
Review Article
Austin J Endocrinol Diabetes. 2023; 10(2): 1103.
Adipokines in Obesity: A Neuroendocrine Perspective on Female Reproductive Health
Chalikkaran Thilakan Rejani; Venugopal Bhuvarahamurthy*
Department of Medical Biochemistry, Dr. ALM PG Institute of Basic Medical Sciences, University of Madras, India
*Corresponding author: V Bhuvarahamurthy Department of Medical Biochemistry, Dr. ALM PG Institute of Basic Medical Sciences, University of Madras, Taramani, Chennai – 600 113, India. Tel: +91-44-2454-7080/7081 Email: murthyboston@gmail.com
Received: November 24, 2023 Accepted: December 07, 2023 Published: December 17, 2023
Abstract
Obesity-related disruptions in female reproduction are a growing concern. The neuroendocrine regulation of female reproduction in the context of obesity involves the intricate interplay between adipokines and the Hypothalamus-Pituitary- Ovarian (HPO ) axis. In this review, we explore the role of adipokines, particularly leptin and adiponectin, in regulating neuroendocrine female reproduction in the context of obesity. Leptin, primarily produced by adipocytes, plays a pivotal role in signaling energy status to the brain. However, excessive levels of obesity can lead to leptin resistance, affecting the HPO axis and causing menstrual irregularities and fertility issues. Adiponectin, on the other hand, is reduced in obesity and influences insulin sensitivity and ovarian function. We delve into the impact of altered adipokine levels on the HPO axis, discussing disruptions in hormone secretion, oocyte maturation, and ovarian steroidogenesis. This study paves the way for further exploration of adipokine-related pathways and potential therapeutic targets, enhancing our understanding of the intricate relationship between obesity and female reproduction.
Keywords: Adipokines; Adiponectin; Leptin; HPO axis; Neuroendocrine regulation; Obesity; Reproductive disorder
Abbreviations: GnRH: Gonadotropin-Releasing Hormone, FSH: Follicle-Stimulating Hormone; LH: Luteinizing Hormone; NPY: Neuropeptide Y; AgRP: Agouti-Related Protein; GALP: Galanin-Like Peptide; POMC: Pro-opiomelanocortin; LEPT: Leptin Receptor; (AdipoR1 or AdipoR2): Adipokine receptor 1 or 2; PPARγ: Peroxisome Proliferator-Activated Receptor Gamma; (PGC1): Peroxisome Proliferator-Activated Receptor Gamma Coactivator 1; (C/EBPa): CCAAT/Enhancer-Binding Protein Alpha; (LRH-1): Liver Receptor Homolog-1; (FoxO1): Forkhead box O1; (SREBP-1c): Sterol-Regulatory Element-Binding Protein1c; (ATF3): Activating Transcription Tactor 3; (NFATc4): Nuclear Factor of Activated T Cells 4; (Id3): Inhibitor of Differentiation 3; (STAT5 or STAT3): Signal Transducer and Activator of Transcription 5 or 3; (CLOCK): Circadian Locomotor Output Cycles Kaput; (BMAL1): Brain and Muscle ARNT-Like 1; (COX2): Cyclooxygenase-2; (PGE2): Prostaglandin E2; (EGF): Epidermal Growth Factor; (FABP): Fatty Acid-Binding Protein; (PAI-1): Plasminogen Activator Inhibitor-1; (AMPK): AMP-Activated Protein Kinase; (GHS-R): GH Secretagogue Receptor; (GHRH-R): Growth Hormone-Releasing Hormone Receptor; (GH): Growth Hormone; (GHS-R): GH Secretagogue Receptor; (cAMP): Cyclic Adenosine Monophosphate; (MAPK): Mitogen-Activated Protein Kinase; (ERK1/2): Extracellular Regulated Kinase 1 or 2; (JAK): Janus Kinase; (P13K/Akt): Phosphatidylinositol 3-Kinase/Protein Kinase B; (FGF21): Fibroblast Growth Factor 21; (TNFa): Tumor Necrosis Factor-a; (IL-6): Interleukin-6; (IL-1Β): Interleukin 1Β; (a-MSH): a-Melanocyte-Stimulating Hormone; (GABA): Gamma-Aminobutyric Acid; (CART): Cocaine and Amphetamine-Regulated Transcript.
Highlights
• Adipokines serve as messengers, conveying energy status to the brain and influencing reproductive functions.
• Altered adipokine levels disrupt hormone secretion within the HPO axis, impacting various aspects of female reproductive health.
• Adipokines action in the HPO axis may lead to targeted interventions for improved fertility in obese individuals.
Introduction
Obesity, characterized by abnormal or excessive fat accumulation, presents a formidable global health threat. Both the World Health Organization (WHO) and the World Obesity Federation (WOF) classify it as a grave concern, with the latter designating it as a chronic, recurring ailment [1-2]. This multifaceted condition emerges from a complex interplay of socio-psychological influences, genetic predisposition, and dietary choices [3]. Notably, diets abundant in hypercaloric, high-fat [4], low-fiber [5], and sugary foods fuel the escalating obesity rates [6-8]. However, obesity transcends mere body weight concerns; it intricately intertwines with metabolic irregularities and a plethora of health issues, including type 2 diabetes, dyslipidaemia, non-alcoholic fatty liver disease, hypertension, and diverse reproductive disorders [2,9]. This global epidemic affects over 600 million adults, with its prevalence soaring in recent decades [10]. Tragically, it contributes to millions of annual deaths and substantial healthcare costs worldwide, reaching nearly $150 billion annually in the United States alone [11]. India, too, has witnessed a surge in obesity, propelled by shifting dietary patterns, reduced physical activity, and urbanization. According to the 2019-21 National Family Health Survey (NFHS-5), approximately 40.8% of Indian women and 32.2% of men are now overweight or obese. Obesity rates tend to be higher in urban areas compared to rural regions, and prevalence varies among states and regions [12]. The worrisome association between obesity and type 2 diabetes is particularly concerning, with projections indicating a further escalation in these numbers [13].
Obesity poses multifaceted challenges that extend far beyond its well-documented association with metabolic disorders and cardiovascular diseases. Recent research has unveiled a complex interplay between obesity and various physiological systems, including the intricate web of neuroendocrine regulation governing female reproduction. Within this context, the role of adipokines, bioactive molecules secreted by adipose tissue, has garnered significant attention as a key mediator of the profound impact of excess adiposity on reproductive health [14,15]. The regulation of female reproduction is an exquisitely orchestrated process involving a delicate balance of hormones, neural signals, and environmental cues [16]. Disruptions in this finely tuned system can lead to a spectrum of reproductive disorders, ranging from menstrual irregularities and anovulation to infertility [17]. Obesity, characterized by an excessive accumulation of adipose tissue, has been linked to disturbances in this intricate neuroendocrine network, with a growing body of evidence implicating adipokines as central players in these disruptions [18,19].
Adipose tissue, the body's largest reservoir for triacylglycerols, serves as a pivotal player in energy metabolism. Through lipolysis, it breaks down stored triacylglycerols, providing essential fuel for organs and substrates for critical processes like gluconeogenesis in the liver [20]. White Adipose Tissue (WAT) is central to adipose tissue homeostasis, and disruptions in its metabolism can lead to insulin resistance and other health complications [21]. Beyond its role as a storage site, WAT functions as an active endocrine organ. It secretes a diverse array of proteins and signaling molecules known as adipokines, which exert influence over metabolic processes throughout the body. WAT establishes extensive interactions with various organs and metabolic systems via these adipokines [22]. Obesity frequently results in excessive lipid storage in visceral adipose tissue, primarily due to high-calorie diets. Lipids released from obese adipose tissue can accumulate in peripheral tissues such as the liver, pancreas, and muscles, ultimately impairing insulin sensitivity and contributing to Type 2 Diabetes Mellitus (T2DM) [23]. Key mechanisms driving obesity and its associated health issues encompass adipocyte hypertrophy and hyperplasia, inflammation within adipose tissue, altered extracellular matrix remodeling, fibrosis, and changes in adipokine production [24]. Comprehending these processes holds paramount importance in addressing obesity-related reproductive complications.
By examining the pivotal roles played by adipokines in this complex interplay, we aim to shed light on the mechanisms through which obesity exerts its influence on female reproductive health. Additionally, we will explore the potential implications of these findings for clinical practice, emphasizing the need for a comprehensive understanding of adipokine-mediated regulation in order to develop targeted interventions and personalized therapeutic strategies for women grappling with obesity-related reproductive challenges [25]. As we embark on this journey into the intricate crossroads of adipokine biology and neuroendocrine female reproduction, we hope to gain deeper insights into the mechanisms at play and inspire further research endeavors that promise to improve the lives of countless women affected by these complexes and often overlapping conditions.
Adipokines and Its Impact on Neuroendocrine Regulation
Adipokines exert substantial influence over the HPO axis, a crucial regulatory pathway in the context of reproductive functions. Leptin, a key player among these adipokines, orchestrates the regulation of hypothalamic Gonadotropin-Releasing Hormone (GnRH) and the secretion of Follicle-Stimulating Hormone (FSH) and Luteinizing Gormone (LH) from the adenohypophysis. In parallel, adiponectin enhances insulin sensitivity and impacts ovarian sex hormone synthesis [26]. These adipokines, particularly leptin and adiponectin, act as vital markers of the body's energy status, establishing a pivotal link between energy status and reproductive competence. Their intricate control over the HPO axis encompasses various aspects of female reproduction, influencing hormone production, folliculogenesis, oocyte maturation, steroidogenesis, and other critical processes, all significantly shaped by prevailing energy status (Figure 1) [27,28].
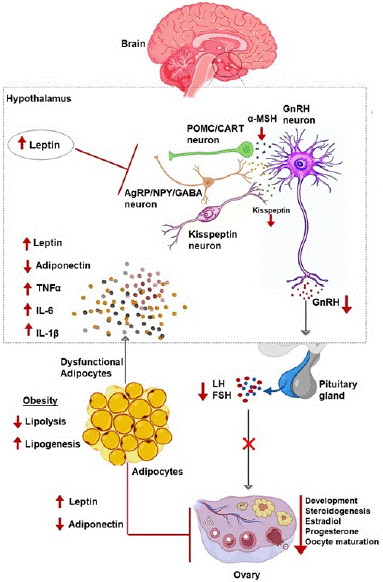
Figure 1: Schematic diagram shows the effect of altered adipokines secretion on the hypothalamus-pituitary-ovarian axis in obese condition. The red upper arrows indicate stimulatory effects. The red down arrows indicate down-regulation.
The regulation of the HPG axis involves the central participation of the hypothalamus and pituitary as neuroendocrine organs, overseeing ovarian functions [29,30]. Adipokines, in synergy with insulin, finely modulate ovarian functions by influencing gonadotropins (LH and FSH) [31,32]. This intricate hormonal interplay further regulates the secretion of pituitary gonadotropins and modulates sex steroids, including testosterone (T) and estradiol (E2), ultimately shaping ovarian functions [33]. The hypothalamus, as a central regulator, not only governs reproduction but also plays multifaceted roles in various physiological processes beyond reproduction [34]. It regulates appetite by orchestrating food intake reduction and increased energy expenditure through the activation of anorexigenic factors and the suppression of orexigenic neuropeptides. Additionally, the hypothalamus oversees bone mass regulation, hypothalamic-pituitary-adrenal hormone secretion, basal metabolism, pancreatic Β-cell function, insulin secretion, endothelial cell angiogenesis, and influences innate and adaptive immunity [34-39]. The complex modulation of nutrient absorption and induction of satiety by the hypothalamus is achieved through the activation of POMC neurons, resulting in the release of anorexigenic peptides, while hyperpolarization inhibits NPY neurons, leading to the release of orexigenic peptides [40]. Furthermore, in the intestine, enterocytes employ mechanisms to reduce glucose absorption, involving the activation of PKC and subsequent signaling pathways like p38, PI3K, and ERK, ultimately inhibiting glucose uptake [38].
a) Leptin Regulation of HPO Axis
Leptin exerts regulatory control over all levels of the HPO axis, including the hypothalamus, pituitary gland, and ovaries. While leptin does not directly interact with GnRH due to the absence of its receptor on GnRH neurons, it exerts its influence indirectly through intermediaries that bridge the gap between metabolic status and reproduction [41]. Key players in this interaction include kisspeptin, NPY, AgRP, and GALP, which modulate metabolic cues and reproductive functions [42]. NPY, originating from POMC, serves a dual role by stimulating food intake and negatively regulating reproduction, including the inhibition of LH secretion [43]. NPY is also associated with the production of the pre-ovulatory surge of LH [44].
Kisspeptin neurons, highly sensitive to energy status and metabolic signals, act as downstream mediators of leptin's positive impact on gonadotropin secretion, making them crucial in coordinating nutritional and reproductive axes [45]. Studies in ob/ob mice, characterized by low Kiss1 mRNA levels, have shown that leptin treatment can increase Kiss1 expression [46]. Deletion of kisspeptin and its receptor (GPR54) in mice results in infertility and abnormal sexual maturation [48,49]. Additionally, kisspeptin administration stimulated reproductive axis maturation in hyperleptinemic prepubertal rats and leptin receptor (LEPR)-mutated fa/fa Zucker rats [50]. While transgenic GPR54 expression in LEPR-deficient mice did not induce sterility, these findings support the kisspeptin-leptin intermediate hypothesis. Leptin's actions extend beyond the hypothalamus to extra-hypothalamic brain regions, contributing to Selective Leptin Resistance (SLR), particularly in Diet-Induced Obese (DIO) mice within the Arcuate nucleus (ARC) [51-53]. Further research is needed to elucidate the precise mechanisms underlying leptin's effects on the ARC, NPY, and POMC in the context of obesity in females.
Leptin receptors are indeed present in the pituitary gland, highlighting the hormone's involvement in regulating reproductive processes [54]. Evidence demonstrates that leptin plays a crucial role in promoting reproductive permissiveness. Mice unable to produce leptin (Lep/Lep) are known to be infertile [55]. Furthermore, when the signaling portion of LEPR is specifically deleted in gonadotrophs, it reduces fertility in female mice. Leptin is a pulsatile hormone whose variations are linked to changes in LH and E2 levels. During fasting, LH pulsatility is suppressed, but leptin restores it, indicating its hypothalamic site of action since LH is controlled by GnRH [56]. Targeting leptin in obesity can potentially restore hormonal balance within the Hypothalamic-Pituitary-Gonadal (HPG) axis, thereby improving reproductive health. Leptin's regulatory influence extends to the ovaries, where it acts through its own receptors located in the ovary. Leptin is involved in regulating key aspects of ovarian function, including the menstrual cycle, ovulation, oocyte maturation, and ovarian steroidogenesis in granulosa and thecal cells, all of which are essential for fertility. Leptin stimulates ovarian 17-hydroxylase activity, a crucial enzyme in ovarian steroidogenesis [56]. Despite adipose tissue being the primary source of leptin, the ovary also produces it locally. Studies suggest that LEPR plays a direct role in normal ovarian function, particularly in ovulation [57]
b) Adiponectin Regulation of HPO Axis
Adiponectin, exerts a significant influence on the neuroendocrine reproductive axis, impacting all three pillars of the HPO axis: the hypothalamus, pituitary gland, and ovaries. Both adiponectin receptors, AdipoR1 and AdipoR2, are expressed in these key reproductive organs, indicating their involvement in the regulation of reproductive processes [58-59]. In the hypothalamus, AdipoR1 is located in the arcuate and lateral nuclei, suggesting adiponectin-mediated modulation of the neuroendocrine reproductive axis [60-61]. Evidence supports the idea that adiponectin can inhibit GnRH secretion through AMPK activation in hypothalamic GT1-7 cell lines [62].
Within the pituitary gland, somatotrophs and gonadotrophs express AdipoR1 and AdipoR2, indicating the presence of a regulatory system for adiponectin. Adiponectin has been shown to regulate somatotroph cell function, inhibiting basal GH release in rats while simultaneously increasing the expression of GHS-R and GHRH-R mRNA [63]. This dual short-term action, reducing hormone secretion while enhancing key stimulatory receptors in somatotrophs, underscores the complexity of adiponectin's effects [64]. However, these stimulatory effects were not observed in gonadotropic GnRHR expression [65]. Adiponectin also regulates its own receptor expression (AdipoR1 and AdipoR2) within the pituitary [63].
Adiponectin significantly impacts ovarian functions, influencing steroidogenesis either independently or in synergy with gonadotropins through cross-talk mechanisms [62]. AdipoR1 is found in various ovarian components, including follicular fluid, oocytes, corpus luteum, GCs, and theca cells, highlighting its broad influence on ovarian steroidogenesis and oocyte maturation [66]. Adiponectin also acts as an insulin sensitizer, enhancing insulin action and lowering glucose production. Obesity-related conditions can lead to a reduction in oocyte quality and maturity, potentially contributing to infertility due to abnormal adiponectin production [67,68].
Adiponectin's effects extend to steroid hormone secretion. It has been observed to increase progesterone and estradiol secretion in rat and human GCs in response to Insulin-Like Growth Factor-1 (IGF-1), likely through the activation of the IGF-1 receptor and increased aromatase expression [62,69]. AdipoR2, on the other hand, plays a role in steroidogenesis in human GCs [70]. In vitro studies have demonstrated that adiponectin increases the expression of ovulation-related molecules such as COX2, PGE2, and EGF in GCs and enhances embryo development [71]. Conversely, adiponectin suppresses androstenedione production in ovarian theca cells by reducing the expression of LHR and the steroidogenic enzymes CYP11A1 and CYP17A1 [72]. Knockdown of AdipoR1 and AdipoR2 genes has revealed increased androstenedione secretion [73]. The elevation of adiponectin levels in ovarian follicular fluid in response to recombinant LH further emphasizes its role in the regulation of ovarian functions [74]. While numerous studies have explored the influence of adiponectin on various physiological processes, its direct impact on the HPO axis remains an active and expanding area of research.
Adipokines: Key Players in Obesity and Reproduction
Adipokines are a diverse group of hormones and signaling molecules secreted by adipose tissue, collectively exerting substantial influence over the body's physiological processes, including inflammation and metabolism. These adipose-derived molecules operate both locally and systemically, contributing significantly to overall health. Leptin, a well-established adipokine, serves as a critical messenger to the brain, regulating appetite and energy balance, thus playing a pivotal role in weight regulation [75]. Adiponectin, another significant adipokine, is known for its anti-inflammatory properties and ability to enhance insulin sensitivity, aiding blood glucose control and reducing the risk of type 2 diabetes [76]. Resistin, while still undergoing research, is suspected of possessing pro-inflammatory characteristics and potential connections to metabolic disorders. These multifaceted adipokines exemplify the intricate orchestration of biochemical signals originating from adipose tissue, governing essential aspects of metabolic and immune regulation within the body [77].
Furthermore, several other adipokines, such as TNF-a, IL-6, RBP-4, Visfatin, Omentin, Apelin, and FGF21, play distinctive roles in various physiological processes. For instance, TNF-a, a pro-inflammatory adipokine, contributes to insulin resistance and chronic low-grade inflammation in obesity, linking it to metabolic dysfunction [78]. IL-6, produced by adipose tissue, exhibits dual functions, promoting inflammation while also having anti-inflammatory effects within adipose tissue. Elevated IL-6 levels are associated with chronic inflammation, underpinning metabolic disorders [79]. RBP-4, secreted by adipocytes, is implicated in insulin resistance, elevating the risk of type 2 diabetes by impairing insulin sensitivity [80].
Visfatin initially considered an insulin-mimicking adipokine, is still under scrutiny for its precise metabolic role [81]. In contrast, Omentin stands as an anti-inflammatory adipokine, potentially enhancing insulin sensitivity and cardiovascular health by reducing inflammation [82]. Apelin influences blood pressure, fluid balance, and energy metabolism, with a role in cardiovascular function and appetite regulation [83]. Lastly, FGF21 is a key player in regulating glucose and lipid metabolism, aiding in fasting adaptation and maintaining energy balance and body weight [84]. Beyond these, other adipokines like Chemerin, FABP, and PAI-1 have specific roles in glucose metabolism, vitamin A transport, blood pressure regulation, adipocyte function, and cardiovascular health. Adipokines exert significant control over various aspects of female reproductive health, ranging from hormone regulation and ovarian function to inflammation and metabolic factors. Dysregulation of adipokine levels, often observed in obesity, can disrupt the delicate neuroendocrine balance essential for female reproduction, leading to a range of reproductive disorders and fertility challenges. Understanding the role of adipokines in this context is crucial for diagnosing and managing reproductive issues in women, especially those with obesity-related complications.
a) Leptin: Structure and Function
Leptin, often referred to as the "satiety hormone," plays a vital role in regulating energy balance and controlling appetite by signaling a sense of fullness to the brain. Elevated levels of leptin are frequently observed in individuals with a higher amount of adipose tissue. In terms of its structure, leptin is a hormone synthesized by adipocytes and is encoded by the human leptin gene located on chromosome 7 [75]. Its discovery was facilitated through the positional cloning of ob/ob mice [85]. Leptin exerts its effects by interacting with its receptor, ObR or LEPR, found in both peripheral tissues and the brain. The leptin receptor generates multiple isoforms through alternative splicing, including LEPR-a, LEPR-b, LEPR-c, LEPR-d, LEPR-e, and LEPR-f [86]. Among these isoforms, LEPRb, the long leptin receptor isoform, is the most crucial and efficient for signaling [87]. It is highly expressed in the hypothalamus, a pivotal brain region responsible for regulating energy balance and neuroendocrine functions [88-90]. Conversely, the ObRa isoform, the short leptin receptor isoform, is believed to facilitate leptin transport across the blood-brain barrier [91]. This complex leptin-receptor system underscores its critical role in appetite regulation and the maintenance of energy homeostasis.
Role of Leptin in Energy and Metabolism Regulation
Leptin, a pivotal neurohormone, plays a multifaceted role in regulating the body's energy status and metabolism. Extensive research, predominantly in rodent models, has underscored the correlation between leptin levels and fat mass, emphasizing its significance as a metabolic signal [92-93]. Beyond its metabolic functions, leptin is intricately intertwined with reproductive maturation and fertility, as evidenced by studies involving ob/ob mice and normal mice [41]. The interplay between leptin, growth, energy homeostasis, and reproductive processes is highly intricate [44]. A reduced leptin signal can disrupt energetically demanding reproductive events like pregnancy and lactation [55]. The interaction of circulating leptin with brain receptors initiates signaling pathways that suppress appetite and enhance energy expenditure [94-96]. Mutations affecting the leptin gene or its regulatory regions can result in severe obesity and morbid obesity in humans, often accompanied by hypogonadism [97]. Moreover, leptin's biological functions have expanded beyond combating obesity to encompass various effects on reproduction, hematopoiesis, angiogenesis, bone mass maintenance, T lymphocyte systems, lymphoid organ homeostasis, and blood pressure regulation.
Leptin and Female Reproductive Abnormalities
Elevated leptin levels have been linked to various reproductive abnormalities, including infertility, polycystic ovarian syndrome (PCOS), disruptions in the menstrual cycle, and hyperinsulinemia [98-101]. Leptin can inhibit hCG-stimulated steroidogenesis by interfering with the stimulatory action of gonadotropins [102]. It negatively impacts ovarian steroidogenesis, inhibiting progesterone production by decreasing StAR (Steroidogenic Acute Regulatory) protein expression [103]. Leptin also binds to its receptor on granulosa cells (GCs) and inhibits cAMP-stimulated steroidogenesis by activating the signaling pathway [103]. Notably, in obese animals, leptin inhibits progesterone production in GCs when leptin levels exceed a certain threshold, typically around 100 ng/ml [104]. These findings emphasize the intricate role of leptin in regulating ovarian function and its potential impact on reproductive health in different physiological and pathological contexts.
The influence of leptin on ovarian function varies depending on dosage and context. Acute leptin treatment has been observed to inhibit the ovulatory process, reducing the expression of the P450scc enzyme in the ovary, while not affecting StAR or 3ΒHSD. In contrast, daily leptin treatment can induce ovulation and elevate 3ΒHSD levels without significantly affecting other components of the steroidogenic apparatus [105]. Physiologically normal leptin levels tend to increase P450scc and 3ΒHSD expression, whereas an increase in leptin levels results in decreased 3ΒHSD expression [106]. Moreover, disrupting the LEPR in GCs diminishes leptin's ability to inhibit cAMP-stimulated progesterone production [107]. Leptin has a documented role in reducing estradiol and progesterone production in ovarian GCs [108]. In women with PCOS, leptin is found in the walls of polycystic follicles, and hormonal dysregulation in GCs is thought to contribute to their reduced pregnancy rates [109]. Obesity-related dysregulation of leptin signaling can lead to hormonal abnormalities, resulting in menstrual irregularities, anovulation, and infertility. Targeting leptin may offer a potential avenue to restore hormonal balance within the HPO axis, ultimately enhancing reproductive health.
Role of Leptin in Obesity
Leptin's role as a messenger for maintaining Body Mass Index (BMI) primarily involves communication with the hypothalamus [110]. In mice, a polymorphism in the leptin gene has been discovered to hinder functional leptin protein production [111]. Leptin's activity hinges on its interaction with the LEPR, particularly LEPR-b, which is crucial for robust signaling [112]. Dysfunctional leptin signaling is a major driver of severe obesity (Figure 2) [87]. Missense mutations in the LEPR can disrupt LEPR signaling and are linked to human obesity [113]. Experimental studies with obese mice have also demonstrated that polymorphisms in the ob gene can alter leptin protein function, leading to morbid obesity [114-115], and similar polymorphisms in the LEP-R gene can induce obesity in mice [90, 116]. In humans, a single-nucleotide polymorphism in the leptin gene (LEP-2548 G/A polymorphism) is associated with obesity. Additionally, research has explored genetic and epigenetic factors influencing leptin expression, including the distant Leptin Enhancer 1 (LE1) sequence, critical for fat-regulated expression [117].
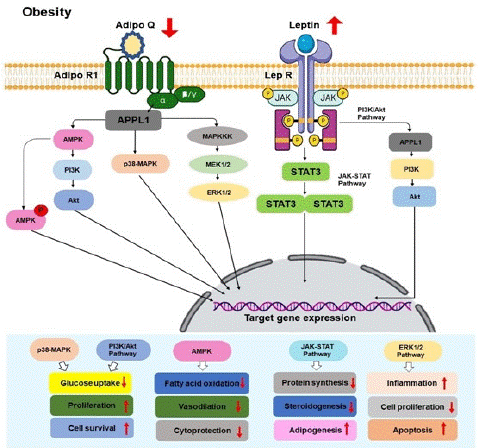
Figure 2: Signaling pathways show the interaction of leptin and adiponectin and their downstream signaling pathways in the ovary in the obese condition. Red upward-pointing arrows indicate stimulatory effects, while red downward-pointing arrows signify a decrease or inhibitory effect. Leptin exerts its stimulatory effects in obesity through multiple signaling pathways upon binding to its receptor. The JAK-STAT3 pathway involves phosphorylation of LepR, leading to STAT3 activation. Leptin also inactivates FoxO1 by sequestering it in the cytoplasm, activating the PI3K pathway by recruiting IRS proteins and triggering the ERK pathway. In contrast, adiponectin downregulates signaling through AdipoR1 receptors, initiating phosphorylation of AMPK, activation of MAPK pathways like ERK1/2, and the induction or repression of target gene expression. Additionally, adiponectin’s signal transduction may involve the translocation of APPL1 to the nucleus, where it interacts with chromatin-remodeling complexes, influencing transcription processes. These intricate pathways mediate the biological effects of leptin and adiponectin in various physiological processes.
Non-coding RNAs have emerged as vital contributors to obesity, including hypothalamic leptin insensitivity, where the brain becomes less responsive to leptin signaling [118]. Leptin itself influences the expression of microRNAs targeting Pro-Opiomelanocortin (POMC) mRNA, a neuropeptide involved in appetite regulation [119]. These findings emphasize the intricate molecular mechanisms governing energy balance and metabolism. In the context of female health, obesity is closely linked to PCOS, a reproductive condition characterized by elevated androgen levels, menstrual irregularities, and ovarian cysts [120]. Leptin plays a pivotal role in PCOS by regulating gonadotropin production, promoting ovarian androgen synthesis, and influencing insulin resistance. Leptin resistance, often observed in obesity, is a common feature in PCOS patients, potentially contributing to its pathogenesis. Elevated leptin levels in the presence of insulin resistance further exacerbate hormonal imbalances associated with PCOS-related symptoms [121]. Leptin's involvement in obesity extends to reproductive diseases like PCOS, highlighting its significance in understanding and addressing the complexities of obesity-related health conditions, particularly those affecting female reproductive health.
b) Adiponectin: Structure and Function
Adiponectin, a multifaceted adipokine, holds a central position in regulating metabolic homeostasis and insulin sensitivity. Its structural diversity, including trimeric (67 kDa), hexameric (a complex of two trimers, 130 kDa), and high molecular weight complexes (300 kDa), plays a significant role in determining its biological activity. The globular domain of adiponectin shares structural homology with various proteins, underscoring its versatility in interacting with different receptors and molecular partners [122]. Genetic mutations affecting the gene responsible for adiponectin production can lead to adiponectin deficiency. Intriguingly, there are multiple alternatively spliced variants of this gene, all encoding the same protein [123]. The adiponectin protein itself comprises a secretory signal sequence, a collagenous region featuring Gly-X-Y repeats, and a globular domain. Notably, the globular domain shares structural similarities with complement factor C1q, collagen alpha 1(X), and the brain-specific factor cerebellin [124]. Adiponectin, known by various aliases such as ACRP30, GBP28, ADIPOQ, and apM1, is a pivotal regulator of glucose metabolism and a crucial enhancer of insulin sensitivity [125]. Its profound properties play a critical role in sensitizing the body to insulin and mitigating inflammation. Research consistently demonstrates a strong link between reduced adiponectin levels and insulin resistance in the context of obesity. Adiponectin's structural complexity underscores its multifaceted role in glucose metabolism regulation and its potential significance in addressing insulin resistance-related conditions.
Adiponectin Signaling Pathways and Physiological Effects
Adiponectin is subject to the influence of numerous factors and physiological processes. The human adiponectin gene features binding sites for various transcription factors, including PPARγ and its coactivator PGC1, C/EBPa, LRH-1, FoxO1, SREBP-1c, ATF3, NFATc4, Id3, STAT5, CLOCK, and BMAL1 [126-134]. The regulation of these transcription factors by endogenous and exogenous signals initiates various signaling pathways within adiponectin-secreting cells. Upon binding to its specific receptors, adiponectin triggers multiple signaling pathways, including MAPKs (p38, ERK1/2), Akt, AMPK, and phosphorylation of PPAR-a [135]. Adiponectin exhibits several beneficial properties, such as anti-diabetic, anti-atherogenic, and anti-inflammatory effects. It enhances glucose utilization and fatty acid combustion through AMPK activation [136]. Additionally, it inhibits pro-inflammatory cytokine TNF-a expression and interferes with endothelial NF-kappa-B signaling [137]. Adiponectin also inhibits gluconeogenic enzymes and fatty acid oxidation in the liver, contributing to glucose and lipid metabolism regulation [138].
Adiponectin Role in Glucose and Lipid Metabolism Regulation
AdipoR1, an adiponectin receptor, is expressed not only in adipose tissue but also in pancreatic islet cells. Adiponectin's impact on insulin production in islet cells varies, particularly in insulin-resistant states. It enhances fatty acid oxidation in the liver and reduces CD36 expression, ultimately reducing fatty acid circulation and hepatic triglyceride levels. In skeletal muscle, adiponectin reduces triglyceride accumulation and promotes energy dissipation [139-141]. Post-receptor signaling pathways involving AMPK, ACC, p38 MAPK, and PPAR-a have been identified in various tissues, contributing to glucose and lipid metabolism regulation [74, 142-143]. Clinical studies consistently show that elevated Adiponectin levels are negatively associated with insulin resistance and the development of Type 2 Diabetes Mellitus (T2DM), especially when adjusted for BMI [144]. Reduced plasma Adiponectin levels are strongly linked to T2DM progression. Adiponectin enhances insulin sensitivity by reducing free fatty acid concentrations, particularly evident in lipoatrophic mice and animal obesity models. It may also activate AMPK [145].
Insulin resistance triggers endoplasmic reticulum stress, suppressing Adiponectin synthesis. Inflammation and oxidative stress in obesity hinder Adiponectin maturation [123]. Thiazolidinediones, a class of anti-diabetic drugs, elevate Adiponectin levels [145]. The Pro12Ala PPAR gene variant, known for its protective effect against T2DM, is associated with higher Adiponectin levels, while PPAR mutants with a dominant negative effect are linked to reduced Adiponectin levels, especially in diabetes, insulin resistance, and hypertension [146]. Adiponectin deficiency is consistently associated with insulin resistance and increased atherosclerosis susceptibility in animal models. Conversely, administering Adiponectin improves insulin resistance, especially in lipodystrophic or obese mice [147]. Polymorphisms in the Adiponectin gene have been identified as factors contributing to insulin resistance and an elevated risk of T2DM by altering Adiponectin expression [148]. Genetic studies support the role of reduced Adiponectin levels in increasing the risk of T2DM, emphasizing its significance in insulin resistance, T2DM, and cardiovascular pathologies [149-150].
Adiponectin and Female Reproductive Abnormalities
Adiponectin has garnered significant attention in the context of obesity and its intricate relationship with female reproductive health. Molecular studies have elucidated various mechanisms through which adiponectin is intricately involved in the pathophysiology of metabolic disorders, particularly in the context of female reproductive health. The presence of adiponectin receptors in ovarian tissues and their roles in modulating steroidogenesis, ovulation-related processes, and the impact of obesity-related conditions underscore the significance of adiponectin in maintaining reproductive homeostasis [151]. Adiponectin receptors, notably AdipoR1, are abundantly expressed in various ovarian components, including GCs, theca cells, follicular fluid, oocytes, and the corpus luteum [152]. This widespread receptor distribution underscores the broad influence of adiponectin on ovarian steroidogenesis and oocyte maturation, two crucial determinants of fertility.
Molecular studies have delved into the specific mechanisms through which adiponectin impacts ovarian function. Notably, adiponectin has been shown to modulate steroidogenesis in GCs by regulating key enzymes such as Cytochrome P450 (CYP) family members, including CYP11A1 and CYP17A1 [153]. Additionally, it can influence the expression of the LHR, a pivotal component of the reproductive axis. These findings emphasize the intricate role of adiponectin in fine-tuning the hormonal milieu necessary for successful reproduction. Furthermore, molecular investigations have revealed that adiponectin can regulate the expression of ovulation-related molecules within GCs, including COX2, PGE2, and EGF [154-155]. This suggests that adiponectin may play a role in facilitating the ovulatory process, which is critical for fertility. Further research in this field promises to provide valuable insights into potential therapeutic interventions for obesity-related female reproductive abnormalities.
Adiponectin in Obesity
Studies have shown that obesity can lead to reduced expression of the Adiponectin gene (ADIPOQ) in adipose tissue. This downregulation is associated with increased fat mass and insulin resistance. Epigenetic modifications, such as DNA methylation and histone acetylation, have been implicated in the regulation of ADIPOQ expression in obesity [156]. Obesity can disrupt the equilibrium between different Adiponectin multimers, favoring the formation of lower molecular weight isoforms over high molecular weight multimers. This shift in multimerization is closely associated with insulin resistance and metabolic dysfunction [157]. Molecular studies have highlighted the significance of post-translational modifications, such as glycosylation, in Adiponectin biology. Alterations in glycosylation patterns in obesity can impact the bioactivity of Adiponectin isoforms, affecting their ability to modulate insulin sensitivity [158]. The downstream signaling pathways initiated upon Adiponectin binding to its receptors, AdipoR1 and AdipoR2, and activating the AMPK pathway, are central to the regulation of glucose and lipid metabolism. Obesity-related alterations in these signaling cascades contribute to insulin resistance [159]. Obesity-induced changes in Adiponectin production and function also have significant implications for inflammation. Decreased Adiponectin levels are associated with increased production of pro-inflammatory cytokines, exacerbating metabolic dysfunction and insulin resistance [160]. In the context of obesity-related reproductive abnormalities, such as PCOS, molecular studies have unveiled alterations in adiponectin signaling. Reduced expression of AdipoR1 and AdipoR2, along with disrupted intracellular signaling pathways, has been observed in ovarian tissues of individuals with PCOS, particularly those who are obese [161]. These findings highlight the potential impact of obesity-induced disruptions in adiponectin signaling on the hormonal dysregulation commonly associated with PCOS.
Clinical Implications and Therapeutic Potential
Understanding the regulation of the neuroendocrine regulation of reproduction by adipokines in the context of obesity holds paramount clinical relevance. It serves as a critical window for early diagnosis and risk assessment, allowing healthcare providers to identify individuals at higher risk of metabolic and reproductive disorders. Tailored interventions can then be designed based on a patient's specific adipokine profile, addressing the root causes of their reproductive issues. This personalized approach not only enhances fertility management but also minimizes the emotional and financial burdens associated with infertility treatments. Furthermore, leveraging the protective effects of adipokines like adiponectin during pregnancy can help prevent complications such as gestational diabetes and preeclampsia, improving maternal and fetal health outcomes. Individualized treatment plans based on adipokine regulation pave the way for more effective interventions, while ongoing monitoring of adipokine levels allows for real-time adjustments, optimizing treatment efficacy. Ultimately, this comprehensive understanding of adipokine-mediated neuroendocrine regulation in obesity-related reproductive issues translates into improved long-term health outcomes by addressing the intricate interplay between obesity, adipokines, and reproductive health.
Mitigating disruptions in obesity-related reproductive issues necessitates a comprehensive approach that addresses the complex interplay of metabolic and hormonal factors. Lifestyle modifications are foundational, emphasizing weight management through a balanced diet and regular physical activity. Behavioral interventions can help individuals overcome psychological barriers to healthy habits. In cases of insulin resistance, medications like metformin may be prescribed, while hormonal treatments can regulate menstrual cycles and improve fertility. Bariatric surgery offers a viable option for severe obesity. Multidisciplinary care, including nutrition counseling and psychological support, plays a pivotal role in long-term success. Future prospects involve potential therapies targeting adipokines and continued research into personalized interventions. Ultimately, understanding the clinical relevance of adipokine regulation and implementing tailored therapeutic strategies are essential for restoring reproductive health in individuals with obesity-related issues.
Future Directions and Research Gaps
Future research in the study of obesity-related adipokines and their impact on the neuroendocrine control of female fertility holds substantial promise and presents several important avenues for exploration. Understanding the intricate mechanisms underlying adipokine dysregulation in obesity, including the role of inflammation, oxidative stress, and genetic factors, is essential for devising effective therapeutic strategies. Investigating the intracellular signaling pathways triggered by adipokine receptors, particularly in reproductive tissues, can reveal novel targets for intervention. Epigenetic modifications and their influence on adipokine-related genes should be further examined to comprehend how environmental factors affect fertility. The bidirectional relationship between the gut microbiome and adipokines in the context of reproductive health is an emerging field with significant potential. Precision medicine approaches that tailor interventions based on an individual's adipokine profile and genetic background may enhance treatment outcomes. Long-term health implications for offspring born to obese mothers require extensive investigation, encompassing metabolic health, neurodevelopment, and the risk of obesity-related conditions. Additionally, studying the role of adipokines in male fertility within the context of obesity is warranted. Clinical trials assessing therapeutic interventions targeting adipokine pathways in obese individuals with fertility issues should be prioritized, and interdisciplinary collaboration across various fields is essential for a comprehensive understanding of this multifaceted issue. In sum, future research endeavors have the potential to deepen our understanding of obesity-related adipokines and female fertility, leading to innovative diagnostic and therapeutic advancements to improve the reproductive health of affected individuals.
Conclusion
Adipokines are critical to normal reproductive physiology and are directly associated with obesity, a disease of global health concern. Adipokines possess complex interactions at all levels of the reproductive neuroendocrine axis or hypothalamus-pituitary-ovary axis. Numerous studies have witnessed that the deficiency, resistance, or excess of adipokines leads to reproductive abnormalities, such as PCOS, endometriosis, and female infertility in obese individuals. Thus, the current discussion infers that the adipokines-mediated neuroendocrine regulation of reproductive functions in obesity is essential for understanding the mechanisms underlying female reproductive disorders.
Adipokines and reproductive hormones interact within the HPO axis, and studying this interaction could reveal complex regulatory mechanisms and give insight into metabolic signaling and reproduction. In addition, adipokine profiles or signaling pathways may help in the identification of obesity-associated reproductive failure on adipokines-mediated regulation of the HPO axis, and this information may be used to develop personalized treatment action plans against reproductive diseases. Moreover, it could also be possible to discover novel biomarkers for diagnosing reproductive diseases in obese individuals. The development of new therapeutics could also be a choice by targeting the adipokine signaling pathway which may resolve the reproductive issues by positively enhancing the fertility outcomes.
Author Statements
Acknowledgment
We thank Biorender.com for being helpful in creating the illustrations and also thanks to Divyarathna Bhuvarahamurthy for editing the manuscript.
Funding
This study received no external funding.
Declaration of Competing Interest
The authors declare no conflict of interest.
Data Availability Statement
Not applicable; no unpublished data is included.
Ethical Approval
Not applicable; this is a review manuscript.
References
- World Health Organization. Obesity: preventing and managing the global epidemic: report of a WHO consultation on obesity. Geneva, Switzerland: World Health Organization; 1998.
- Bray GA, Kim KK, Wilding JPH, World Obesity Federation. Obesity: A chronic relapsing progressive disease process. A position statement of the World Obesity Federation. Obes Rev. 2017; 18: 715-23.
- Bartolomucci A, Cabassi A, Govoni P, Ceresini G, Cero C, Berra D, et al. Metabolic consequences and vulnerability to diet-induced obesity in male mice under chronic social stress. PLOS ONE. 2009; 4: e4331.
- Piernas C, Popkin BM. Snacking increased among U.S. adults between 1977 and 2006. J Nutr. 2010; 140: 325-32.
- Ledikwe JH, Blanck HM, Khan LK, Serdula MK, Seymour JD, Tohill BC, et al. Low-energy-density diets are associated with high diet quality in adults in the United States. J Am Diet Assoc. 2006; 106: 1172-80.
- Malik VS, Hu FB. The role of sugar-sweetened beverages in the global epidemics of obesity and chronic diseases. Nat Rev Endocrinol. 2022; 18: 205-18.
- Young LR, Nestle M. The contribution of expanding portion sizes to the U.S. obesity epidemic. Am J Public Health. 2002; 92: 246-9.
- Akyol A, McMullen S, Langley-Evans SC. Glucose intolerance associated with early-life exposure to maternal cafeteria feeding is dependent upon post-weaning diet. Br J Nutr. 2012; 107: 964-78.
- Klop B, Elte JW, Cabezas MC. Dyslipidemia in obesity: mechanisms and potential targets. Nutrients. 2013; 5: 1218-40.
- GBD 2015 Obesity Collaborators, Afshin A, Forouzanfar MH, Reitsma MB, Sur P, Estep K, et al. Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med. 2017; 377: 13-27.
- Practice Committee of the American Society for Reproductive Medicine. Electronic address: asrm@asrm.org, Practice Committee of the American Society for Reproductive Medicine. Obesity and reproduction: a committee opinion. Fertil Steril. 2021; 116: 1266-85.
- Nianogo RA, Arah OA. Forecasting obesity and Type 2 diabetes incidence and burden: the ViLA-obesity simulation model. Front Public Health. 2022; 10: 818816.
- Risk NCD, Factor Collaboration. Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4.4 million participants. Lancet. 2016; 387: 1513-30.
- Egan N, Bartels Å, Krettek A, Nguyen TV, O’Byrne KT. The endocrinology of human pregnancy and fetal-placental neuroendocrine development. J Clin Endocrinol Metab. 2020; 105: e2854-e2869.
- Iwasa T, Matsuzaki T, Yano K, Munkhzaya M, Tungalagsuvd A, Kawami T, et al. Developmental changes in the hypothalamic mRNA expression levels of brain-derived neurotrophic factor and serum leptin levels: their responses to fasting in male and female rats. Int J Dev Neurosci. 2014; 36: 33-9.
- Tang T, Glanville J, Hayden CJ. Anovulation due to obesity: interaction between insulin sensitivity and anti-Müllerian hormone. Fertil Steril. 2018; 110: e27.
- Tersigni C, Di Simone N, D’Ippolito S, Di Nicuolo F, Castellani R, Scambia G. Adipokines: new emerging roles in fertility and reproduction. Obstet Gynecol Int. 2018: 1-10.
- Van Vliet-Ostaptchouk JV, Nuotio ML, Slagter SN, Doiron D, Fischer K, Foco L, et al. The prevalence of metabolic syndrome and metabolically healthy obesity in Europe: A collaborative analysis of ten large cohort studies. BMC Endocr Disord. 2012; 12: 1-13.
- Rosen ED, Spiegelman BM. Adipocytes as regulators of energy balance and glucose homeostasis. Nature. 2006; 444: 847-53.
- Chait A, den Hartigh LJ. Adipose tissue distribution, inflammation and its metabolic consequences, including diabetes and cardiovascular disease. Front Cardiovasc Med. 2020; 7: 22.
- Kelesidis T, Kelesidis I, Chou S, Mantzoros CS. Narrative review: the role of leptin in human physiology: emerging clinical applications. Ann Intern Med. 2010; 152: 93-100.
- Hausman DB, DiGirolamo M, Bartness TJ, Hausman GJ, Martin RJ. The biology of white adipocyte proliferation. Obes Rev. 2001; 2: 239-54.
- Unamuno X, Gómez-Ambrosi J, Rodríguez A, Becerril S, Frühbeck G, Catalán V. Adipokine dysregulation and adipose tissue inflammation in human obesity. Eur J Clin Investig. 2018; 48: e12997.
- Brewer CJ, Balen AH. The adverse effects of obesity on conception and implantation. Reproduction. 2010; 140: 347-64.
- Teede HJ, Hutchison SK, Zoungas S, Meyer C. Insulin resistance, the metabolic syndrome, diabetes, and cardiovascular disease risk in women with PCOS. Endocrine. 2010; 38: 141-9.
- Blair JA, McGee H, Bhatta S, Palm R, Casadesus G. Hypothalamic-pituitary-gonadal axis involvement in learning and memory and Alzheimer’s disease: more than ”just” estrogen. Front Endocrinol (Lausanne). 2015; 6: 45.
- Orbetzova MM. Clinical impact of insulin resistance in women with polycystic ovary syndrome. In: London: IntechOpen. Polycystic ovarian syndrome [internet] Wang Z, editor. 2020.
- Tsutsumi R, Webster NJ. GnRH pulsatility, the pituitary response and reproductive dysfunction. Endocr J. 2009; 56: 729-37.
- Rejani CT, Navin AK, Mumthaz TMV, Bhuvarahamurthy V. High fat-high fructose diet elicits hypogonadotropism culminating in autophagy-mediated defective differentiation of ovarian follicles. Cells. 2022; 11: 3447.
- Navin AK, Aruldhas MM, Navaneethabalakrishnan S, Mani K, Michael FM, Srinivasan N, et al. Prenatal exposure to hexavalent chromium disrupts testicular steroidogenic pathway in peripubertal F1 rats. Reprod Toxicol. 2021; 101: 63-73.
- Navin AK, Shobana N, Shankar V, Akbarsha MA, Banu SK, Aruldhas MM. Transient gestational exposure to hexavalent chromium (Cr(VI)) adversely affects testicular differentiation: A study in rat model. J Endocrinol Reprod. 2017; 21: 93-108.
- Vuolteenaho K, Koskinen A, Kukkonen M, Nieminen R, Päivärinta U, Moilanen T, et al. Leptin enhances synthesis of proinflammatory mediators in human osteoarthritic cartilage--mediator role of NO in leptin-induced PGE2, IL-6, and IL-8 production. Mediators Inflamm. 2009; 2009: 345838.
- El-Zein O, Kreydiyyeh SI. Leptin inhibits glucose intestinal absorption via PKC, p38MAPK, PI3K and MEK/ERK. PLOS ONE. 2013; 8: e83360.
- Cassano S, Pucino V, La Rocca C, Procaccini C, De Rosa V, Marone G, et al. Leptin modulates autophagy in human CD4+CD25- conventional T cells. Metabolism. 2014; 63: 1272-9.
- Ahima RS, Antwi DA. Brain regulation of appetite and satiety. Endocrinol Metab Clin North Am. 2008; 37: 811-23.
- Quennell JH, Howell CS, Roa J, Augustine RA, Grattan DR, Anderson GM. Leptin deficiency and diet-induced obesity reduce hypothalamic kisspeptin expression in mice. Endocrinology. 2011; 152: 1541-50.
- Harno E, Gali Ramamoorthy T, Coll AP, White A. POMC: the physiological power of hormone processing. Physiol Rev. 2018; 98: 2381-430.
- Childs GV, Odle AK, MacNicol MC, MacNicol AM. The importance of leptin to reproduction. Endocrinology. 2021; 162.
- Grodstein F, Goldman MB, Cramer DW. Body mass index and ovulatory infertility. Epidemiology. 1994; 5: 247-50.
- Frisch RE. The right weight: body fat, menarche and ovulation. Baillieres Clin Obstet Gynaecol. 1990; 4: 419-39.
- Cao Z, Li J, Luo L, Li X, Liu M, Gao M, et al. Molecular cloning and expression analysis of adiponectin and its receptors (AdipoR1 and AdipoR2) in the hypothalamus of the Huoyan goose during different stages of the egg-laying cycle. Reprod Biol Endocrinol. 2015; 13: 87.
- Wen JP, Lv WS, Yang J, Nie AF, Cheng XB, Yang Y, et al. Globular adiponectin inhibits GnRH secretion from GT1-7 hypothalamic GnRH neurons by induction of hyperpolarization of membrane potential. Biochem Biophys Res Commun. 2008; 371: 756-61.
- Jin L, Burguera BG, Couce ME, Scheithauer BW, Lamsan J, Eberhardt NL, et al. Leptin and leptin receptor expression in normal and neoplastic human pituitary: evidence of a regulatory role for leptin on pituitary cell proliferation. J Clin Endocrinol Metab. 1999; 84: 2903-11.
- Popovic V, Damjanovic S, Dieguez C, Casanueva FF. Leptin and the pituitary. Pituitary. 2001; 4: 7-14.
- Chan JL, Mantzoros CS. Leptin and the hypothalamic-pituitary regulation of the gonadotropin-gonadal axis. Pituitary. 2001; 4: 87-92.
- Odle AK, Akhter N, Syed MM, Allensworth-James ML, Beneš H, Melgar Castillo AI, et al. Leptin regulation of gonadotrope gonadotropin-releasing hormone receptors as a metabolic checkpoint and gateway to reproductive competence. Front Endocrinol. 2017; 8: 367.
- Childs GV, Odle AK, MacNicol MC, MacNicol AM. The importance of leptin to reproduction. Endocrinology. 2021; 162.
- Estrada KM, Pompolo S, Morris MJ, Tilbrook AJ, Clarke IJ. Neuropeptide Y (NPY) Delays the oestrogen-Induced Luteinizing Hormone (LH) Surge in the Ovariectomized Ewe: further Evidence That NPY has a Predominant Negative Effect on LH Secretion in the Ewe. J Neuroendocrinol. 2003; 15: 1011-20.
- Chehab FF. 20 years of leptin: leptin and reproduction: past milestones, present undertakings, and future endeavors. J Endocrinol. 2014; 223: T37-48.
- Pinilla L, Aguilar E, Dieguez C, Millar RP, Tena-Sempere M. Kisspeptins and reproduction: physiological roles and regulatory mechanisms. Physiol Rev. 2012; 92: 1235-316.
- Backholer K, Smith J, Clarke IJ. Melanocortins may stimulate reproduction by activating orexin neurons in the dorsomedial hypothalamus and kisspeptin neurons in the preoptic area of the ewe. Endocrinology. 2009; 150: 5488-97.
- Roa J, García-Galiano D, Castellano JM, Gaytan F, Pinilla L, Tena-Sempere M. Metabolic control of puberty onset: new players, new mechanisms. Mol Cell Endocrinol. 2010; 324: 87-94.
- Smith JT, Acohido BV, Clifton DK, Steiner RA. KiSS-1 neurons are direct targets for leptin in the ob/ob mouse. J Neuroendocrinol. 2006; 18: 298-303.
- Lapatto R, Pallais JC, Zhang D, Chan YM, Mahan A, Cerrato F, et al. Kiss1-/- mice exhibit more variable hypogonadism than Gpr54-/- mice. Endocrinology. 2007; 148: 4927-36.
- Navarro VM, Fernández-Fernández R, Castellano JM, Roa J, Mayen A, Barreiro ML, et al. Advanced vaginal opening and precocious activation of the reproductive axis by KiSS-1 peptide, the endogenous ligand of GPR54. J Physiol. 2004; 561: 379-86.
- Cravo RM, Frazão R, Perello M, Osborne-Lawrence S, Williams KW, Zigman JM, et al. Leptin signaling in Kiss1 neurons arises after pubertal development. PLOS ONE. 2013; 8: e58698.
- Fruhwürth S, Vogel H, Schürmann A, Williams KJ. Novel insights into how overnutrition disrupts the hypothalamic actions of leptin. Front Endocrinol. 2018; 9: 89.
- Mark AL. Selective leptin resistance revisited. Am J Physiol Regul Integr Comp Physiol. 2013; 305: R566-81.
- Matheny M, Shapiro A, Tümer N, Scarpace PJ. Region-specific diet-induced and leptin-induced cellular leptin resistance includes the ventral tegmental area in rats. Neuropharmacology. 2011; 60: 480-7.
- Jin L, Burguera BG, Couce ME, Scheithauer BW, Lamsan J, Eberhardt NL, et al. Leptin and leptin receptor expression in normal and neoplastic human pituitary: evidence of a regulatory role for leptin on pituitary cell proliferation. J Clin Endocrinol Metab. 1999; 84: 2903-11.
- Li C, Li Q, Li J, Zhang N, Li Y, Li Y, et al. Expression and localization of adiponectin and its receptors (AdipoR1 and AdipoR2) in the hypothalamic-pituitary-ovarian axis of laying hens. Theriogenology. 2021; 159: 35-44.
- Padmanabhan V, Cardoso RC. Neuroendocrine, autocrine, and paracrine control of follicle-stimulating hormone secretion. Mol Cell Endocrinol. 2020; 500: 110632.
- Rodriguez-Pacheco F, Martinez-Fuentes AJ, Tovar S, Pinilla L, Tena-Sempere M, Dieguez C, et al. Regulation of pituitary cell function by adiponectin. Endocrinology. 2007; 148: 401-10.
- Ibrahim Abdalla MM. Ghrelin - physiological functions and regulation. Eur Endocrinol. 2015; 11: 90-5.
- Petridou E, Mantzoros C, Dessypris N, Koukoulomatis P, Addy C, Voulgaris Z, et al. Plasma adiponectin concentrations in relation to endometrial cancer: a case-control study in Greece. J Clin Endocrinol Metab. 2003; 88: 993-7.
- Silvestris E, de Pergola G, Rosania R, Loverro G. Obesity as disruptor of the female fertility. Reprod Biol Endocrinol. 2018; 16: 22.
- Chabrolle C, Tosca L, Ramé C, Lecomte P, Royère D, Dupont J. Adiponectin increases insulin-like growth factor I-induced progesterone and estradiol secretion in human granulosa cells. Fertil Steril. 2009; 92: 1988-96.
- Pierre P, Froment P, Nègre D, Ramé C, Barateau V, Chabrolle C, et al. Role of adiponectin receptors, AdipoR1 and AdipoR2, in the steroidogenesis of the human granulosa tumor cell line, KGN. Hum Reprod. 2009; 24: 2890-901.
- Chen P, Jia R, Liu Y, Cao M, Zhou L, Zhao Z. Progress of Adipokines in the female reproductive system: A focus on polycystic ovary syndrome. Front Endocrinol (Lausanne). 2022; 13: 881684.
- Parida S, Siddharth S, Sharma D. Adiponectin, Obesity, and cancer: clash of the bigwigs in health and disease. Int J Mol Sci. 2019; 20: 2519.
- Ledoux S, Campos DB, Lopes FL, Dobias-Goff M, Palin MF, Murphy BD. Adiponectin induces periovulatory changes in ovarian follicular cells. Endocrinology. 2006; 147: 5178-86.
- Lagaly DV, Aad PY, Grado-Ahuir JA, Hulsey LB, Spicer LJ. Role of adiponectin in regulating ovarian theca and granulosa cell function. Mol Cell Endocrinol. 2008; 284: 38-45.
- Comim FV, Hardy K, Franks S. Adiponectin and its receptors in the ovary: further evidence for a link between obesity and hyperandrogenism in polycystic ovary syndrome. PLOS ONE. 2013; 8: e80416.
- Gutman G, Barak V, Maslovitz S, Amit A, Lessing JB, Geva E. Recombinant luteinizing hormone induces increased production of ovarian follicular adiponectin in vivo: implications for enhanced insulin sensitivity. Fertil Steril. 2009; 91: 1837-41.
- Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994; 372: 425-32.
- Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, et al. Adiponectin stimulates glucose utilization and fatty acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002; 8: 1288-95.
- Steppan CM, Bailey ST, Bhat S, Brown EJ, Banerjee RR, Wright CM, et al. The hormone resistin links obesity to diabetes. Nature. 2001; 409: 307-12.
- Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-a: direct role in obesity-linked insulin resistance. Science. 1993; 259: 87-91.
- Mohamed-Ali V, Goodrick S, Rawesh A, Katz DR, Miles JM, Yudkin JS, et al. Subcutaneous adipose tissue releases interleukin-6, but not tumor necrosis factor-a, in vivo. J Clin Endocrinol Metab. 1997; 82: 4196-200.
- Yang Q, Graham TE, Mody N, Preitner F, Peroni OD, Zabolotny JM, et al. Serum retinol-binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature. 2005; 436: 356-62.
- Fukuhara A, Matsuda M, Nishizawa M, Segawa K, Tanaka M, Kishimoto K, et al. Visfatin: a protein secreted by visceral fat that mimics the effects of insulin. Science. 2005; 307: 426-30.
- Yang RZ, Lee MJ, Hu H, Pollin TI, Ryan AS, Nicklas BJ, et al. Acute-phase serum amyloid A: an inflammatory adipokine and potential link between obesity and its metabolic complications. PLOS Med. 2006; 3: e287.
- Tatemoto K, Hosoya M, Habata Y, Fujii R, Kakegawa T, Zou MX, et al. Isolation and characterization of a novel endogenous peptide ligand for the human APJ receptor. Biochem Biophys Res Commun. 1998; 251: 471-6.
- Kharitonenkov A, Shiyanova TL, Koester A, Ford AM, Micanovic R, Galbreath EJ, et al. FGF-21 as a novel metabolic regulator. J Clin Invest. 2005; 115: 1627-35.
- Münzberg H, Morrison CD. Structure, production and signaling of leptin. Metabolism. 2015; 64: 13-23.
- Gorska E, Popko K, Stelmaszczyk-Emmel A, Ciepiela O, Kucharska A, Wasik M. Leptin receptors. Eur J Med Res. 2010; 15: 50-4.
- Nanjappa V, Raju R, Muthusamy B, Sharma J, Thomas JK, Haridas Nidhina PAH, et al. A comprehensive curated reaction map of leptin signaling pathway. J Proteomics Bioinform. 2011; 4: 184-9.
- Park HK, Ahima RS. Physiology of leptin: energy homeostasis, neuroendocrine function and metabolism. Metabolism. 2015; 64: 24-34.
- Elmquist JK, Bjørbaek C, Ahima RS, Flier JS, Saper CB. Distributions of leptin receptor mRNA isoforms in the rat brain. J Comp Neurol. 1998; 395: 535-47.
- Lee GH, Proenca R, Montez JM, Carroll KM, Darvishzadeh JG, Lee JI, et al. Abnormal splicing of the leptin receptor in diabetic mice. Nature. 1996; 379: 632-5.
- Bjørbaek C. Central leptin receptor action and resistance in obesity. J Investig Med. 2009; 57: 789-94.
- Li MD. Leptin and beyond: an odyssey to the central control of body weight. Yale J Biol Med. 2011; 84: 1-7.
- Chen G, Koyama K, Yuan X, Lee Y, Zhou YT, O’Doherty R, et al. Disappearance of body fat in normal rats induced by adenovirus-mediated leptin gene therapy. Proc Natl Acad Sci USA. 1996; 93: 14795-9.
- Saxena NK, Vertino PM, Anania FA, Sharma D. Leptin-induced growth stimulation of breast cancer cells involves recruitment of histone acetyltransferases and mediator complex to cyclin D1 promoter via activation of Stat3. J Biol Chem. 2007; 282: 13316-25.
- Vuolteenaho K, Koskinen A, Kukkonen M, Nieminen R, Päivärinta U, Moilanen T, et al. Leptin enhances synthesis of proinflammatory mediators in human osteoarthritic cartilage--mediator role of NO in leptin-induced PGE2, IL-6, and IL-8 production. Mediators Inflamm. 2009; 2009: 345838.
- Otero M, Lago R, Lago F, Reino JJ, Gualillo O. Signalling pathway involved in nitric oxide synthase type II activation in chondrocytes: synergistic effect of leptin with interleukin-1. Arthritis Res Ther. 2005; 7: R581-91.
- Zhang F, Chen Y, Heiman M, DiMarchi R. Leptin: structure, function and biology. Vitam Horm. 2005; 71: 345-72.
- Rojas J, Chávez M, Olivar L, Rojas M, Morillo J, Mejías J, et al. Polycystic ovary syndrome, insulin resistance, and obesity: navigating the pathophysiologic labyrinth. Int J Reprod Med. 2014; 2014: 719050.
- Grodstein F, Goldman MB, Cramer DW. Body mass index and ovulatory infertility. Epidemiology. 1994; 5: 247-50.
- Frisch RE. The right weight: body fat, menarche and ovulation. Baillieres Clin Obstet Gynaecol. 1990; 4: 419-39.
- Hartz AJ, Barboriak PN, Wong A, Katayama KP, Rimm AA. The association of obesity with infertility and related menstrual abnormalities in women. Int J Obes. 1979; 3: 57-73.
- Spicer LJ, Francisco CC. Adipose obese gene product, leptin, inhibits bovine ovarian thecal cell steroidogenesis. Biol Reprod. 1998; 58: 207-12.
- Procaccini C, Lourenco EV, Matarese G, La Cava A. Leptin signaling: A key pathway in immune responses. Curr Signal Transduct Ther. 2009; 4: 22-30.
- Misch M, Puthanveetil P. The head-to-toe hormone: leptin as an extensive modulator of physiologic systems. Int J Mol Sci. 2022; 23: 5439.
- Bilbao MG, Di Yorio MP, Faletti AG. Different levels of leptin regulate different target enzymes involved in progesterone synthesis. Fertil Steril. 2013; 99: 1460-6.
- Zhang J, Li K, Yuan M, Zhang J, Huang G, Ao J, et al. A high-fat diet impairs reproduction by decreasing the IL1β level in mice treated at immature stage. Sci Rep. 2017; 7: 567.
- Wolodko K, Castillo-Fernandez J, Kelsey G, Galvão A. Revisiting the impact of local leptin signaling in folliculogenesis and oocyte maturation in obese mothers. Int J Mol Sci. 2021; 22: 4270.
- Tsai EM, Yang CH, Chen SC, Liu YH, Chen HS, Hsu SC, et al. Leptin affects pregnancy outcome of in vitro fertilization and steroidogenesis of human granulosa cells. J Assist Reprod Genet. 2002; 19: 169-76.
- Chakrabarti J. Serum leptin level in women with polycystic ovary syndrome: correlation with adiposity, insulin, and circulating testosterone. Ann Med Health Sci Res. 2013; 3: 191-6.
- Ramos-Lobo AM, Donato J. The role of leptin in health and disease. Temperature (Austin). 2017; 4: 258-91.
- Gruzdeva O, Borodkina D, Uchasova E, Dyleva Y, Barbarash O. Leptin resistance: underlying mechanisms and diagnosis. Diabetes Metab Syndr Obes. 2019; 12: 191-8.
- Tsirigotaki A, Dansercoer A, Verschueren KHG, Markovic I, Pollmann C, Hafer M, et al. Mechanism of receptor assembly via the pleiotropic adipokine Leptin. Nat Struct Mol Biol. 2023; 30: 551-63.
- Kimber W, Peelman F, Prieur X, Wangensteen T, O’Rahilly S, Tavernier J, et al. Functional characterization of naturally occurring pathogenic mutations in the human leptin receptor. Endocrinology. 2008; 149: 6043-52.
- Hirasawa T, Ohara T, Makino S. Genetic typing of the mouse ob mutation by PCR and restriction enzyme analysis. Exp Anim. 1997; 46: 75-8.
- Igel M, Becker W, Herberg L, Joost HG. Hyperleptinemia, leptin resistance, and polymorphic leptin receptor in the New Zealand obese mouse. Endocrinology. 1997; 138: 4234-9.
- Vaisse C, Halaas JL, Horvath CM, Darnell JE, Stoffel M, Friedman JM. Leptin activation of Stat3 in the hypothalamus of wild-type and ob/ob mice but not db/db mice. Nat Genet. 1996; 14: 95-7.
- Zhang Y, Dallner OS, Nakadai T, Fayzikhodjaeva G, Lu YH, Lazar MA, et al. A noncanonical PPARγ/RXRa-binding sequence regulates leptin expression in response to changes in adipose tissue mass. Proc Natl Acad Sci USA. 2018; 115: E6039-47.
- Derghal A, Djelloul M, Azzarelli M, Degonon S, Tourniaire F, Landrier JF, et al. MicroRNAs are involved in the hypothalamic leptin sensitivity. Epigenetics. 2018; 13: 1127-40.
- Derghal A, Astier J, Sicard F, Couturier C, Landrier JF, Mounien L. Leptin modulates the expression of miRNAs-targeting POMC mRNA by the JAK2-STAT3 and PI3K-Akt pathways. J Clin Med. 2019; 8.
- Singh S, Pal N, Shubham S, Sarma DK, Verma V, Marotta F, et al. Polycystic ovary syndrome: etiology, current management, and future therapeutics. J Clin Med. 2023; 12: 1454.
- Purwar A, Nagpure S. Insulin resistance in polycystic ovarian syndrome. Cureus. 2022; 14: e30351.
- Hada Y, Yamauchi T, Waki H, Tsuchida A, Hara K, Yago H, et al. Selective purification and characterization of adiponectin multimer species from human plasma. Biochem Biophys Res Commun. 2007; 356: 487-93.
- Achari AE, Jain SK. Adiponectin, a therapeutic target for obesity, diabetes, and endothelial dysfunction. Int J Mol Sci. 2017; 18.
- Hu E, Liang P, Spiegelman BM. AdipoQ is a novel adipose-specific gene dysregulated in obesity. J Biol Chem. 1996; 271: 10697-703.
- Tianxin S. Kangjuan Y. Adiponectin and its association with insulin resistance and type 2 diabetes. J Genet Genomics. 2008; 35: 321-6.
- Berg AH, Combs TP, Scherer PE. ACRP30/adiponectin: an adipokine regulating glucose and lipid metabolism. Trends Endocrinol Metab. 2002; 13: 84-9.
- Qiao L, Maclean PS, Schaack J, Orlicky DJ, Darimont C, Pagliassotti M, et al. C/EBPa regulates human adiponectin gene transcription through an intronic enhancer. Diabetes. 2005; 54: 1744-54.
- Iwaki M, Matsuda M, Maeda N, Funahashi T, Matsuzawa Y, Makishima M, et al. Induction of adiponectin, a fat-derived antidiabetic and antiatherogenic factor, by nuclear receptors. Diabetes. 2003; 52: 1655-63.
- Qiao L, Shao J. SIRT1 regulates adiponectin gene expression through FoxO1-C/enhancer-binding protein alpha transcriptional complex. J Biol Chem. 2006; 281: 39915-24.
- Seo HY, Kim MK, Jung YA, Jang BK, Yoo EK, Park KG, et al. Clusterin decreases hepatic SREBP-1c expression and lipid accumulation. Endocrinology. 2013; 154: 1722-30.
- Kim HB, Kong M, Kim TM, Suh YH, Kim WH, Lim JH, et al. NFATc4 and ATF3 negatively regulate adiponectin gene expression in 3T3-L1 adipocytes. Diabetes. 2006; 55: 1342-52.
- Doran AC, Meller N, Cutchins A, Deliri H, Slayton RP, Oldham SN, et al. The helix-loop-helix factors Id3 and E47 are novel regulators of adiponectin. Circ Res. 2008; 103: 624-34.
- White UA, Maier J, Zhao P, Richard AJ, Stephens JM. The modulation of adiponectin by STAT5-activating hormones. Am J Physiol Endocrinol Metab. 2016; 310: E129-36.
- Barnea M, Chapnik N, Genzer Y, Froy O. The circadian clock machinery controls adiponectin expression. Mol Cell Endocrinol. 2015; 399: 284-7.
- Kadowaki T, Yamauchi T. Adiponectin and adiponectin receptors. Endocr Rev. 2005; 26: 439-51.
- Esfahani M, Movahedian A, Baranchi M, Goodarzi MT. Adiponectin: an adipokine with protective features against metabolic syndrome. Iran J Basic Med Sci. 2015; 18: 430-42.
- Ouchi N, Walsh K. Adiponectin as an anti-inflammatory factor. Clin Chim Acta. 2007; 380: 24-30.
- Combs TP, Berg AH, Obici S, Scherer PE, Rossetti L. Endogenous glucose production is inhibited by the adipose-derived protein Acrp30. J Clin Invest. 2001; 108: 1875-81.
- Li M, Chi X, Wang Y, Setrerrahmane S, Xie W, Xu H. Trends in insulin resistance: insights into mechanisms and therapeutic strategy. Signal Transduct Target Ther. 2022; 7: 216.
- Kharroubi I, Rasschaert J, Eizirik DL, Cnop M. Expression of adiponectin receptors in pancreatic beta cells. Biochem Biophys Res Commun. 2003; 312: 1118-22.
- Winzell MS, Nogueiras R, Dieguez C, Ahrén B. Dual action of adiponectin on insulin secretion in insulin-resistant mice. Biochem Biophys Res Commun. 2004; 321: 154-60.
- Tomas E, Tsao TS, Saha AK, Murrey HE, Zhang Cc Ebbits, Itani SI, Lodish HF, Ruderman NB. Enhanced muscle fat oxidation and glucose transport by ACRP30 globular domain: acetyl-CoA carboxylase inhibition and AMP-activated protein kinase activation. Proc Natl Acad Sci USA. 2002; 99: 16309-13.
- Wu X, Motoshima H, Mahadev K, Stalker TJ, Scalia R, Goldstein BJ. Involvement of AMP-activated protein kinase in glucose uptake stimulated by the globular domain of adiponectin in primary rat adipocytes. Diabetes. 2003; 52: 1355-63.
- Wondmkun YT. Obesity, insulin resistance, and Type 2 diabetes: associations and therapeutic implications. Diabetes Metab Syndr Obes. 2020; 13: 3611-6.
- Chiarelli F, Di Marzio D. Peroxisome proliferator-activated receptor-gamma agonists and diabetes: current evidence and future perspectives. Vasc Health Risk Manag. 2008; 4: 297-304.
- Sarhangi N, Sharifi F, Hashemian L, Hassani Doabsari M, Heshmatzad K, Rahbaran M, et al. PPARG (Pro12Ala) genetic variant and risk of T2DM: a systematic review and meta-analysis. Sci Rep. 2020; 10: 12764.
- Witka BZ, Oktaviani DJ, Marcellino M, Barliana MI, Abdulah R. Type 2 diabetes-associated genetic polymorphisms as potential disease predictors. Diabetes Metab Syndr Obes. 2019; 12: 2689-706.
- Nguyen TMD. Adiponectin: role in physiology and pathophysiology. Int J Prev Med. 2020; 11: 136.
- Lindsay RS, Funahashi T, Hanson RL, Matsuzawa Y, Tanaka S, Tataranni PA, et al. Adiponectin and development of type 2 diabetes in the Pima Indian population. Lancet. 2002; 360: 57-8.
- Palit SP, Patel R, Jadeja SD, Rathwa N, Mahajan A, Ramachandran AV, et al. A genetic analysis identifies a haplotype at adiponectin locus: association with obesity and type 2 diabetes. Sci Rep. 2020; 10: 2904.
- Palit SP, Patel R, Jadeja S, Rathwa N, Mahajan A, et al. A genetic analysis identifies a haplotype at adiponectin locus: Association with obesity and type 2 diabetes. Sci Rep. Sci Rep. 2020; 10: 7017.
- Ejarque M, et al. Adiponectin: A relevant marker to oversee metabolic syndrome. Nutrients. 2019; 11: 719.
- Estienne A, Brossaud A, Reverchon M, Ramé C, Froment P, Dupont J. Adipokines expression and effects in oocyte maturation, fertilization and early embryo development: lessons from mammals and birds. Int J Mol Sci. 2020; 21: 3581.
- Comim FV, Hardy K. Franks S. Adiponectin and its receptors in the ovary: further evidence for a link between obesity and hyperandrogenism in polycystic ovary syndrome. PLOS ONE. 2020; 15: e0243518.
- Nigro E, Scudiero O, Monaco ML, Palmieri A, Mazzarella G, Costagliola C, et al. New insight into adiponectin role in obesity and obesity-related diseases. BioMed Res Int. 2017; 2017: 1-11.
- Stepien M, Rosniak-Bak K, Paradowski M, Misztal M, Kujawa A, Banach M. Serum concentrations of adiponectin, leptin, resistin, ghrelin, and insulin and their association with obesity indices in obese normo- and hypertensive patients—pilot study. Arch Med Sci. 2019; 15: 614-22.
- Wang ZV, Scherer PE, Gupta RK. Improving metabolism by increasing energy expenditure. Mol Metab. 2015; 4: 663-71.
- Pajvani UB, Hawkins M, Combs TP, Rajala MW, Doebber T, Berger JP, et al. Complex distribution, not absolute amount of adiponectin, correlates with thiazolidinedione-mediated improvement in insulin sensitivity. J Biol Chem. 2004; 279: 12152-62.
- Waki H, Yamauchi T, Kamon J, Ito Y, Uchida S, Kita S, et al. Impaired multimerization of human adiponectin mutants associated with diabetes. Molecular structure and multimer formation of adiponectin. J Biol Chem. 2003; 278: 40352-63.
- Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K, et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med. 2001; 7: 941-6.
- Kern PA, Di Gregorio GB, Lu T, Rassouli N, Ranganathan G. Adiponectin expression from human adipose tissue: relation to obesity, insulin resistance, and tumor necrosis factor-a expression. Diabetes. 2003; 52: 1779-85.
- Wu X, et al. Adiponectin: mechanisms, diagnostics, and therapeutics. Int J Endocrinol. 2020; 2020: 1-8.