP-exposure effects, where the intercept is constrained to zero [28]. MR analyses were performed using MR-Base (default settings) (http://app.MrBase.html.org/). In addition to simple lookup requests for individual SNPs across multiple GWAS, MR-Base automates the implementation of two-sample MR, including effect allele harmonization across separate studies, linkage disequilibrium pruning to ensure independence of genetic variants, and diagnostic and sensitivity analyses [29]. Statistical significance was set at P<0.05.
Expression Omnibus (GEO) Database
Gene profiles (GSE6872, GSE55200) were obtained from the GEO database (https://www.ncbi.nlm.nih.gov/geo/). GSE6872 datasets [30] prepared from the semen samples of 21 individuals. They included semen samples from 13 normally fertile males who had fathered at least one child and from eight infertile individuals with severe and consistent heterogeneous teratozoospermia who showed no other abnormal semen parameters. Subcutaneous adipose tissue samples of GSE55200 datasets were obtained from the periumbilical region under local anesthesia and after an overnight fast. Previously described microarrays [31] were used to examine differences in subcutaneous adipose tissue gene expression in seven lean healthy controls and 16 obese individuals.
Gene Function Analysis
All datasets were downloaded from the GEO database through the “GEOquery” (v. 2.54.1) package [32]. The probes corresponding to multiple molecules were removed. If probes corresponding to the same molecule were encountered, only the probe with the highest signal value was retained. Statistical analysis and visualization were performed using the R package modules “limma” (v. 3.42.2) [33], “umap” (v. 0.2.7.0), “ComplexHeatmap” (v. 2.2.0) [34], and “ggplot2” (v. 3.3.3). Differentially Expressed Genes (DEGs) were identified with |log2FC|>1 and an adjusted P-value<0.05. Gene Ontology (GO) enrichment analysis [35] was performed to enrich DEGs according to Biological Process (BP), Cellular Component (CC), and Molecular Function (MF). Enrichment analysis of the Kyoto Encyclopedia of Genes and Genomes (KEGG) (https://www.genome.jp/kegg/) [36] was performed to identify and confirm the related signaling pathways of DEGs. GO and KEGG enrichment analyses were performed using the R package modules of “org.Hs.eg.db” (v. 3.11.4) and clusterProfiler” (v. 3.17.3). Gene Set Enrichment Analysis (GSEA) [37] was used to focus on group of genes that share common biological function, chromosomal location, or regulation. GSEA was performed using GSEA (v. 3.0). The Broad Molecular Signatures Database (MSigDB v. 7.4, https://www.gsea-msigdb.org/gsea/msigdb/) C2: curated gene sets (KEGG pathway) summarize and represent specific well-defined biological states or processes.
Animals and Diets
C57BL/6J mice were purchased from Xi’an Jiao Tong University Animal Experiment Center (ID: SCXK 2021-153). The mice were housed using alternating 12-h light/dark cycles with ad libitum access to food and water. Male mice were randomly assigned to a HFD (60% fat, Supplementary Information 1, n=8) to induce obesity or a normal chow diet (n=8). Body weights were recorded per two weeks. All animal experiments took place at Scientific Research and Experiment Center of Xi’an Jiaotong University Second Affiliated Hospital. This study was approved by the Ethics Committee of Xi’an Jiaotong University Second Affiliated Hospital. We confirm that all methods were performed in accordance with the relevant guidelines and regulations.
Serological Indicators Detection of Mice
C57BL/6J mice were starved overnight one day before sacrifice at 18 weeks of age. All mice were anaesthetized by intraperitoneal injection with 0.3% pentobarbital sodium injection (50mg/kg, NDC 67386-501-55). At the completion of the study, mice were euthanized with pentobarbital sodium (100 mg/kg). Blood samples were obtained for further biochemical analyses and were centrifuged at 3000g for 10 min at 4°C after standing for 2h in room temperature; serum was isolated and stored at -80°C. The levels of Free Fatty Acids (FFA), insulin, FSH and testosterone in serum were detected by Enzyme-Linked Immunosorbent Assay (ELISA). ELISA requires several experimental steps, including antibody immobilization, target binding, labeling, substrate incubation, signal production, and multiple washing steps.
Tissue Collection and Histological Analyses
Before tissue collection, the anesthetic (0.3% pentobarbital sodium) was continued until the mice were euthanized. Epididymal white adipose tissue (eWAT), liver, and testis tissues of mice were harvested for histological analyses and RNA extraction. The eWAT tissue was fixed in formalin for 24 h, followed by washing in 70% ethanol. Paraffin-embedded sections (5 μm) were cut, dewaxed, and stained with hematoxylin and eosin. Images were scanned using an Aperio ImageScope and analyzed using ImageScope software (Leica). To determine testicular lipid accumulation, frozen sections of the testes (10 μm) were fixed in 95% ethanol for 10s, washed with distilled water for 10s, stained with Oil Red O for 10 min, washed again with distilled water for 10s, and counterstained with hematoxylin for 30s. To evaluate the consequences of the previously described delay in spermatogenic development at the level of sperm chromatin condensation and DNA damage, we performed the terminal deoxynucleotidyl Transferase dUTP Nick End Labelling (TUNEL) to examine DNA damage.
Gene Expression
Total RNA was extracted from the tissues. cDNA was synthesized using a high-capacity cDNA synthesis kit (Takara). Targeted RT-qPCR assays were run in 20-μL triplicate reactions using iTaq SYBR Green Supermix (Takara). Gene expression levels were calculated after normalization to the housekeeping gene Β-tubulin using the 2–ΔΔCt method. The expression levels were expressed as relative mRNA levels compared to the control. The primers used are listed in Table 1.
Gene
Sequence (5'- 3')
CYP19A1
Fwd
TGGCAAGCTCTCCTCATCAA
Rev
TCTCCACGTCTCTCAGCGA
SRD5a
Fwd
CGCCCGACTATCTCTCACCTA
Rev
GACAGATTGAGGAGGTTGTCA
FSHR
Fwd
TTGCAACTCTCCACCATGAC
Rev
TTGTGACGAAGGTCTGTTCC
SHBG
Fwd
GCAACCTGGACTGTTCTTCC
Rev
AGTTGTCCTGAGCCATCCAC
STAT3
Fwd
CACCTTGGATTGAGAGTCAAGAC
Rev
AGGAATCGGCTATATTGCTGGT
ELF1
Fwd
ATGGCTGCTGTTGTCCAACAGAAC
Rev
CTAAAAAGAGTTGGGTTCCAGCAGTTC
CREB
Fwd
AGCAGCTCATGCAACATCATC
Rev
AGTCCTTACAGGAAGACTGAACT
β-tublin
Fwd
CAGCGATGAGCACGGCATAGAC
Rev
CCAGGTTCC- AAGTCCACCAGAATG
Table 1: Primer sequence.
Western Blotting
For western blotting, adipose tissues were lysed using 1× Radioimmunoprecipitation Aassay (RIPA) buffer. The supernatant protein concentration was determined using the Pierce BCA protein assay kit (Thermo Fisher Scientific). Briefly, 15 or 30 ng of supernatant was separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis. The resolved proteins were transferred onto nitrocellulose membranes and detected using primary antibodies that included mouse anti-chicken Β-tubulin, rabbit anti-mouse cytochrome P450 family 19 subfamily A member 1 (CYP19A1), and rabbit anti-mouse signal transducer and activator of transcription 3 (STAT3).
Results
Causal Relationship between Obesity and Male Infertility
MR results suggested causal relationships between male infertility and childhood obesity (IVW Β=0.4632, SE=0.2233, P=0.038) and obesity class 1 (IVW Β=0.4933, SE=0.2429, P=0.0423), obesity class 2 (IVW Β=0.5750, SE=0.2002, P=0.0041), and obesity class 3 (IVW Β=0.4933, SE=0.2429, P=0.0423) (Table 2, Figure 1).
Exposure group
Outcome group
Number of SNP
Analytical method
β
SE
P
Childhood obesity
Male infertility
6
IVW
0.4632
0.2233
0.0380a
Obesity class 1
14
0.4933
0.2429
0.0423a
Obesity class 2
9
0.5750
0.2002
0.0041b
Obesity class 3
2
0.2852
0.2232
0.2014
β: effect sizes for each SNP; SE: standard errors.
aP<0.05, the difference is statistically significant.
bP<0.0125 (0.05/4), the difference is statistically significant after multiple comparison correction.
Table 2: MR results of the causal relationship between different kinds of obesity and male infertility.
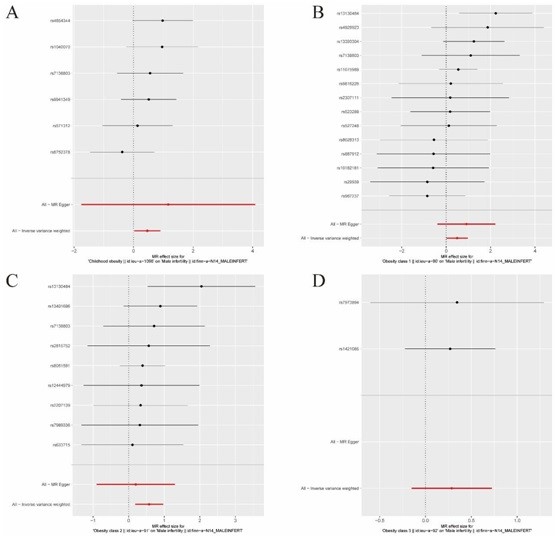
Figure 1: MR plot. A, Forest plot of childhood obesity and male infertility SNP MR-estimated 287 effects sizes. B, Forest plot of Obesity class1 and male infertility SNP MR-estimated effects sizes. C, Forest plot of Obesity class2 and male infertility SNP MR-estimated effects sizes. D, Forest plot of Obesity class3 and male infertility SNP MR-estimated effects sizes.
Exploration of Gene Function in Male Infertility
To identify the key genes affecting male infertility, we selected and analyzed GSE6872 datasets from the GEO database. The datasets were normalized and corrected. Principal component analysis revealed significant differences between subgroups, suggesting that a follow-up analysis of variance would be meaningful. GSE6872 (Figure 2) included 8162 DEGs. Enrichment analysis implicated four major pathways (cell proliferation, hormone-related, inflammatory, and metabolic pathways).
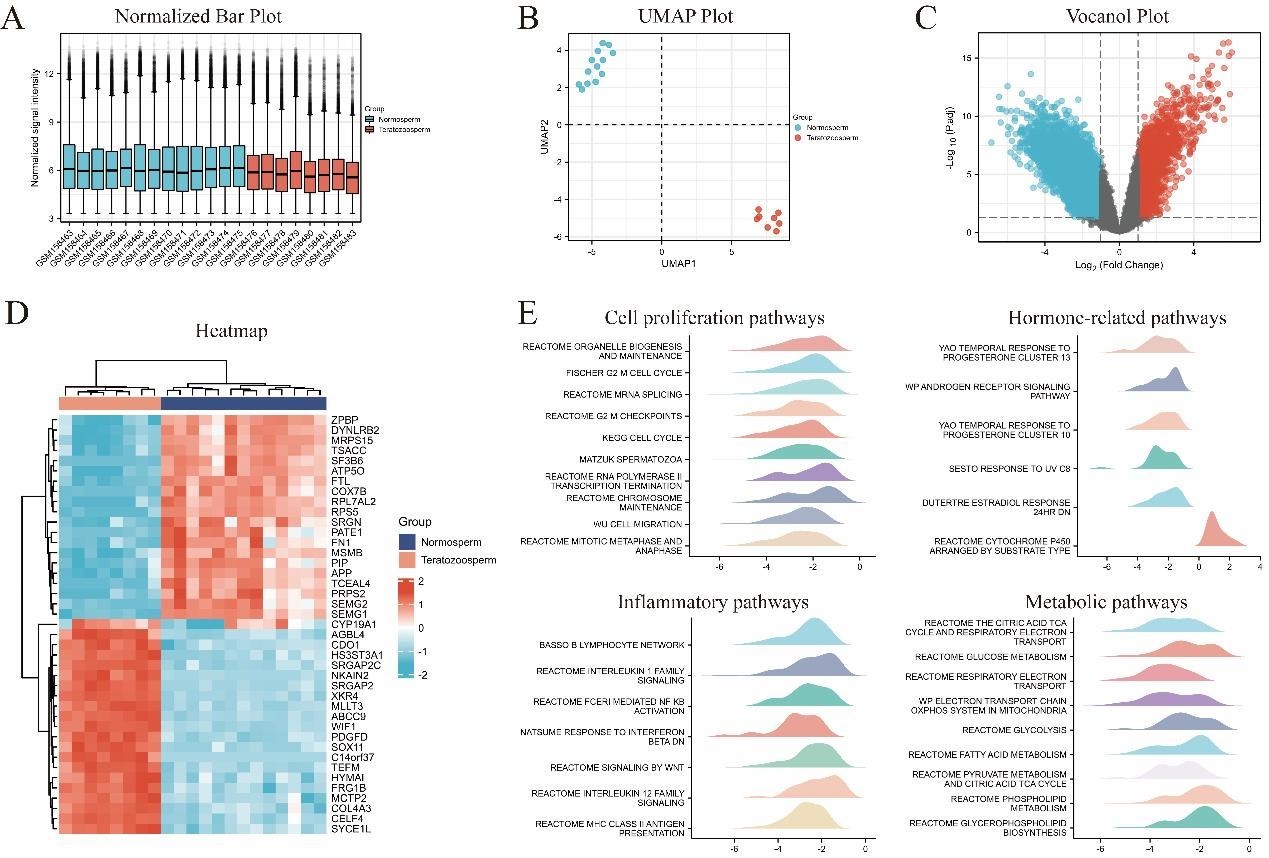
Figure 2: Analyses of GSE6872. A. Normalized Bar Plot; B. UMAP Plot; C. Vocanol Plot; D. Heatmap; E. GSEA analyses.
The single most striking difference in the data comparison was CYP19A1, which was explored as the key to deciphering obesity-induced male fertility impairment.
Impaired Fertility in Obese Mice
Differences in body weight between mice fed the regular diet and those fed the HFD began to appear at week 6. Significant differences in body weight were present at week 10 (Figure 3A). ELISA was used to detect serological indices in mice. Serum-free fatty acid and insulin levels tended to decrease in HFD-fed mice, while serum FSH and testosterone levels decreased significantly in HFD-fed mice. The differences were statistically significant (Figure 3B). The disordered arrangement of seminiferous units and decreased number of mature spermatozoa were observed in HFD-fed mice. In contrast, the seminiferous epithelial cells of mice fed the normal diet were closely arranged, spermatogenic cells existed at all levels, and spermatogenic function was good (Figure 3C). Oil Red O staining of testicular tissue revealed increased ectopic lipid deposition in the testicular interstitial tissue of HFD-fed mice (Figure 3D). TUNEL staining revealed increased apoptosis of seminiferous epithelial cells in the testes of HFD-fed mice (Figure 3E). PCR was used to examine mRNA expression levels of CYP19A1, SRD5a, and FSHR in mouse testes. The CYP19A1 levels tended to be elevated in the testes of obese mice, whereas SRD5a and FSHR levels were decreased.
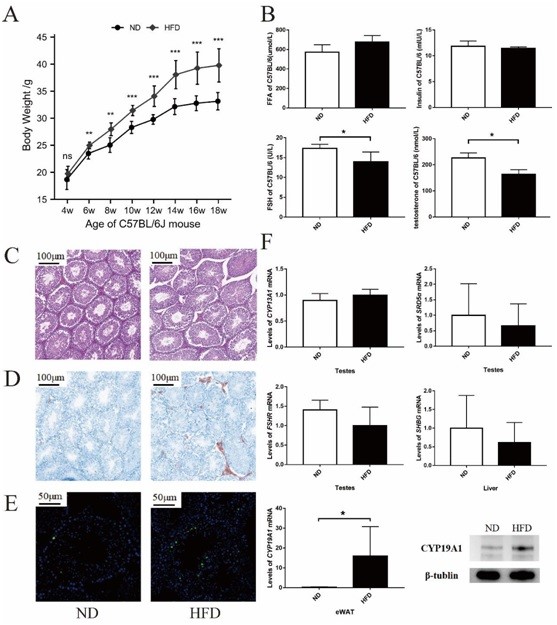
Figure 3: Fertility is impaired in obese mice. A. Body weight of C57BL/6J mice; B. ELISA results for serological indexes; C. H&E staining analysis of testes; D. Oil Red O staining analysis of testes; E. TUNEL staining analysis of testes; F. PCR and western blotting results for testes, liver and epididymal white adipose tissue of C57BL/6J mice. The samples derive from the same experiment and that gels/blots were processed in parallel. The gels have been cropped to improve the clarity and conciseness of the presentation. ND: Normal diet; HFD: High fat diet. *P<0.05; **P<0.01; ***P<0.001.
We also examined the mRNA expression of SHBG in liver tissue of mice. We found that SHBG was reduced in liver from HFD mice. This is com-pletely in line with previous papers showing that shbg is reduced in metabolic diseases while reflecting Non-Alcoholic Steato Hepatitis (NASH) [38]. Surprisingly, mRNA ex-pression level of CYP19A1 in eWAT was significantly increased in obese mice (P<0.05), which was confirmed by western blotting (Figure 3F).
CYP19A1 Transcription Factor Prediction
To further investigate the association between obesity and male infertility, we analyzed the GSE55200 dataset. GSE55200 harbored 176 DEGs, including upregulated and 81 down regulated DEGs (Figure 4A-C). Analyses of the 176 DEGs using KEGG and GO enrichment identified some important pathways. These included “regulation of interleukin-6 production,” “response to leptin,” “organic acid biosynthetic process,” “lymphocyte activation involved in immune response,” “regulation of inflammatory response,” “response to steroid hormone,” “response to nutrient,” “regulation of sequestering of triglyceride,” “regulation of cytokine-mediated signaling pathway,” “cellular response to peptide hormone stimulus,” and “regulation of lipid storage” regulation of lipid storage (Figure 4D). The JASPAR database was examined to predict transcription factors CYP19A1.
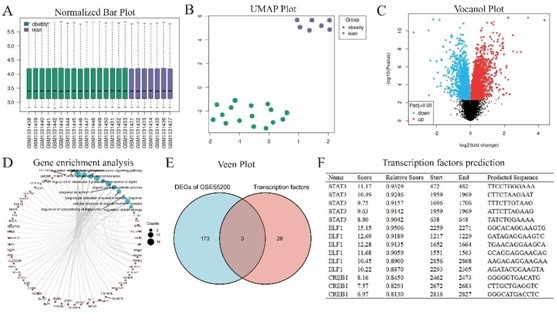
Figure 4: Analyses of GSE55200. A. Normalized Bar Plot of GSE55200; B. UMAP Plot of GSE55200; C. Vocanol Plot of GSE55200; D. Gene enrichment analysis of GSE55200 DEGs; E. Veen plot of GSE55200 DEGs and transcription factors predicted by JASPAR. F. Transcription factors predicted by JASPAR.
Thirty-one different transcription factors were identified (Supplementary Information 2). Venn plots identified the common genes of GSE55200 DEGs and the predicted transcription factors (Figure 4E). The three common transcription factor genes and their predicted scores are shown in Figure 4F.
STAT3 as the Implied Transcription Factor for CYP19A1
To verify the regulatory effects of different transcription factors on CYP19A1 expression, we examined their expression in eWAT. STAT3 expression level was similar to that of CYP19A1 and was significantly different between the obese and control groups (Figure 5A). We further showed the pattern of STAT3 and CYP19A1 transcriptional binding sites predicted using the JASPAR database (Figure 5B).
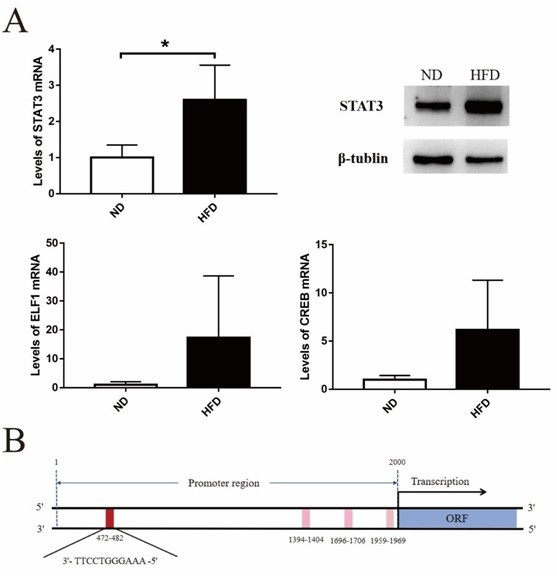
Figure 5: Analyses of transcription factors. A. PCR and western blotting results for transcription factors in epididymal white adipose tissue of C57BL/6J mice.; B. Transcription factor binding sites prediction. The samples derive from the same experiment and that gels/blots were processed in parallel. The gels have been cropped to improve the clarity and conciseness of the presentation.
Discussion
Male obesity leads to subfertility, mainly because of disruption of the HPG axis, increased testicular temperature, decreased sperm quality, and erectile dysfunction [39]. However, the mechanisms of obesity-mediated reduced male fertility are not well understood. In this study, obese boys had significantly lower testicular volume and penile length, and significantly lower total testosterone levels than normal-weight boys. These findings suggest that lower testosterone levels are an important cause of external genital hypoplasia in obese boys. Therefore, determining the causes of obesity-related lower serum testosterone levels will be crucial in elucidating the mechanisms underlying obesity-induced external genital hypoplasia in boys. MR and GEO dataset analyses were performed to predict the causal relationship between obesity and male infertility, and the possible role of STAT3/CYP19A1 upregulation in male obesity. A mouse model of HFD-induced obesity was established and used to implicate the STAT3/CYP19A1/testosterone pathway as a candidate pathway for lowered serum testosterone levels due to obesity. MR and GEO dataset analyses were performed to predict the causal relationship between obesity and male infertility, and the possible role of STAT3/CYP19A1 upregulation in male obesity. A mouse model of HFD-induced obesity was established and used to implicate the STAT3/CYP19A1/testosterone pathway as a candidate pathway for lowered serum testosterone levels due to obesity.
Obesity is associated with impaired male reproductive function. In addition to signs and symptoms directly stemming from reduced circulating testosterone levels, obese men have poor fertility outcomes [40]. MR analysis of large GWAS database predicted a causal relationship between obesity, adult obesity, childhood obesity, and male infertility. An observational study in 1130 boys from birth to 20 years of age showed that compared with normal-weight boys, penile length growth was significantly decreased (by approximately 10%) in obese boys with concomitantly reduced testosterone levels [41]. We observed a disordered arrangement of the seminiferous epithelium and increased apoptosis of seminiferous tubules and germinal epithelial cells in the testes of HFD mice. What is surprising is that there were significantly increased ectopic lipid deposi-tion in the testes of HFD-fed mice. The results in ectopic lipid deposition via increased lipid deposition in eWAT and non-adipose tissues leading to lipotoxic effects and the development of metabolic [42].
Metabolic syndrome is associated with testis ultrasound inhomogeneity, hypogonadism, and poor sperm morphology [43]. Previous study demonstrated that in an animal model of HFD-induced metabolic syndrome tes-tis and epididymis were affected by the metabolic-syndrome induced inflammation and by hyperestrogenism and that blocking estrogen activity (by using tamoxifen) was able to revert these alterations [44]. A previous study [45] demonstrated that ectopic li-pid deposition in the testis leads to abnormal testicular lipid metabolism and lipotoxi-city. The authors also described that, with the decline in FSH level, fertility decreased, and the blood–testis barrier was impaired in HFD mice. EWAT is a type of WAT sur-rounding the epididymis and testis [46]. When HFD is consumed for an extended period, triglycerides first accumulate in inguinal WAT and eWAT [47]. The testis and testosterone are both essential for male spermatogenesis and are regulated by the HPG axis [48]. The importance of non-classical androgen and estrogen signaling has been described [49]. LH stimulates Leydig cells to produce testosterone, whereas FSH acts on the seminiferous tubules and enhances spermatogenesis [50]. Aromatase is a rate-limiting enzyme in the irreversible conversion of androgens to estrogens. This leads to the overconversion of androgens to estrogen when their activity increases and disturbs the balance of hormones. High expression level of adipose tissue aromatase in obese men leads to abnormal serum testosterone levels with abnormal regulation of testicular structures and Sertoli cells [51]. However, the mechanism underlying increased aromatase expression level in WAT remains unclear and requires further study.
Obesity is associated with chronic low-grade inflammation that affects multiple systems, including the endocrine, skeletal muscular, and reproductive systems [52]. The present findings support previous observations that HFD alters the WAT immune response and influences inflammation by altering signaling pathways, including increased mRNA expression levels of interleukin-6, Kruppel-like factor 4, and STAT3 during obesity [53]. STAT3 is an important member of the STAT protein family and is involved in the regulation of energy homeostasis, glucose and lipid metabolism, immune function, and other systems [54]. The STAT protein family comprises STAT 1, STAT 2, STAT 3, STAT 4, STAT 5a, STAT 5b, and STAT 6. They have highly conserved structural domains, including the N-Terminal Domain (NTD), coiled-coil domain, DNA-Binding Domain (DBD), linker domain, Src homology 2 domain, and transactivation domain [55]. NTD can stabilize the dimer formed by STAT proteins and initiate transcription in concert with DBD binding to the tandem gamma activator sequence (GAS, TTCNNNNGAA) element during nuclear import [56,57]. Recent work has established that kynurenine, an aberrant amino acid excessive accumulation in obesity, impairs lipid homeostasis in adipocytes via activating the aryl hydrocarbon receptor/STAT3/interleukin-6 signaling [58]. The results of this study showed the elevated expression of STAT3 and CYP19A1. Since most aromatase tran-scription in adipose tissue is mediated by promoter I.4 and aromatase contains GAS elements [59], we hypothesized that STAT3 may be a transcriptional activator of CYP19A1. We examined the expression levels of CTBP1, CREB, and STAT3 in pe-ri-testicular adipose tissue of HFD mice. The expression level of STAT3 was significantly elevated, whereas the expression levels of CTBP1 and CREB did not change. These findings indicate that STAT3 might be the main transcription factor that pro-motes the expression of CYP19A1 in eWAT. In addition, expression of the 17Β-estradiol stimulates enzyme aromatase is highly upregulated [60] mainly via GPR30 receptor [61]. This observation may support the hypothesis that since the elevation of STAT3 expression in obesity, the promoted expressed CYP19A1 could increase a shift from testosterone to estrogens. The persistently elevated level of estrogen will further stimulate STAT3, which may constitute an apparent positive feedback loop. The obesity–inflammation–aromatase axis severely impairs male fertility and causes male in-fertility [62]. This relationship has attracted considerable attention. We presently identified the TAT3/CYP19A1/testosterone pathway, which provide new insights into the mechanisms by which obesity causes infertility in males. This study had some limitations. First, we were unable to directly obtain subcutaneous abdominal fat tissue from children to verify our conclusions owing to the protection of children and an ethical review. Second, the mechanism of action of STAT3 is complex and requires further study, including examination of the binding and regulatory modes of STAT3 and CYP19A1. Finally, since the synthesis and metabolism of testosterone is a complex process, and aromatase only provides a partial explanation of the changes in serum testosterone, caution is needed when applying the results of this study. Nevertheless, to the best of our knowledge, this is the first study to report the biological meaning of STAT3/CYP19A1 and its potential possibility in obesity-related male fertility.
Conclusions
Obesity might lead to male infertility from a genomic perspective. HFD mice had reduced serum testosterone and FSH levels along with structural abnormalities, ectopic lipid deposition, and chronic inflammation in the testes. Our observation may support the hypothesis that since the elevation of STAT3 expression in obesity, the promoted expressed CYP19A1 could increase a shift from testosterone to estrogens. The persistently elevated level of estrogen will further stimulate STAT3, which may constitute a positive feedback loop. These findings provide new insights into the mechanisms of obesity-mediated male fertility impairment.
Author Statements
Author Contributions
Author Mengnan Lu and Ruoyang Feng completed the experiments and wrote the article. Chunyan Yin and Yanfeng Xiao designed the study.
Acknowledgments
We are indebted to all the individuals who participated in and helped with our research. We consent for publication.
Funding
This work was supported by the National Natural Scientific Foundation of China (81903340).
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Ethics Approval
This study was approved by the Ethics Committee of Xi’an Jiaotong University Second Affiliated Hospital. We confirm that all methods were performed in accordance with the relevant guidelines and regulations.
Consent to Participate
All of authors participated this study.
Consent for Publication
The authors consent for publication as not applicable.
Availability of Data and Material
The datasets analyzed during the current study are available from the UK biobank (http://geneatlas.roslin.ed.ac.uk/) (fields: 20002); the Gene Expression Omnibus database (https://www.ncbi.nlm.nih.gov/gds) (accession numbers GSE6872 and GSE55200).
Code Availability
All codes are available from the R package modules.
References
- Bailly L, Fabre R, Pradier C, Iannelli A. Colorectal cancer risk following bariatric surgery in a nationwide study of French individuals with obesity. JAMA Surg. 2020; 155: 395-402.
- Lee EY, Yoon KH. Epidemic obesity in children and adolescents: risk factors and prevention. Front Med. 2018; 12: 658-66.
- Aris IM, Block JP. Childhood obesity interventions-going beyond the individual. JAMA Pediatr. 2022; 176: e214388.
- Zhang YD, Tan LN, Luo SY, Chen YX, Wei HY. [Status of penis and testicular development and effects of overweight/obesity on them in boys in the Zhengzhou area]. Zhongguo Dang Dai Er Ke Za Zhi. 2015; 17: 72-6.
- Zhang LD, Li HC, Gao M, Wang L, Deng Q, Shi T, et al. [Sexual development characteristics and sex hormone levels in obese male adolescents]. Zhonghua Nan Ke Xue. 2013; 19: 434-8.
- Chambers TJ, Richard RA. The impact of obesity on male fertility. Hormones (Athens). 2015; 14: 563-8.
- Sermondade N, Faure C, Fezeu L, Shayeb AG, Bonde JP, Jensen TK, et al. BMI in relation to sperm count: an updated systematic review and collaborative meta-analysis. Hum Reprod Update. 2013; 19: 221-31.
- MacDonald AA, Herbison GP, Showell M, Farquhar CM. The impact of body mass index on semen parameters and reproductive hormones in human males: a systematic review with meta-analysis. Hum Reprod Update. 2010; 16: 293-311.
- Wang S, Sun J, Wang J, Ping Z, Liu L. Does obesity based on body mass index affect semen quality?-A meta-analysis and systematic review from the general population rather than the infertile population. Andrologia. 2021; 53: e14099.
- Liu Y, Ding Z. Obesity, a serious etiologic factor for male subfertility in modern society. Reproduction. 2017; 154: R123-31-r131.
- Khodamoradi K, Khosravizadeh Z, Seetharam D, Mallepalli S, Farber N, Arora H. The role of leptin and low testosterone in obesity. Int J Impot Res. 2022; 34: 704-13.
- Benatta M, Kettache R, Buchholz N, Trinchieri A. The impact of nutrition and lifestyle on male fertility. Arch Ital Urol Androl. 2020; 92.
- Ferramosca A, Zara V. Diet and male fertility: the impact of nutrients and antioxidants on sperm energetic metabolism. Int J Mol Sci. 2022; 23: 2542.
- Signorini C, Moretti E, Noto D, Micheli L, Ponchia R, Collodel G. Fatty acid oxidation and pro-resolving lipid mediators are related to male infertility. Antioxidants. 2022; 11: 107.
- Crisóstomo L, Videira RA, Jarak I, Starcevic K, Mašek T, Rato L, et al. Diet during early life defines testicular lipid content and sperm quality in adulthood. Am J Physiol Endocrinol Metab. 2020; 319: E1061-73-e1073.
- Crisóstomo L, Videira RA, Jarak I, Starcevic K, Mašek T, Rato L, et al. Inherited metabolic memory of high-fat diet impairs testicular fatty acid content and sperm parameters. Mol Nutr Food Res. 2022; 66: e2100680.
- Crisóstomo L, Jarak I, Rato LP, Raposo JF, Batterham RL, Oliveira PF, et al. Inheritable testicular metabolic memory of high-fat diet causes transgenerational sperm defects in mice. Sci Rep. 2021; 11: 9444.
- Minas A, Fernandes ACC, Maciel Júnior VL, Adami L, Intasqui P, Bertolla RP. Influence of physical activity on male fertility. Andrologia. 2022; 54: e14433.
- Leisegang K, Sengupta P, Agarwal A, Henkel R. Obesity and male infertility: mechanisms and management. Andrologia. 2021; 53: e13617.
- Yeap BB, Wu FCW. Clinical practice update on testosterone therapy for male hypogonadism: contrasting perspectives to optimize care. Clin Endocrinol. 2019; 90: 56-65.
- Pihlajamaa P, Sahu B, Jänne OA. Determinants of receptor- and tissue-specific actions in androgen signaling. Endocr Rev. 2015; 36: 357-84.
- Tchernof A, Brochu D, Maltais-Payette I, Mansour MF, Marchand GB, Carreau AM, et al. Androgens and the regulation of adiposity and body fat distribution in humans. Compr Physiol. 2018; 8: 1253-90.
- Mintziori G, Nigdelis MP, Mathew H, Mousiolis A, Goulis DG, Mantzoros CS. The effect of excess body fat on female and male reproduction. Metabolism. 2020; 107: 154193.
- Yang C, Li P, Li Z. Clinical application of aromatase inhibitors to treat male infertility. Hum Reprod Update. 2021; 28: 30-50.
- Buniello A, MacArthur JAL, Cerezo M, Harris LW, Hayhurst J, Malangone C, et al. The NHGRI-EBI GWAS Catalog of published genome-wide association studies, targeted arrays and summary statistics 2019. Nucleic Acids Res. 2019; 47: D1005-12.
- Bradfield JP, Taal HR, Timpson NJ, Scherag A, Lecoeur C, Warrington NM, et al. A genome-wide association meta-analysis identifies new childhood obesity loci. Nat Genet. 2012; 44: 526-31.
- Berndt SI, Gustafsson S, Mägi R, Ganna A, Wheeler E, Feitosa MF, et al. Genome-wide meta-analysis identifies 11 new loci for anthropometric traits and provides insights into genetic architecture. Nat Genet. 2013; 45: 501-12.
- Choi KW, Chen CY, Stein MB, Klimentidis YC, Wang MJ, Koenen KC, et al. Assessment of bidirectional relationships between physical activity and depression among adults: A 2-sample Mendelian randomization study. JAMA Psychiatry. 2019; 76: 399-408.
- Hemani G, Zheng J, Elsworth B, Wade KH, Haberland V, Baird D, et al. The MR-Base platform supports systematic causal inference across the human phenome. eLife. 2018; 7: e34408.
- Platts AE, Dix DJ, Chemes HE, Thompson KE, Goodrich R, Rockett JC, et al. Success and failure in human spermatogenesis as revealed by teratozoospermic RNAs. Hum Mol Genet. 2007; 16: 763-73.
- Badoud F, Lam KP, DiBattista A, Perreault M, Zulyniak MA, Cattrysse B, et al. Serum and adipose tissue amino acid homeostasis in the metabolically healthy obese. J Proteome Res. 2014; 13: 3455-66.
- Davis S, Meltzer PS. GEOquery: a bridge between the Gene Expression Omnibus (GEO) and BioConductor. Bioinformatics. 2007; 23: 1846-7.
- Smyth GK. limma: linear Models for microarray Data. In: Gentleman R, editors. Bioinformatics and Computational Biology solutions using R and bioconductor. New York: Springer. NY: New York; 2005; 397-420.
- Gu Z, Eils R, Schlesner M. Complex heatmaps reveal patterns and correlations in multidimensional genomic data. Bioinformatics. 2016; 32: 2847-9.
- Hill DP, Blake JA, Richardson JE, Ringwald M. Extension and integration of the gene ontology (GO): combining GO vocabularies with external vocabularies. Genome Res. 2002; 12: 1982-91.
- Kanehisa M, Goto S. KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000; 28: 27-30.
- Mooney MA, Wilmot B. Gene set analysis: A step-by-step guide. Am J Med Genet B Neuropsychiatr Genet. 2015; 168: 517-27.
- Di Stasi V, Maseroli E, Rastrelli G, Scavello I, Cipriani S, Todisco T, et al. SHBG as a Marker of NAFLD and Metabolic Impairments in Women Referred for oligomenorrhea and/or hirsutism and in Women with Sexual Dysfunction. Front Endocrinol (Lausanne). 2021; 12: 641446.
- Amiri M, Ramezani Tehrani F. Potential adverse effects of female and male obesity on fertility: A narrative review. Int J Endocrinol Metab. 2020; 18: e101776.
- Carrageta DF, Oliveira PF, Alves MG, Monteiro MP. Obesity and male hypogonadism: tales of a vicious cycle. Obes Rev. 2019; 20: 1148-58.
- Mancini M, Pecori Giraldi F, Andreassi A, Mantellassi G, Salvioni M, Berra CC, et al. Obesity is strongly associated with low testosterone and reduced penis growth during development. J Clin Endocrinol Metab. 2021; 106: 3151-9.
- Spalding KL, Bernard S, Näslund E, Salehpour M, Possnert G, Appelsved L, et al. Impact of fat mass and distribution on lipid turnover in human adipose tissue. Nat Commun. 2017; 8: 15253.
- Lotti F, Corona G, Degli Innocenti S, Filimberti E, Scognamiglio V, Vignozzi L, et al. Seminal, ultrasound and psychobiological parameters correlate with metabolic syndrome in male members of infertile couples. Andrology. 2013; 1: 229-39.
- Marchiani S, Vignozzi L, Filippi S, Gurrieri B, Comeglio P, Morelli A, et al. Metabolic syndrome-associated sperm alterations in an experimental rabbit model: relation with metabolic profile, testis and epididymis gene expression and effect of tamoxifen treatment. Mol Cell Endocrinol. 2015; 401: 12-24.
- Ye J, Luo D, Xu X, Sun M, Su X, Tian Z, et al. Metformin improves fertility in obese males by alleviating oxidative tress-induced blood-testis barrier damage. Oxid Med Cell Longev. 2019; 2019: 9151067.
- Swanson GM, Estill M, Diamond MP, Legro RS, Coutifaris C, Barnhart KT, et al. Human chromatin remodeler cofactor, RNA interactor, eraser and writer sperm RNAs responding to obesity. Epigenetics. 2020; 15: 32-46.
- Zhao Y, Zhao MF, Jiang S, Wu J, Liu J, Yuan XW, et al. Liver governs adipose remodelling via extracellular vesicles in response to lipid overload. Nat Commun. 2020; 11: 719.
- Guo J, Nie X, Giebler M, Mlcochova H, Wang Y, Grow EJ, et al. The dynamic transcriptional cell atlas of testis development during human puberty. Cell Stem Cell. 2020; 26: 262-276.e4.
- Cooke PS, Walker WH. Nonclassical androgen and estrogen signaling is essential for normal spermatogenesis. Semin Cell Dev Biol. 2022; 121: 71-81.
- Sato Y, Tajima A, Katsurayama M, Nozawa S, Yoshiike M, Koh E, et al. A replication study of a candidate locus for follicle-stimulating hormone levels and association analysis for semen quality traits in Japanese men. J Hum Genet. 2016; 61: 911-5.
- Komninos D, Ramos L, van der Heijden GW, Morrison MC, Kleemann R, van Herwaarden AE, et al. High fat diet-induced obesity prolongs critical stages of the spermatogenic cycle in a Ldlr(-/-). Leiden mouse model. Sci Rep. 2022; 12: 430.
- Wijesinghe SN, Nicholson T, Tsintzas K, Jones SW. Involvements of long noncoding RNAs in obesity-associated inflammatory diseases. Obes Rev. 2021; 22: e13156.
- Kiran S, Rakib A, Kodidela S, Kumar S, Singh UP. High-fat diet-induced dysregulation of immune cells correlates with macrophage phenotypes and chronic inflammation in adipose tissue. Cells. 2022; 11: 1327.
- Bharadwaj U, Kasembeli MM, Robinson P, Tweardy DJ. Targeting Janus kinases and signal transducer and activator of transcription 3 to treat inflammation, fibrosis, and cancer: rationale, progress, and caution. Pharmacol Rev. 2020; 72: 486-526.
- Waldmann TA, Chen J. Disorders of the JAK/STAT pathway in T cell lymphoma pathogenesis: implications for immunotherapy. Annu Rev Immunol. 2017; 35: 533-50.
- Zheng J, Chang MR, Stites RE, Wang Y, Bruning JB, Pascal BD, et al. HDX reveals the conformational dynamics of DNA sequence specific VDR co-activator interactions. Nat Commun. 2017; 8: 923.
- Roy B, Zuo Z, Stormo GD. Quantitative specificity of STAT1 and several variants. Nucleic Acids Res. 2017; 45: 8199-207.
- Huang T, Song J, Gao J, Cheng J, Xie H, Zhang L, et al. Adipocyte-derived kynurenine promotes obesity 618 and insulin resistance by activating the AhR/STAT3/IL-6 signaling. Nat Commun. 2022; 13: 3489.
- Zhao Y, Nichols JE, Bulun SE, Mendelson CR, Simpson ER. Aromatase P450 gene expression in human adipose tissue. Role of a Jak/STAT pathway in regulation of the adipose-specific promoter. J Biol Chem. 1995; 270: 16449-57.
- Wang J, Sareddy GR, Lu Y, Pratap UP, Tang F, Greene KM, et al. Astrocyte-Derived estrogen Regulates Reactive astrogliosis and is neuroprotective following ischemic Brain Injury. J Neurosci. 2020; 40: 9751-71.
- Wang L, Liu J, Xu J, Zhang W, Wang R. Coupling of GPR30 mediated neurogenesis and protection with astroglial aromatase-STAT3 signaling in rat hippocampus after global cerebral ischemia. Mol Cell Endocrinol. 2021; 535: 111394.
- Yuxin L, Chen L, Xiaoxia L, Yue L, Junjie L, Youzhu L, et al. Research progress on the relationship between obesity-inflammation-aromatase axis and male infertility. Oxid Med Cell Longev. 2021; 2021: 6612796.