
Mini Review
Austin J Endocrinol Diabetes. 2015;2(1): 1034.
Advanced Glycation End Products and Retinal Vascular Lesions in Diabetes Mellitus
Wautier MP¹ and Wautier JL¹*
¹Faculty of medicine, University Denis Diderot Paris 7, Paris, France
*Corresponding author: Wautier JL, University Denis Diderot, 8 Avenue Leopold II, 75016 Paris, France
Received: April 10, 2015;Accepted: May 18, 2015; Published: May 29, 2015
Abstract
Diabetic retinopathy is the first cause of blindness. Two types are preeminent, dial macular edema and proliferative retinopathy. One common factor which leads to retinal lesions is hyperglycemia. High glucose level is responsible for Advanced Glycation End Products (AGE) formation. AGE bind to a receptor (RAGE) which is present on different cell types. RAGE engagement by AGE is responsible for NADPH oxidase stimulation, resulting in oxygen species formation and genes NFκB dependent transcription. Consequently a series of reactions occurred inducing endothelial dysfunction: increase in vascular permeability, pericyte apoptosis associated to enhanced Vascular Endothelial Cell Growth Factor (VEGF) secretion. All these factors are involved in the development of diabetic vascular retinopathy. Laser treatment limited vascular dysfunction leakage and proliferation. Local anti-VEGF treatment, more recently introduced as a therapeutic approach, appears to be also beneficial for patients.
Keywords: Diabetes mellitus; vascular retinopathy; Glycation; VEGF; Endothelial cells; NADPH oxidase
Retinal Vascular Lesion in Diabetes Mellitus
Main reasons for loss of vision in patients with diabetes mellitus are diabetic macular edema and Proliferative Diabetic Retinopathy (PDR). Macular edema caused disruption of the inner bloodretinal barrier [1]. Diabetic maculopathy may develop in the nonproliferative and the proliferative stage of Diabetic Retinopathy (DR). DR involves both morphological and functional changes in the retinal capillaries, including basement membrane thickening, loss of pericytes, increased permeability and vascular dysfunction .The risk of PDR is higher in type 1 diabetes than in type 2, while diabetic macular edema is more commonly found in type 2 diabetes. Retinal Vein Occlusion (RVO) is an important cause of visual loss among older adults throughout the world. RVO is the second cause of visual loss from retinal vascular disease following diabetic retinopathy. The anatomic classification includes three groups: Branch Retinal Vein Occlusion (BRVO), Central Retinal Vein Occlusion (CRVO) and Hemi Retinal Vein Occlusion (HRVO). RVO was diagnosed by the presence of retinal hemorrhages and venous dilation/tortuosity with or without retinal/disc swelling in a defined venous retinal territory: diffuse for CRVO, and located within a hemi retina for HRVO or within a retinal quadrant or less for BRVO. The most frequent and less severe type of RVO, BRVO) [2] is possibly driven by a mechanical factor because it generally occurs at an arteriovenous crossing. The most rare and sight-threatening form, Central Retinal Vein Occlusion (CRVO) remains of unknown pathophysiology. However recent work demonstrated an increased adhesiveness of Red Blood Cells (RBC) due to phosphatidylserine exposure which binds to endothelial annexin V [3]. The prevalence of BRVO is 0.6% and is 0.1% for CRVO. The prevalence of BRVO is associated with hypertension (odd ratio (OR) 5.42, 95% confidence interval (CI) 2.18, 13.47, diabetes mellitus OR 2.43, 95% CI 1.04, 5.70) [4]. Central Retinal Artery Occlusion (CRAO) is analogous to an acute stroke of the eye. The incidence is estimated to be 1 in 100 000 people and accounts for 1 in 10 000 ophthalmological outpatient visits [5]. In a previous article we observed that patients were diagnosed as CRAO on the following criteria: sudden vision loss, presence of diffuse retinal pallor with delayed arterial filling during angiography, and a cherry red foveola [3]. The retinal artery can become blocked. The most common cause is an embolus alternatively there may a sudden narrowing of the vessel by hemorrhage into an atherosclerotic plaque or inflammation (giant cell arthritis.). Retinal artery occlusion is frequently associated to hypertension, dyslipidemia and metabolic syndrome. The metabolic syndrome is a cluster of the most dangerous heart attack risk factors: diabetes and raised fasting plasma glucose, abdominal obesity, high cholesterol and high blood pressure [6].
Pathophysiology
Advanced Glycation End Products (AGEs) deleterious effect
AGEs exert deleterious effect by acting directly to induce cross linking of long-lived proteins to promote vascular stiffness and interacting with receptor for AGE (RAGE) to induce intracellular signaling leading to enhanced oxidative stress and production of pro-inflammatory cytokines [7].RAGE is a member of the immunoglobulin superfamily of molecules and the gene coding for RAGE is located on chromosome six in the Major Histocompatibility Complex region. RAGE was originally described as a transmembrane multiligand receptor [8]. In diabetes mellitus AGE present on proteins or cell membranes bind to the receptor RAGE. Engagement of endothelial RAGE induced Tissue Factor (TF) production, Intercellular Cell Adhesion Molecule-1 (ICAM-1) and Vascular Cell Adhesion Molecule-1 (VCAM-1) expression. Furthermore the binding of AGE to RAGE induced IL-6, Vascular Endothelial Growth Factor (VEGF), and Macrophage Chemoattractant Protein-1 (MCP-1) release. (Figure 1). In humans increased AGE accumulation has been found in cataract lenses. Furthermore glycation of vitreal collagen fibrils producing dissociation from hyaluronan leading to gel structure destabilization associated with vitreous liquefaction and posterior vitreous detachment in diabetes [9].
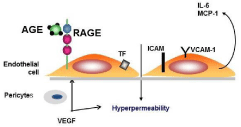
Figure 1: Endothelial dysfunction induced by Advanced Glycation End
Products (AGE and Consequences in diabetic retinopathy.
Engagement of endothelial RAGE induced Tissue Factor (TF) production,
Intercellular Cell Adhesion Molecule-1(ICAM-1) and Vascular Cell Adhesion
Molecule-1 (VCAM-1) expression, and IL-6, Vascular Endothelial Growth
Factor (VEGF), Macrophage Chemoattractant protein-1 (MCP-1) release
and increase in vascular permeability. Hyperglycemia via AGE and reactive
oxygen species lead to pericyte apoptosis.
Increased Vascular Endothelial Cell Growth Factor (VEGF) expression
The retinal alterations result in two components: macular edema due to the leakage of macromolecules such as lipoproteins into the retinal layers, and progressive capillary closure related to micro thrombosis. Biochemical alterations such as oxidative stress, activation of protein kinase C and formation of advanced glycation end products have been detected as a response of the retina to hyperglycemia [10]. Capillary closure leads to non-perfused hypoxemic retinal areas (ischemic retinopathy) which, in turn, induces the secretion of Vascular Endothelial Growth Factor (VEGF) and the development of new vessels (proliferative retinopathy) [11]. The relationship between the extent of retinal ischemia in PDR and angle neovascularization was explored by panoramic fundus fluorescein angiography and 360-degree fluorescein gonioangiography. The study revealed that retinal non perfusion in the mid periphery, capillary occlusion in the radial peripapillary capillaries; temporal raph and optic disk were risk factors for angle neovascularization [12]. VEGF, a potent vascular permeability and proangiogenic factor, has various isoforms, with VEGF165 or VEGF-A being the predominant form in humans. VEGF-A exerts its important actions on vascular endothelial cells through two specific cell surface receptor tyrosine kinases, VEGFreceptor 1 (VEGF-R1 [Flt-1]) and VEGF receptor -2 (VEGFR-2 [Flk- 1/KDR]) of which VEGFR-2 has been reported to transduce the major signals for angiogenesis [13]. The role of chronic hyperglycemia in the development of diabetic retinopathy has been established both in type 1 diabetes mellitus by the Diabetes Control and Complications Trial, [14] and in type 2 diabetes mellitus [15]. Glycated hemoglobin has been used for years for monitoring diabetic treatment. Hemoglobin is glycated at two sites: on the valine residue of the N Terminal beta chains at the epsilon amino group of the alpha and beta chains, and at the N termini of the alpha chains [16]. Moreover, a positive correlation has been established between the level of hyperglycemia as indicated by elevated glycated hemoglobin (HbA1c) and the prevalence and the severity of retinal lesions [17]. The role of AGE in the development of retinopathy has been suspected for years. In diabetic patients, an increase in skin concentration of pentosidine is associated with the development of proliferative retinopathy [18]. The same holds true for 2-(2-fuoryl)-4(5)-(2-furanyl)-1H-imidazole (FFI), N-epsilon Carboxy Methyl Lysine (CML) and total fluorescence, which increase in parallel with the increasing severity of retinopathy [19]. Other intracellular and membrane proteins of Red Blood Cells (RBC) are also glycated, for example spectrin, a major RBC membrane protein, band 3 transmembrane protein, and band 4–1 [20]. The glycation results in reduced RBC deformability and an increased adherence to endothelium [11,21] . Anti-AGE antibodies or soluble RAGE (sRAGE) inhibit the enhanced adhesion to endothelium when incubated with RBC from diabetic patients. The accelerated clearance of diabetic rat RBC when infused in normal rats is prevented by the infusion of anti-RAGE antibody in the animal. These results support the concept that the abnormal adhesion of RBC taken from diabetics is mediated by AGE present on RBC and RAGE expressed at the endothelial cell surface [22].
Consequences of NADPH oxidase stimulation
Engagement of RAGE by products of nonenzymatic glycation/ oxidation triggers the generation of reactive oxygen species (ROS), thereby altering gene expression. A major consequence of ligand engagement of RAGE is activation of multiple signaling pathway, including p21ras, erk 1/2 (p44/p42) MAP kinases, and downstream effectors such as NF-kB (Figure 2). We showed that incubation of human endothelial cells with AGEs on the surface of diabetic red blood cells, or specific AGEs, CML-modified adducts, prompted intracellular generation of hydrogen peroxide, cell surface expression of vascular cell adhesion molecule-1, and generation of tissue factor in a manner suppressed by treatment with diphenyliodonium, but not by inhibitors of nitric oxide. Consistent with an important role for NADPH oxidase, although macrophages derived from wild-type mice expressed enhanced levels of tissue factor upon stimulation with AGE, macrophages derived from mice deficient in a central subunit of NADPH oxidase, gp91phox, failed to display enhanced tissue factor in the presence of AGE [23]. These findings demonstrate a major role of NADPH oxidase in AGE-RAGE-mediated generation of Reactive Oxygen Species (ROS) and provide a mechanism for altered gene expression in AGE-related disorders. The increase in permeability found in several organs has been shown to be secondary to the AGE– RAGE interaction and to the ROS [24] but it is also known that ketone bodies may alter intestinal vascular permeability. Other studies have addressed the specific putative mechanisms of AGE toxicity. In vitro, the toxic effects of high glucose concentrations on capillary pericytes have been shown to be inhibited by aminoguanidine, suggesting a role for AGE. The importance of Endothelial Cells (EC) and pericytes in DR has led to research into therapies to prevent their dysfunction, before the onset of clinically overt vascular abnormalities, which become resistant to therapy over time [25]. An improved understanding of the role played by pericyte and EC dysfunction can open new avenues for treatments that reverse their dysfunction and lead to enhanced insights into retinal vascular regulation in DR. Further insight as to the mechanisms involved has come from recent studies which indicate that the toxic effects of AGE on retinal capillary pericytes and endothelial cells in culture can be blocked by RAGE antibodies. A role for VEGF in vascular dysfunction (increased blood flow and vascular permeability) related to pseudo-hypoxemic changes have been suggested by recent experiments. These effects were prevented by neutralizing VEGF antibodies.
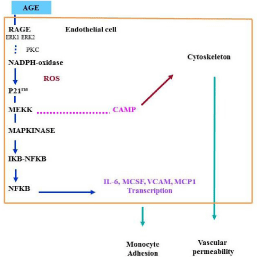
Figure 2: NADPH-oxidase stimulation after binding of AGE to RAGE,
transduction mechanism, NFKB activation and gene transcription.
Role of soluble RAGE (sRAGE)
Retinopathy and glomerulosclerosis are common complications of diabetes mellitus. There is substantial evidence to support the involvement of Advanced Glycation End-Products (AGE) binding to its receptor (RAGE) in the development of diabetic microvascular complications [26]. Activation of RAGE induces oxidative stress, increased permeability and an inflammatory response in the vessel wall [27]. Recently, a soluble isoform of RAGE (sRAGE), generated by splicing the RAGE gene transcript, has been described [28]. In diabetic patients with end-stage renal disease, low circulating sRAGE is a predictor of cardiovascular mortality suggesting that sRAGE may protect against AGE-mediated vessel damage. We previously observed that N-epsilon Carboxy Methyl Lysine (CML)-protein was increased in diabetic patients with microvascular complications. Lower levels of sRAGE were found in patients who developed both diabetic retinopathy and nephropathy. CML-protein was increased in all diabetic patients, but was highest in those with microvascular complications [28,29]. The association between low levels of sRAGE and high levels of CML-protein in patients with microvascular complications suggests that the production of sRAGE was probably insufficient to clear the excess CML-protein. This unbalanced ratio allowed circulating AGE to bind to cell RAGE, leading to endothelial dysfunction, an early step in diabetic microvascular complications. On the other hand, patients with higher sRAGE and lower CMLprotein levels did not exhibit vascular complications. sRAGE may act as a decoy of AGE to maintain vascular homeostasis. This hypothesis is consistent with the results showing that, in type-2 diabetes, a decrease of sRAGE is associated to a high incidence of cardiovascular mortality. In type-1 diabetes, a low sRAGE blood level is related to the severity of retinopathy and to vessel-wall thickness [30]. In type- 2 diabetes, sRAGE is correlated with serum levels of macrophage colony-stimulating factor (MCSF) and tumor necrosis factor alpha (TNF-alpha). sRAGE has been proposed as a possible marker of vascular inflammation [31]. Blocking RAGE activation with the use of recombinant sRAGE limited the development of atherosclerotic lesions in ApoE-null mice, in which RAGE ligands mediate vascular and inflammatory stress [32]. sRAGE expression is affected by drugs such as Angiotensin Converting Enzyme (ACE) inhibitors and rosiglitazone, and patients treated with these drugs have significantly higher levels of circulating sRAGE [33]. Thus, stimulation of sRAGE production should be considered as a potential therapeutic in diabetes and AGE related vascular disease.
The renin-angiotensin system
The Renin-Angiotensin System (RAS) is known to play an important role in controlling blood pressure, fluid homeostasis, and salt balance [34]. Angiotensin II (Ang II) is the most physiologically active component of RAS. The various effects of Ang II depend on time and on the cells/tissues upon which acts. Beside G-protein and non-G protein related signaling pathways, Ang II, via AT 1 receptors (AT1R) carries out its functions via MAP kinases (erk 1/2, JNK, p38MAP kinase). AT1 receptor mediated NADPH oxidase activation leads to generation of reactive oxygen species, widely implicated in vascular inflammation [35]. Pro-renin has long been considered an inactive precursor of renin, without any biologic function of its own. However it binds to the (pro) renin receptor (PRR), which has a high homology with an accessory protein of vacuolar-ATPase, H+ Transporting Lysosomal accessory protein 2 (ATP6AP2). ATP6AP2 has recently been reported to exert the biologic effects in the neural retina and Retinal Pigment Epithelium (RPE) [36]. A local RAS with all its components is expressed in the retina, Müller cells, RPE, and retinal endothelial cells [37].
Therapy for diabetic retinopathy
In developed countries, Dial Macular Edema (DME) has now overtaken PDR as the more common vision-threatening form of diabetic retinopathy, particularly among patients with type 2 diabetes. The incidence of visual impairment among people with diabetic retinopathy has halved, likely as a result of a lower risk of DME and PDR among patients with recently diagnosed diabetes [38,39]. Systemic management of hyperglycemia, hypertension, and dyslipidemia remains the most important and effective strategy for preventing the development and progression of diabetic retinopathy [40]. According to Cochrane Database, laser photocoagulation is beneficial in treating PDR, but the evidence is moderate or low. However this is based on trials conducted many years ago. Now panretinal photocoagulation is the most currently used technique [41]. The evidence of the efficacy and safety of anti-VEGF for the treatment of PDR is low. However the results of control trials suggest that anti-VEGF can reduce the risk of intra-ocular bleeding in patients with PDR [42]. Future research on laser photocoagulation should investigate the combination of laser photocoagulation with anti-VEGF. Pars-plana vitrectomy is performed in advanced cases of PDR, the presence of extensive tractional membranes, vitreous hemorrage and tractional retinal detachment [1]. Over the last decade, intraocular administration of pharmacological agents (e.g., steroid and anti-VEGF agents) has been evaluated as a new treatment modality for DME and PDR. Delivery of these agents is achieved by direct injection into the vitreal cavity. Although intraocular injections of long-acting steroids (e.g., triamcinolone) have demonstrated ability to reduce DME and improve vision, these beneficial effects appear to be short-lived, and long-term visual outcome was generally not better than conventional laser therapy [8].
In recent years, there has been a surge of clinical trials investigating the use of anti-VEGF therapy for DME [43-45]. These trials provide robust evidence that intraocular administration of anti-VEGF agents is better than laser therapy both in preserving and in improving vision for patients with DME. Ocular safety concerns include cataract formation, infection (endophthalmitis), vitreous hemorrhage, and retinal detachment. The rates of serious sight-threatening complications are acceptably low, as shown in studies of not only patients with diabetic retinopathy, but also of patients with age-related macular degeneration. Cost-effectiveness analyses have shown that substantial cost savings (40–88%) could be achieved by individualized treatment strategies for DME [46]. Assuming equivalent effectiveness and similar safety profiles between bevacizumab and ranibizumab injections, the use of bevacizumab confers much greater value among different treatment options for DME [47]. This is due to the substantial cost differential between the two anti-VEGF agents.
Conclusion
Systemic management of metabolic syndrome remains the most important effective strategy for preventing the development and progression of diabetic retinopathy. Hyperglycemia induced AGE formation; AGEs bind to RAGE, stimulating NADPH oxidase, ROS formation and gene transcription. Limitation of AGE formation by antidiabetic treatment limited retinal vessel damage. In experimental models in vitro or in vivo blocking RAGE (anti RAGE, peptides) prevented microvascular consequences. RAGE blood level appears to be linked to the risk of microvascular complications. Laser treatment and local anti VEGF treatment reduced significantly the rate of retinopathy evolution. Modulation of RAGE gene expression may be a future way for preventing microvascular lesions.
References
- Nentwich MM, Ulbig MW. Diabetic retinopathy - ocular complications of diabetes mellitus. World J Diabetes. 2015; 6: 489-499.
- Rogers S, McIntosh RL, Cheung N, Lim L, Wang JJ, Mitchell P, et al. The prevalence of retinal vein occlusion: pooled data from population studies from the United States, Europe, Asia, and Australia. Ophthalmology. 2010; 117: 313-319.
- Wautier MP, Heron E, Picot J, Colin Y, Hermine O, Wautier JL. Red blood cell phosphatidylserine exposure is responsible for increased erythrocyte adhesion to endothelium in central retinal vein occlusion. J Thromb Haemost. 2011; 9: 1049-1055.
- Klein R, Klein BE, Moss SE, Meuer SM. The epidemiology of retinal vein occlusion: the Beaver Dam Eye Study. Trans Am Ophthalmol Soc. 2000; 98:133-141.
- Varma DD, Cugati S, Lee AW, Chen CS. A review of central retinal artery occlusion: clinical presentation and management. Eye (Lond). 2013; 27: 688-697.
- Alberti KG, Zimmet P, Shaw J, Group Idfetfc. The metabolic syndrome--a new worldwide definition Lancet. 2005; 366: 1059-1062.
- Sharma Y, Saxena S, Mishra A, Saxena A, Natu SM. Advanced glycation end products and diabetic retinopathy. J Ocul Biol Dis Infor. 2012; 5: 63-69.
- Schmidt AM, Yan SD, Wautier JL, Stern D. Activation of receptor for advanced glycation end products: a mechanism for chronic vascular dysfunction in diabetic vasculopathy and atherosclerosis. Circ Res. 1999; 84: 489-497.
- Peppa M, Vlassara H. Advanced glycation end products and diabetic complications: a general overview. Hormones (Athens). 2005; 4: 28-37.
- Ahsan H. Diabetic retinopathy-biomolecules and multiple pathophysiology. Diabetes Metab Syndr. 2015; 9: 51-54.
- Wautier JL, Guillausseau PJ. Diabetes advanced glycation endproducts and vascular disease. Vasc Med. 1998; 3:131-137.
- Hamanaka T, Akabane N, Yajima T, Takahashi T, Tanabe A. Retinal ischemia and angle neovascularization in proliferative diabetic retinopathy. Am J Ophthalmol. 2001; 132: 648-658.
- Haque R, Hur EH, Farrell AN, Iuvone PM, Howell JC. MicroRNA-152 represses VEGF and TGFbeta1 expressions through post-transcriptional inhibition of (Pro)renin receptor in human retinal endothelial cells. Mol Vis. 2015; 21: 224-235.
- The relationship of glycemic exposure (HbA1c) to the risk of development and progression of retinopathy in the diabetes control and complications trial. Diabetes. 1995; 44: 968-983.
- Guillausseau PJ, Massin P, Charles MA, Allaguy H, Guvenli Z, Virally M, et al. Glycaemic control and development of retinopathy in type 2 diabetes mellitus: a longitudinal study. Diabet Med. 1998; 15: 151-155.
- McDonald MJ, Shapiro R, Bleichman M, Solway J, Bunn HF. Glycosylated minor components of human adult hemoglobin. Purification, identification, and partial structural analysis. J Biol Chem. 1978; 253: 2327-2332.
- Chase HP, Jackson WE, Hoops SL, Cockerham RS, Archer PG, O'Brien D. Glucose control and the renal and retinal complications of insulin-dependent diabetes. JAMA. 1989; 261: 1155-1160.
- Beisswenger PJ, Moore LL, Brinck-Johnsen T, Curphey TJ. Increased collagen-linked pentosidine levels and advanced glycosylation end products in early diabetic nephropathy. J Clin Invest. 1993; 92: 212-217.
- McCance DR, Dyer DG, Dunn JA, Bailie KE, Thorpe SR, Baynes JW, et al. Maillard reaction products and their relation to complications in insulin-dependent diabetes mellitus. J Clin Invest. 1993; 92: 212-217.
- Miller JA, Gravallese E, Bunn HF. Nonenzymatic glycosylation of erythrocyte membrane proteins. Relevance to diabetes. J Clin Invest. 1980; 65: 896-901.
- Wautier JL, Paton RC, Wautier MP, Pintigny D, Abadie E, Passa P, et al. Increased adhesion of erythrocytes to endothelial cells in diabetes mellitus and its relation to vascular complications. N Engl J Med. 1981; 305: 237-242.
- Wautier JL, Wautier MP, Schmidt AM, Anderson GM, Hori O, Zoukourian C, et al. Advanced Glycation End Products (AGEs) on the surface of diabetic erythrocytes bind to the vessel wall via a specific receptor inducing oxidant stress in the vasculature: a link between surface-associated AGEs and diabetic complications. Proc Natl Acad Sci USA. 1994; 91: 7742-7746.
- Wautier MP, Chappey O, Corda S, Stern DM, Schmidt AM, Wautier JL. Activation of NADPH oxidase by AGE links oxidant stress to altered gene expression via RAGE. Am J Physiol Endocrinol Metab. 2001; 280: 685-694.
- Wautier JL, Zoukourian C, Chappey O, Wautier MP, Guillausseau PJ, Cao R, et al. Receptor-mediated endothelial cell dysfunction in diabetic vasculopathy. Soluble receptor for advanced glycation end products blocks hyperpermeability in diabetic rats. J Clin Invest. 1996; 97: 238-243.
- Chou J, Rollins S, Fawzi AA. Role of endothelial cell and pericyte dysfunction in diabetic retinopathy: review of techniques in rodent models. Adv Exp Med Biol. 2014; 801: 669-675.
- Wautier JL, Schmidt AM. Protein glycation: a firm link to endothelial cell dysfunction. Circ Res. 2004; 95: 233-238.
- Wautier JL, Guillausseau PJ. Advanced glycation end products, their receptors and diabetic angiopathy. Diabetes Metab. 2001; 27: 535-542.
- Grossin N, Wautier MP, Meas T, Guillausseau PJ, Massin P, Wautier JL. Severity of diabetic microvascular complications is associated with a low soluble RAGE level. Diabetes Metab. 2008; 34: 392-395.
- Wautier MP, Massin P, Guillausseau PJ, Huijberts M, Levy B, Boulanger E, et al. N(carboxymethyl)lysine as a biomarker for microvascular complications in type 2 diabetic patients. Diabetes Metab. 2003; 29: 44-52.
- Katakami N, Matsuhisa M, Kaneto H, Matsuoka TA, Sakamoto K, Nakatani Y, et al. Decreased endogenous secretory advanced glycation end product receptor in type 1 diabetic patients: its possible association with diabetic vascular complications. Diabetes Care. 2005; 28: 2716-2721.
- Nakamura K, Yamagishi S, Adachi H, Kurita-Nakamura Y, Matsui T, Yoshida T, et al. Serum levels of sRAGE, the soluble form of receptor for advanced glycation end products, are associated with inflammatory markers in patients with type 2 diabetes. Mol Med. 2007; 13: 185-189.
- Harja E, Bu DX, Hudson BI, Chang JS, Shen X, Hallam K, et al. Vascular and inflammatory stresses mediate atherosclerosis via RAGE and its ligands in apoE-/- mice. J Clin Invest. 2008; 118: 183-194.
- Forbes JM, Thorpe SR, Thallas-Bonke V, Pete J, Thomas MC, Deemer ER, et al. Modulation of soluble receptor for advanced glycation end products by angiotensin-converting enzyme-1 inhibition in diabetic nephropathy. J Am Soc Nephrol. 2005; 16: 2363-2372.
- Lavoie JL, Sigmund CD. Minireview: overview of the renin-angiotensin system--an endocrine and paracrine system. Endocrinology. 2003; 144: 2179-2183.
- Mehta PK, Griendling KK. Angiotensin II cell signaling: physiological and pathological effects in the cardiovascular system. Am J Physiol Cell Physiol. 2007; 292: 82-97.
- Nguyen G. Renin, (pro)renin and receptor: an update. Clin Sci (Lond). 2011; 120: 169-178.
- Wagner J, Jan Danser AH, Derkx FH, de Jong TV, Paul M, Mullins JJ, et al. Demonstration of renin mRNA, angiotensinogen mRNA, and angiotensin converting enzyme mRNA expression in the human eye: evidence for an intraocular renin-angiotensin system. Br J Ophthalmol. 1996; 80: 159-163.
- Cheung N, Wong IY, Wong TY. Ocular anti-VEGF therapy for diabetic retinopathy: overview of clinical efficacy and evolving applications. Diabetes Care. 2014; 37: 900-905.
- Antonetti DA, Klein R, Gardner TW. Diabetic retinopathy. N Engl J Med. 2012; 366: 1227-1239.
- Cheung N, Mitchell P, Wong TY. Diabetic retinopathy. Lancet. 2010; 376: 124-136.
- Evans JR, Michelessi M, Virgili G. Laser photocoagulation for proliferative diabetic retinopathy. Cochrane Database Syst Rev. 2014; 11: 011234.
- Martinez-Zapata MJ, Marti-Carvajal AJ, Sola I, Pijoan JI, Buil-Calvo JA, Cordero JA, et al. Anti-vascular endothelial growth factor for proliferative diabetic retinopathy. Cochrane Database Syst Rev. 2014; 11: 008721.
- Bandello F, Cunha-Vaz J, Chong NV, Lang GE, Massin P, Mitchell P, et al. New approaches for the treatment of diabetic macular oedema: recommendations by an expert panel. Eye (Lond). 2012; 26: 485-493.
- Zechmeister-Koss I, Huic M. Vascular endothelial growth factor inhibitors (anti-VEGF) in the management of diabetic macular oedema: a systematic review. Br J Ophthalmol. 2012; 96: 167-178.
- Virgili G, Parravano M, Menchini F, Brunetti M. Antiangiogenic therapy with anti-vascular endothelial growth factor modalities for diabetic macular oedema. Cochrane Database Syst Rev. 2012; 12: 007419.
- Smiddy WE. Clinical applications of cost analysis of diabetic macular edema treatments. Ophthalmology. 2012; 119: 2558-2562.
- Stein JD, Newman-Casey PA, Kim DD, Nwanyanwu KH, Johnson MW, Hutton DW. Cost-effectiveness of various interventions for newly diagnosed diabetic macular edema. Ophthalmology. 2013; 120: 1835-1842.