
Research Article
Austin J Endocrinol Diabetes. 2016; 3(1): 1037.
Down-Regulation of Zinc Transporter 8 (SLC30A8) in Pancreatic Beta-Cells Promotes Cell Survival
Liping Huang1,2* and Catherine P Kirschke¹
¹Obesity and Metabolism Research Unit, USDA/ARS/ Western Human Nutrition Research Center, USA
²Department of Nutrition, University of California Davis, USA
*Corresponding author: Liping Huang, USDA/ARS/ Western Human Nutrition Research Center, Davis, CA 95616, USA
Received: November 03, 2015; Accepted: February 23, 2016; Published: March 16, 2016
Abstract
The pancreatic islet contains high levels of zinc in granular vesicles of β-cells where insulin is matured, crystallized, and stored before secretion. Zinc is an essential co-factor for insulin crystallization forming dense cores in secretory granules. In insulin-containing secretory granules, zinc is mainly brought in by ZNT8 (SLC30A8), a zinc transporter predominantly expressed in pancreatic β-cells in the body. A recent study in humans has revealed that haploinsufficiency of ZNT8 may reduce the risk of Type 2 Diabetes (T2D). The mechanism by which ZNT8 haploinsufficiency protects individuals from T2D is not understood. The aim of this study was to investigate expression levels of ZNT8 in human normal and T2D pancreases and in pancreases from mice with diet-induced insulin resistance using immunohistochemistry and to understand the molecular mechanism underlying the protection from T2D by allelic deficiency of ZNT8. Our results showed that ZNT8 expression was upregulated in islets of both diabetic and insulin resistant conditions. shRNA knockdown of Znt8 expression to ~50% of the normal level (a condition which mimicked the allelic deficiency in humans) in MIN6 β-cells stimulated activations of Akt, p70S6K and p38, key kinases involved in cell proliferation and survival. Consistent with these observations, we showed that Znt8KD MIN6 β-cells had increased cell proliferation and were resistant to inflammation-induced cell death. Our results suggest that ZNT8 upregulation due to β-cell compensation during insulin resistance could be harmful for β-cell survival whereas down regulation could be beneficial, pointing to a potential target for T2D prevention and/or treatment.
Keywords: Zinc transporter 8; SLC30A8; ZNT8; MIN6 β-cells; Glucose metabolism; β-cell survival
Abbreviations
SLC30A8: Human Solute Carrier Family 30 Member 8; Slc30a8: Mouse Solute Carrier Family 30 Member 8; ZNT8: Human Zinc Transporter 8; ZnT8: Mouse Zinc Transporter 8; ZnT7: Mouse Zinc Transporter 7; ZIP, ZRT: IRT-Like Protein; MIN6: Mouse Insulinoma Cells; TNFα: Tumor Necrosis Factor α; Akt: V-Akt Murine Thymoma Viral Oncogene Homolog; p38 MAPK: Mitogen-Activated Protein Kinase; p70S6K: S6 Ribosomal Protein Kinase; KD: Knockdown; KO: Knockout
Introduction
Zinc is an essential trace metal for life. Dietary zinc deficiency causes stunted growth, impaired immune function, dermatitis, and hypogonadism. Severe zinc deficiency caused by rare genetic diseases, such as acrodermatitis enteropathica in humans and lethal milk in mice, is life threatening without treatment [1-3]. Cellular zinc homeostasis is largely maintained by two families of zinc transporters, namely SLC30A (ZNT) and SLC39A (ZIP) in humans. The ZNT and ZIP zinc transporter families contain 10 and 14 members, respectively. ZNT and ZIP proteins coordinate each other to maintain cellular zinc levels in a narrow physiological range with ZNTs controlling efflux of excess cytoplasmic zinc into the extracellular space or into intracellular organelles and/or vesicular compartments when zinc is replete and with ZIP functioning in an opposite direction.
Type 2 Diabetes (T2D) affects about 26 million people in the U.S. according to the NIH AMP factsheet of T2D. Diabetes is a leading cause of kidney failure, lower limb amputations, and blindness. Failure to produce adequate insulin from pancreatic β-cells to maintain euglycemia is one of the hallmarks of T2D. The pancreas contains high levels of zinc in the endocrine component of the organ, mostly in the β-cell of the islet of Langerhans [4,5]. Insulin is secreted from β-cells in response to increased blood glucose levels, such as after a meal. It stimulates glucose uptake, glycogenesis, and lipogenesis in insulin-sensitive tissues, such as skeletal muscle, liver, and fat [6]. It is known that zinc co-resides with insulin in secretory granules of β-cells [7] where it is integrated into insulin to form insulin-zinc crystals [8]. The importance of zinc and its roles in insulin synthesis, processing, and secretion has been demonstrated by studying zinc transporters in insulin-secreting cell lines and in knockout animal models. For example, over-expressing Zinc Transporter 7 (ZnT7) in rat insulin-secreting cells stimulates insulin transcription leading to an increase in basal insulin secretion [9]. Consistent with the function of ZnT7 in basal insulin secretion, Znt7 Knockout (KO) mice have abnormalities in fasting insulin secretion [10]. The process of converting proinsulin to insulin requires zincdependent endopeptidases (PC1 and PC2) and carboxypeptidase E [11,12]. ZNT8 has been shown to be associated with the availability of zinc ions for these zinc-dependent enzymes [13]. Inadequate zinc supply to these enzymes along the secretory pathway due to mutations in ZNT8 may have detrimental effects on proinsulin maturation [14]. Additionally, in the secretory granules, processed insulin is crystallized in the presence of zinc ions and stored before secretion [15]. ZNT8 is implicated in this process by transporting zinc ions into the secretory granule [16]. Other zinc transporters, such as ZIP14 may also play roles in insulin and glucose metabolism in the body. Mice with a Zip14-null mutation display hypoglycemia and hyper insulinemia due to decreased gluconeogenesis in the liver and enlarged islets with increased cell mass in the pancreas [17].
A recent study has demonstrated that haploinsufficiency of ZNT8 in humans reduces the risk of T2D [18]. How the haploinsufficiency of ZNT8 protect individuals from T2D is currently not understood. The aim of this study was to understand the molecular mechanism underlying the protection of T2D development from allelic deficiency of ZNT8. Our results demonstrated that the protective effect of allelic ZNT8 deficiency from T2D development could be likely via an Akt-dependent survival mechanism due to increase in β–cell proliferation and/or decrease in β-cell death.
Material and Methods
Human normal and diabetic pancreatic tissue sections
Paraffin embedded human pancreatic tissue sections were purchased from BioChain Inc. (Newark, CA). According to the manufacturer, the normal pancreatic tissue sections were prepared from pancreases of male donors at 66- and 71-year-old. The diabetic diseased pancreatic sections were prepared from a 67-year-old diabetic male donor.
Animals and preparation of mouse pancreas sections
Normal pancreases were isolated from 16-week-old male C57BL/6 (B6) mice fed a standard laboratory chow diet (Laboratory Rodent Diet 5001, Lab Diet, St. Louis, MO). Pancreases with insulin resistance were isolated from 16-week-old male B6 mice fed a high fat diet (45% kcal, Research Diets, New Brunswick, NJ) from 5- to 16-week-old (insulin resistant phenotypes were reported previously) [10]. Mice were housed in a temperature-controlled room at 22-24°C with a 12-h light: dark cycle. All animal experiments were conducted in accordance with National Institutes of Health Guidelines for the Care and Use of Experimental Animals and were approved by the Institutional Animal Care and Use Committee of the University of California Davis. The harvested pancreases were rinsed, fixed (4% paraformaldehyde), embedded, and sectioned (5 μm) as previously described [19].
Antibodies
Mouse anti-human ZNT8 polyclonal antibody was purchased from R&D Systems (Minneapolis, MN). Guinea pig anti-insulin polyclonal antibody was purchased from Abcam (Cambridge, MA). A guinea pig anti-mouse ZnT8 polyclonal antibody was raised against a synthetic peptide (CASRDSQVVRREIAKALSKSFTM) and affinitypurified (Thermo Fisher Scientific, Waltham, MA). The peptide used to generate the mouse ZnT8 antibody came from the same region used for generating the human ZNT8 antibody (R&D Systems). The Actβ antibody was purchased from Sigma-Aldrich (St. Louis, MO). Antibodies against phosphor-AKT (Ser473), phosphor-70S6 kinase (Thr389) and phosphor-p38 MAPK (Thr180/Tyr182) were purchased from Cell Signaling Technology (Danvers, MA). Horseradish peroxide-conjugated secondary antibodies were also purchased from Cell Signaling Technology.
Immunohistochemical Analysis
Immunohistochemical analysis of human and mouse pancreases was performed as previously described [20]. Briefly, following the rehydration steps, tissue sections were incubated in 5% H2O2 to inhibit endogenous peroxidase activity. Non-specific binding was blocked by treating the tissue sections with avidin-biotin blocking solution followed by 3% goat serum treatment (Vector Labs, Burlingame, CA). Primary antibodies were diluted in 1x PBS containing 2% goat serum and incubated at 4°C overnight. Dilution factors for the primary antibodies used in this study were as follows: human ZNT8, 1:100 and mouse ZnT8, 1:2000. Secondary biotinylated goat anti-mouse or anti-guinea pig antibody (Vector Labs) was diluted at 1:200 in 1x PBS containing 2% goat serum. The immunoreactivity was developed in tissue sections using VECTASTAIN elite ABC and HRP substrate ImmPACT DAB kits (Vector Labs) which produced brown deposits within cells.
Cell culture
MIN6 β-cells were purchased from AddexBio (San Diego, CA). Cells were grown in DMEM containing 15% (v/v) Fetal Bovine Serum (FBS), 4.5 g/L glucose, 1.0 mM sodium pyruvate, 50 μM β- Mercaptoethanol (BME), 50 U/mL penicillin, and 50 μg/mL streptomycin (complete medium, Thermo Fisher Scientific). Cells were cultured in a humidified atmosphere at 37 °C with 5% CO2.
Znt8 knockdown in insulin-secreting MIN6 β-cells
MissionTM shRNA lentiviral particles were purchased from Sigma-Aldrich. Lentiviral particles for shRNAs targeting Znt8 (TRCN000979767) or scrambled non-target negative control (SHC002V) were transducted to MIN6 β-cells according to the manufacturer’s protocol. Briefly, 18 h before transduction, MIN6 β-cells were seeded in 6-well plates at ~50% confluence. The next day, hexadimethrine bromide (8 μg/mL, Sigma-Aldrich) was added to the culture medium. Viral particles were subsequently added into the medium at ~2.5 particles/cell. After overnight incubation, the medium containing lentiviral particles were removed. The cells were then cultured in the complete medium plus 450 μg/mL G418 (Znt8KD) or 1 μg/mL puromycin (control). After 5 days, individual cell colonies were selected by the limiting dilution method and expanded by culturing in the complete medium under selection [21]. Cell lines were tested for Znt8 mRNA expression. Two cell lines with a ~50% reduction in Znt8 mRNA expression relative to the controls (also 2 cell lines) were selected for subsequent experiments.
RNA isolation, cDNA synthesis and quantitative PCR
Znt8KD and control MIN6 β-cells were lysed in TRIzol® reagent (Thermo Fisher Scientific). Total RNA were isolated according to the manufacturer’s protocol. cDNA was synthesized using an iScriptTM reverse transcription kit (BioRad, Hercules, CA) following the manufacturer’s protocol. cDNA samples were diluted 10-fold with double-distilled water before being used in quantitative PCR (qPCR). Gene expression for Znt8 and Ins was performed using specific primers (Table 1) with Sso Advanced SYBR Green Supermix (BioRad) and normalized to the expression of Actβ. Fold differences were calculated using the ΔCt method as described previously [22].
Gene
Forward primer (5’→ 3’)
Reverse primer (5’→ 3’)
Znt8
TGCCAAGTGGAGACTCTGTG
AGCCGCATCAGTGAGGATAG
Ins
TGTCAAGCAGCACCTTTGTGG
GGGCCTCCACCCAGCTCCAGTT
Act
TCATGAAGTGTGACGTTGACATCCGT
CCTAGAAGCATTTGCGGTGCACGATG
Table 1: Primer sequences.
Glucose-stimulated insulin secretion
Znt8KD and control MIN6 β-cells were seeded in 12-well cell culture plates in triplicate at 0.6x 106/well and grown for 72 h. Cells were washed once with HEPES-Balanced Krebs-Ringer Bicarbonate buffer (KRB) [19] containing 0.5% Bovine Serum Albumin (BSA). Cells were pre-incubated with 1.0 mL KRB (with 0.5% BSA but without glucose) at 37°C for 30 min and the buffer was then replaced with 0.5 mL KRB containing 0 or 16.7 mM glucose and 0.5% BSA. Cells were incubated at 37°C for 60 min and the buffer was collected for insulin quantification using a mouse insulin ultrasensitive ELISA kit (Alpco, Salem, NH). The remaining cells were washed once with ice-cold 1x PBS and lysed in 300 μL M-PERTM (Thermo Fisher Scientific) containing protease inhibitors in a mini tablet (Roche, Indianapolis, IN). Protein concentration of the cell lysate was determined using a BCA protein assay kit (BioRad). The amount of secreted insulin is expressed as ng insulin/μg total protein.
Quantification of cell-associated insulin
Znt8KD and control MIN6 β-cells were seeded in 12-well cell culture plates in triplicate at 0.6x 106/well and grown for 48 h. Cells were washed once with ice-cold 1x PBS and lysed in 300 μL MPERTM containing protease inhibitors. Protein concentration of the cell lysate was determined using a BCA protein assay kit. Insulin was extracted from 50 μL lysate as described previously [19]. The amount of insulin is expressed as ng insulin/μg total protein.
Cell doubling and viability analysis
Znt8KD and control MIN6 β-cells were seeded at 2x 104/well in 96-well cell culture plates in triplicate and cultured for up to 6 days. Cell proliferation reagent WST-1 was added on days 2, 4, or 6 and incubated at 37°C for 4 h following the manufacture's instructions. Absorbance of the samples (440 nm) against the background control (610 nm) was obtained and cell numbers in the wells were calculated using a standard curve of cell number optical density. Data are the mean ± S.D.
Cell survival analysis
Znt8KD and control MIN6 β-cells were seeded at 3x 104/well in 96-well cell culture plates in triplicate. The cells were allowed to seed at 37°C for 3 h. Vehicle or TNFα (20 ng/mL, PeproTech, Rocky Hill, NJ) was then added into the wells and the cells were further incubated at 37°C for 72 h. Cell viability was measured by WST-1 assay after 72 h incubation. Data are the mean ± S.D.
Western Blot Analysis
Znt8KD and control MIN6 β-cells were seeded in 12-well cell culture plates at 0.6x 106/well in duplicate and cultured for 72 h. The cells were washed with DMEM containing 1.0 mM sodium pyruvate and 50 μM BME without glucose and FBS and then incubated with DMEM containing 1.0 mM sodium pyruvate, 50 μM BME, and 2.0 mM glucose without FBS for 4 h at 37°C. The medium was then changed to DMEM containing 1.0 mM sodium pyruvate and 50 μM BME without glucose and FBS. Insulin was subsequently added at 0, 10 or 100 nM. After 5 min stimulation at 37°C, the cells were washed and lysed in 200 μL M-PERTM containing protease inhibitors and HaltTM phosphatase inhibitor cocktail (Thermo Fisher Scientific). Protein concentrations were determined using a BCA protein assay. Eight μg protein lysate were electrophoresed on Criterion precast gels (4-20% Tris-HCl, BioRad). Separated proteins were subsequently transferred onto PDVF membranes (BioRad) using a TurboBlot system (BioRad) according to the manufacturer’s protocol. Membranes were blocked with 5% nonfat milk powder in 1x PBS with 0.1% Tween 20 (PBST) at Room Temperature (RT) for 1 h. Primary antibody incubations were carried out overnight at 4°C. Secondary antibody incubations were performed at RT for 1 h. Protein bands were visualized using a chemiluminescent substrate (Thermo Fisher Scientific) on a ChemiDoc XRS+ System (BioRad).
Statistical analysis
Significant differences between the two test groups were determined using an unpaired Student’s t-test. Differences were considered significant at P < 0.05. Data are presented as either means ± S.E. or means ± S.D.
Results
Expression of ZNT8 is increased in the human pancreas with T2D and in the mouse pancreas with insulin resistance
Zinc is required for insulin synthesis, processing, and crystallization in pancreatic β-cells. ZNT8 is predominantly expressed in β-cells in the body [16] and is responsive for the uptake of zinc into secretory granules for insulin crystallization in β-cells [13,23]. To determine whether ZNT8 expression was altered under the diabetic condition, we investigated ZNT8 expression in human and mouse pancreatic islets under the normal and insulin resistant/ T2D condition in paraffin embedded pancreatic tissue sections by immunohistochemistry. Our results indicated that the expression of ZNT8 was significantly increased in the islet of the human pancreas with T2D (Figure 1) and in the mouse islet with insulin resistance (Figure 2). Zinc is requried for insulin crystallization which forms dense particles in secretory granules of β-cells [8]. Therefore, the induction of ZNT8 expression may simply be due to a high demand for zinc to be delivered into the granular compartment for insulin maturation and crystallization during β-cell compensation for insulin resistance.
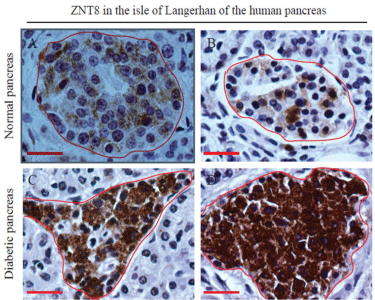
Figure 1: Expression of ZNT8 in normal and T2D human pancreases.
Immunohistochemical staining of ZNT8 in the human pancreas was
performed as described in Materials and Methods. The immunoreactivity for
ZNT8 was examined in pancreatic tissue sections prepared from a 66-yearold
male donor (A) (normal pancreas), a 71-year-old male donor (B) (normal
pancreas), and a 67-year-old male donor (C&D) (T2D pancreas). Sections
were counterstained with hematoxylin. Brown color shows the
immunoreactivity of ZNT8. The islet of Langerhans is encircled in red.
Surrounded by the islet of Langerhans is the exocrine acinar system. Scale
bar = 25 μm.
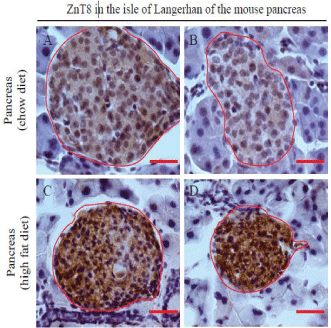
Figure 2: Expression of ZnT8 in the mouse pancreas. Male C57BL/6 mice
were fed a high fat diet (45% kcal fat) at 5-week-old for 11 weeks. The age
and gender-matched control mice were fed a regular chow diet. Mice were
euthanized at 16-week-old. Pancreases were isolated, paraffin embedded,
and sectioned for immunohistochemistry as described in Materials and
Methods. Sections were counterstained with hematoxylin. Brown color
shows the immunoreactivity of ZnT8. The islet of Langerhans is encircled in
red. Surrounded by the islet is the exocrine acinar system. A, B&C,
pancreatic tissues from mice fed a regular chow diet; D, E&F, pancreatic
tissues from mice fed a high fat diet. Scale bar = 25 μm. n = 3 per group.
Insulin expression is down-regulated in Znt8KD MIN6 β-cells
To determine whether insulin expression is altered by Znt8 allelic deficiency in pancreatic β-cells, we used shRNA lentiviral particles (Sigma-Aldrich) to knockdown Znt8 (Znt8KD) in MIN6 β-cells. We reduced the Znt8 mRNA expression in MIN6 β-cells to ~50% of the normal level to mimic the allelic deficiency of ZNT8 observed in humans [18]. Stable control MIN6 β-cell lines were also generated by transduction of vector viral particles (Sigma-Aldrich). A total of four independent stable cell lines were generated, two for the Znt8KD lines and two for the vector controls. Quantitative RT-PCR results indicated that the Znt8 mRNA expression was reduced by 50~60% in the two Znt8KD cell lines compared to the two control lines (Figure 3A). The Znt8 mRNA knockdown remained stable in continuous culture during the study (data not shown). Gross morphology between Znt8KD and control MIN6 β-cells was not distinguishable (data not shown). However, we found that, in Znt8KD MIN6 β-cells, the expression of insulin mRNA trended down by 15% (P = 0.06) (Figure 3B). More importantly, we showed that the insulin content in Znt8KD MIN6 β-cells was reduced significantly compared to the control (15%, P < 0.01) (Figure 3C), consistent with the Znt8 mRNA expression level in Znt8KD MIN6 β-cells (Figure 3B). These data suggest that downregulation of Znt8 expression may have a negative impact on insulin production and/or storage in β-cells.
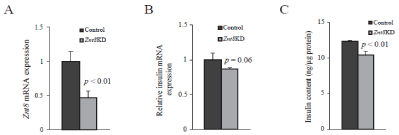
Figure 3: Insulin expression and contents in Znt8KD and control MIN6
β-cells. Znt8KD (2 independent lines) and controls (2 independent lines) MIN6
β-cells were harvested 48 h after cells were seeded. Total RNA was purified.
Transcription of Znt8 and Actβ was quantitated by a SYBR-based quantitative
RT-PCR assay. Actβ was used as the internal reference. Two independent
experiments were performed. (A) Znt8 mRNA expression in Znt8KD and
control MIN6 β-cells. Relative expression of Znt8 mRNA in Znt8KD cells to
the control was plotted. Data are mean ± S.E., n = 8. (B) Expression of Ins
mRNA in Znt8KD and control MIN6 β-cells. Relative expression of Ins
mRNA in Znt8KD cells to the control was plotted. Data are mean ± S.E., n =
4. (C) Insulin contents in Znt8KD and control MIN6 β-cells. Cell-associated
insulin was extracted from cell lysate with acid ethanol [19]. The insulin
content was determined using a mouse insulin ELISA kit (Alpco). Protein
concentration of the cell lysate was determined. The insulin content level is
expressed as ng insulin/μg total protein. Data are mean ± S.E., n = 4.
Glucose-stimulated insulin secretion is decreased in Znt8KD MIN6 β-cells
As ZnT8 is the major zinc transporter to deliver zinc into secretory granules for insulin crystallization and storage in β-cells [16], we next examined glucose-stimulated insulin secretion in Znt8KD and control MIN6 β-cells. Znt8KD and control MIN6 β- cells were stimulated with 16.7 mM glucose for 60 min [19] and the amount of secreted insulin was determined. As shown in Figure 4, Znt8 knockdown in MIN6 β-cells reduced glucose-stimulated insulin secretion by 40% (P < 0.01). This result indicates that ZnT8 is critical for insulin secretion after a glucose load. In addition to the adverse effect of Znt8KD on glucose-stimulated insulin secretion, β- cells with the Znt8 expression knocked-down by half of the normal level displayed significantly lower basal insulin secretion (Figure 4). Taken together, these observations support the previous findings that ZnT8 protein is involved in insulin processing/maturation in the secretory pathway of the β-cell [14,24,25].
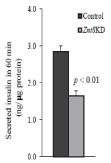
Figure 4: Insulin secretion in Znt8KD and control MIN6 β-cells. Znt8KD
(2 independent lines) and control (2 independent lines) MIN6 β-cells were
seeded and cultured for 72 h. The cells were preincubated with KR buffer
for 30 min at 37°C before glucose (0 or 16.7 mM) stimulation. After 60 min
stimulation at 37°C, the buffer was collected for insulin measurements
and the remaining cells were lysed for protein determination. Data were
summarized from 2 independent lines for Znt8KD or control MIN6 β-cells from
2 independent experiments in duplicate. The amount of secreted insulin is
expressed as ng insulin/μg total protein (mean ± S.E., n = 8).
MIN6 β-cells with Znt8 knockdown grow faster than the control
During the study, we noticed that Znt8KD MIN6 β-cells grew faster than the control. We then examined and compared the cell doubling time between Znt8KD and control MIN6 β-cells. As shown in Figure 5A, Znt8 knockdown in MIN6 β-cells decreased cell doubling time by approximately 2-fold, suggesting that reduction in Znt8 expression could promote β-cell proliferation, growth and/or survival, the key physiological factors important for T2D prevention or delay of the onset of the disease. It is worth noting that the increased Znt8KD β-cell growth/survival may serve as compensatory mechanisms for decreased insulin content and secretion in the Znt8KD β-cell to help maintain normal blood glucose levels.
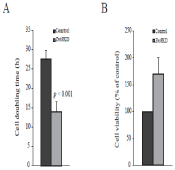
Figure 5: Growth of Znt8KD and control MIN6 β-cells. (A). Cell doubling
time. Znt8KD and control MIN6 β-cells were seeded at 2x 104/well in 96-well
cell culture plates in triplicate and incubated for 2, 4, or 6 days. Cell
proliferation reagent WST-1 was added on days 2, 4, or 6 and incubated at
37°C for 4 h following the manufacture's instructions. Cell numbers in the
wells were calculated using a standard curve of cell number optical density.
Data are the mean ± S.D. (B) Cell viability. Znt8KD and control MIN6 β-cells
were seeded at 3x 104/well in 96-well cell culture plates in triplicate. The
cells were allowed to seed at 37°C for 3 h. TNFα (20 ng/mL) or vehicle was
added and the cells were cultured at 37°C for 72 h. Cell viability was
measured by WST-1 assay. Data are the mean ± S.D.
MIN6 β-cells with Znt8 knockdown are less sensitive to TNFα-induced apoptosis
TNFα is one of the major proinflammatory cytokines involved in the islet cell destruction [26]. To examine whether a decrease in apoptosis played a role in the fast growing Znt8KD MIN6 β-cells, we measured the sensitivity of Znt8KD MIN6 β-cells to TNFα-induced cell apoptosis. Znt8KD and control MIN6 β-cells were cultured in the complete medium containing 20 ng/mL TNFα at 37°C for 72 h and the cell survival rate was determined. As shown in Figure 5B, compared to the control cells, Znt8KD MIN6 β-cells had significantly higher cell viability (~70%), suggesting that Znt8 KD by 50% of the normal level may be beneficial for β-cell survival.
Znt8 knockdown in MIN6 β-cells upregulates the signaling pathways important for β-cell proliferation and survival
Decreased doubling time and increased cell viability after TNFα stimulation in Znt8KD MIN6 β-cells indicated that β-cell proliferation and/or survival might be affected by Znt8 knockdown. It is known that the AKT signaling pathway plays a key role in signal transductions that regulate cellular processes, including glucose metabolism, apoptosis, cell proliferation, and transcription (Figure 6A). We, therefore, analyzed the activation of several key kinases in the insulin signaling pathway, including phosphorylation of Akt, p38 MAPK, and p70S6K by Western blot analysis in Znt8KD MIN6 β-cells. Our results suggested that Znt8 knockdown by 50-60% in MIN6 β-cells increased insulin-stimulated Akt phosphorylation at both10 and 100 nM concentrations (Figure 6B). Insulin stimulation (10 nM) also increased phosphorylation of other downstream target proteins, including p70S6K (Figure 6C) and p38 MAPK (Figure 6D) in Znt8KD MIN6 β-cells relative to the control cells. Taken together, these data indicate that Znt8 knockdown in β-cells affectes the activities of both Akt and p38 MAPK signaling pathways.
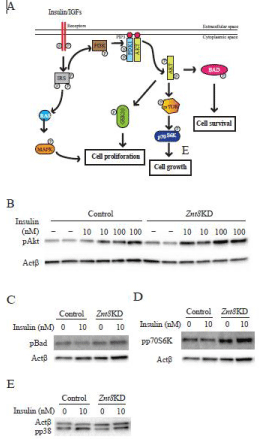
Figure 6: Activities of signaling pathways in Znt8KD and control MIN6 β-cells
after insulin stimulation. (A) Effect of insulin stimulation on downstream
targets. AKT and MAPK signaling activates multiple downstream signaling
pathways that influence β-cell proliferation, growth, and survival. The graph
represents the pathways that were examined in this study. Znt8KD and
control MIN6 β-cells were seeded and cultured for 72 h. Insulin stimulation
was carried out at the indicated concentrations (0, 10 or 100 nM). After 5
min stimulation at 37°C, the cells were washed and lysed for Western blot
analysis of phosphorylation of Akt (pAkt) (B), phosphorylation of p70S6
kinase (pp70S6K) (C), and phosphorylation of p38 MAPK (pp38) (D). Actβ
was used for the loading control. Densities of protein bands were determined
by Image lab software (BioRad) and results were included in the panels B-D.
The expression of pAkt, p70S6, and pp38 was normalized to the expression
of Actβ. The fold increases of pAkt, p70S6, and pp38 were calculated as
the ratio of the expression of pAkt, p70S6, and pp38 at indicated insulin
concentrations to the basal level of the control sample (0 nM insulin). Values
are summarized from two experimental data and expressed as mean ± SD.
*P < 0.05; **P < 0.01.
Discussion
In the current study we investigated the expression of human (ZNT8) and mouse ZnT8 proteins in the pancreas with a T2D or insulin resistant condition. We demonstrated that the expression of ZNT8 was up-regulated in the islet of the human pancreas with T2Dand in the mouse islet with insulin resistance. It is known that insulin resistance precedes the development of overt T2D. Insulin resistance is characterized by reduced insulin response in insulinsensitive organs/tissues, such as liver, skeletal muscle, and fat, accompanied by hyper insulinemia which develops to compensate for the hyperglycemia caused by lower insulin sensitivity in these tissues. Since ZNT8 is the primary transporter responsible for zinc ion delivery into insulin-containing granules for insulin crystallization and storage in pancreatic β-cells [27-29], our finding that ZNT8 expression was up-regulated in islets accompanying with either insulin resistance or T2D is consistent with the biological function of ZNT8. Additionally, it has been shown that increased expression of Znt8 in β-cells can induce a cytosolic zinc deficient condition, as evidenced by the increased expression of Zip zinc transporters, including Zip6, Zip7, and Zip8 [30]. The cellular compartmental/organelle zinc deficient condition in β-cells could be implicated in T2D development by promoting cell death [31]. It is worth noting that ZNT8 expression can eventually be downregulated in the later stage of T2D when β-cells fail to compensate for insulin resistance due to inflammation and cell death [32,33].
Genome-Wide Association Studies (GWAS) in human populations have shown a link of ZNT8 with T2D [34-36]. Individuals carrying a risk allele of ZNT8 (R325; rs13266634, a major allele in human populations) have low basal and glucose-stimulated insulin secretion and are likely to have abnormal glucose tolerance during oral glucose tolerance test [14] while individuals carrying the minor allele of ZNT8 (T325) are associated with modest protection from T2D. However, the mechanism underlying the decrease in T2D risk of the T325 allele of ZNT8 is not completely understood [37]. Mice with Znt8-null mutations (both global and β-cell specific knockouts) have significantly lower zinc content in the secretory granules of the β-cell, consistent with the function of ZNT8 in zinc transport into the secretory granule [23]. Znt8 deficient mice contain less insulincontaining granules but more atypical granules in β-cells [29,38]. As such, Znt8 KO mice are sensitive to diet-induced glucose intolerance [39]. Our study using MIN6 β-cells with reduced Znt8 expression support the view that ZnT8 plays an important role in insulin production and secretion (Figure 4). However, it is worth noting that the physiological impact of the Znt8-null mutation on β-cell function and whole body glucose utilization varies between studies in mice and is largely dependent on the genetic background of the KO mice [37,40].
A recent study has reported that loss-of-function mutations in the ZNT8 gene may actually be beneficial in humans [18]. This study found that individuals with haploinsufficiency for ZNT8 were about 60% less likely to develop T2D than individuals who have both wild type alleles of ZNT8 [18]. However, the underlying mechanism is largely unknown. In the current study, we observed that ZNT8 protein expression was enhanced in the islet of the human T2D pancreas which supports the theory that reduced expression of ZNT8 in humans may have a positive outcome for β-cell function and subsequent whole body glucose controls. It is likely that increased expression of ZNT8 during β-cell compensation for peripheral insulin resistance disturbs cellular zinc homeostasis due to enhanced sequestration of cytoplasmic zinc into insulin-containing secretory granules of the β-cell. This enhancement of zinc sequestration may result in a shortage in zinc supply into other intracellular organelles, such as the ER and Golgi apparatus. As such, insulin synthesis and processing may be interrupted as zinc is required in these processes too [9, 41].
Overt T2D develops when the β-cell fails to compensate for insulin resistance partly due to inflammation and β-cell death [32]. Up-regulation of ZNT8 expression during β-cell compensation for insulin resistance may impair cellular zinc homeostasis in β-cells leading to β-cell apoptosis. Consistent with this notion, we showed that MIN6 β-cells with a lowered Znt8 expression level mimicked allelic deficiency of ZNT8 in humans by growing faster and more resistant to TNFα-induced cell death than the control. These results are in agreement with the findings reported by Egefjord et al. and Muayed et al. that transcription of Znt8 is negatively regulated by TNFα [42,43]. Moreover, we demonstrated that the Akt-mediated signaling pathway, a key pathway that regulates apoptosis and cell proliferation, was more active in MIN6 β-cells when the Znt8 expression was decreased to 50% of the normal level. Therefore, it is conceivable that down-regulation of Znt8 expression in β-cells during T2D development is a defending step towards combating inflammation-induced cell apoptosis. Over-expression of Znt8 may not be attributed to the initial pathogenesis of T2D, however, as insulin resistance progresses, it may play an important role in advancing disease toward full-blown T2D by promoting β-cell death.
In conclusion, our data demonstrated that ZNT8 expression was up-regulated in pancreatic islets during insulin resistance. In addition, we showed that while Znt8 knockdown in β-cells negatively affected cellular insulin concentrations and secretion, it significantly increased β-cell survival rate. The protective effect of allelic deficiency of ZNT8 from T2D development could be likely via an Akt-dependent survival mechanism as well as by reduction in inflammation-induced cell death. These findings have greatly advanced the understanding of the role and significance of ZNT8 in the development of diabetes and provide new insight into developing strategies for T2D prevention and/or treatment.
Acknowledgment
We thank Yimeng Cai for her excellent technical assistance and Pieter Oort for critical reading of the manuscript. This work was supported by the United States Department of Agriculture, ARS intramural project #2032-51000-004-00D. USDA is an equal opportunity provider and employer.
References
- Huang L, Gitschier J. A novel gene involved in zinc transport is deficient in the lethal milk mouse. Nat Genet. 1997; 17: 292-297.
- Wang K, Zhou B, Kuo YM, Zemansky J, Gitschier J. A novel member of a zinc transporter family is defective in acrodermatitis enteropathica. Am J Hum Genet. 2002; 71: 66-73.
- Piletz JE, Ganschow RE. Zinc deficiency in murine milk underlies expression of the lethal milk (lm) mutation. Science. 1978; 199: 181-183.
- Wolters GH, Pasma A, Konijnendijk W, Boom G. Calcium, zinc and other elements in islet and exocrine tissue of the rat pancreas as measured by histochemical methods and electron-probe micro-analysis, Effects of fasting and tolbutamide. Histochemistry. 1979; 62: 1-17.
- Søndergaard LG, Stoltenberg M, Doering P, Flyvbjerg A, Rungby J. Zinc ions in the endocrine and exocrine pancreas of zinc deficient rats. Histol Histopathol. 2006; 21: 619-625.
- Dube S, Errazuriz I, Cobelli C, Basu R, Basu A. Assessment of insulin action on carbohydrate metabolism: physiological and non-physiological methods. Diabet Med. 2013; 30: 664-670.
- Søndergaard LG, Stoltenberg M, Flyvbjerg A, Brock B, Schmitz O, Danscher G, et al. Zinc ions in beta-cells of obese, insulin-resistant, and type 2 diabetic rats traced by autometallography. APMIS. 2003; 111: 1147-1154.
- Wodak SJ, Alard P, Delhaise P, Renneboog-Squilbin C. Simulation of conformational changes in 2 Zn insulin. J Mol Biol. 1985; 181: 317-322.
- Huang L, Yan M, Kirschke CP. Over-expression of ZnT7 increases insulin synthesis and secretion in pancreatic beta-cells by promoting insulin gene transcription. Exp Cell Res. 2010; 316: 2630-2643.
- Huang L, Kirschke CP, Lay YA, Levy LB, Lamirande DE, Zhang PH. Znt7-null mice are more susceptible to diet-induced glucose intolerance and insulin resistance. J Biol Chem. 2012; 287: 33883-33896.
- Davidson HW, Rhodes CJ, Hutton JC. Intraorganellar calcium and pH control proinsulin cleavage in the pancreatic beta cell via two distinct site-specific endopeptidases. Nature. 1988; 333: 93-96.
- Smeekens SP, Montag AG, Thomas G, Albiges-Rizo C, Carroll R, Benig M, et al. Proinsulin processing by the subtilisin-related proprotein convertases furin, PC2, and PC3. Proc Natl Acad Sci USA. 1992; 89: 8822-8826.
- Wijesekara N, Dai FF, Hardy AB, Giglou PR, Bhattacharjee A, Koshkin V, et al. Beta cell-specific Znt8 deletion in mice causes marked defects in insulin processing, crystallisation and secretion. Diabetologia. 2010; 53: 1656-1668.
- Kirchhoff K, Machicao F, Haupt A, Schäfer SA, Tschritter O, Staiger H, et al. Polymorphisms in the TCF7L2, CDKAL1 and SLC30A8 genes are associated with impaired proinsulin conversion. Diabetologia. 2008; 51: 597-601.
- Dunn MF. Zinc-ligand interactions modulate assembly and stability of the insulin hexamer -- a review. Biometals. 2005; 18: 295-303.
- Chimienti F, Devergnas S, Favier A, Seve M. Identification and cloning of a beta-cell-specific zinc transporter, ZnT-8, localized into insulin secretory granules. Diabetes. 2004; 53: 2330-2337.
- Aydemir TB, Sitren HS, Cousins RJ. The zinc transporter Zip14 influences c-Met phosphorylation and hepatocyte proliferation during liver regeneration in mice. Gastroenterology. 2012; 142: 1536-1546.
- Flannick J, Thorleifsson G, Beer NL, Jacobs SB, Grarup N, Burtt NP, et al. Loss-of-function mutations in SLC30A8 protect against type 2 diabetes. Nat Genet. 2014; 46: 357-363.
- Huang L, Yan M, Kirschke CP. Over-expression of ZnT7 increases insulin synthesis and secretion in pancreatic beta-cells by promoting insulin gene transcription. Exp Cell Res. 2010; 316: 2630-2643.
- Yu YY, Kirschke CP, Huang L. Immunohistochemical analysis of ZnT1, 4, 5, 6, and 7 in the mouse gastrointestinal tract. J Histochem Cytochem. 2007; 55: 223-234.
- Fuller SA, Takahashi M, Hurrell JG. Cloning of hybridoma cell lines by limiting dilution. Curr Protoc Mol Biol. 2001.
- Andree KB, Kim J, Kirschke CP, Gregg JP, Paik H, Joung H, et al. Investigation of lymphocyte gene expression for use as biomarkers for zinc status in humans. J Nutr. 2004; 134: 1716-1723.
- Lemaire K, Ravier MA, Schraenen A, Creemers JW, Van de Plas R, Granvik M, et al. Insulin crystallization depends on zinc transporter ZnT8 expression, but is not required for normal glucose homeostasis in mice. Proc Natl Acad Sci USA. 2009; 106: 14872-14877.
- Cauchi S, Proença C, Choquet H, Gaget S, De Graeve F, Marre M, et al. Analysis of novel risk loci for type 2 diabetes in a general French population: the D.E.S.I.R. study. J Mol Med (Berl). 2008; 86: 341-348.
- Staiger H, Machicao F, Stefan N, Tschritter O, Thamer C, Kantartzis K, et al. Polymorphisms within novel risk loci for type 2 diabetes determine beta-cell function. PLoS One. 2007; 2: e832.
- Suk K, Kim S, Kim YH, Kim KA, Chang I, Yagita H, et al. IFN-gamma/TNF-alpha synergism as the final effector in autoimmune diabetes: a key role for STAT1/IFN regulatory factor-1 pathway in pancreatic beta cell death. J Immunol. 2001; 166: 4481-4489.
- Nicolson TJ, Bellomo EA, Wijesekara N, Loder MK, Baldwin JM, Gyulkhandanyan AV, et al. Insulin storage and glucose homeostasis in mice null for the granule zinc transporter ZnT8 and studies of the type 2 diabetes-associated variants. Diabetes. 2009; 58: 2070-2083.
- Pound LD, Sarkar SA, Benninger RK, Wang Y, Suwanichkul A, Shadoan MK, et al. Deletion of the mouse Slc30a8 gene encoding zinc transporter-8 results in impaired insulin secretion. Biochem J. 2009; 421: 371-376.
- Lemaire K, Chimienti F, Schuit F. Zinc transporters and their role in the pancreatic β-cell. J Diabetes Investig. 2012; 3: 202-211.
- Bellomo EA, Meur G, Rutter GA. Glucose regulates free cytosolic Zn²⁺ concentration, Slc39 (ZiP), and metallothionein gene expression in primary pancreatic islet β-cells. J Biol Chem. 2011; 286: 25778-25789.
- Chimienti F, Seve M, Richard S, Mathieu J, Favier A. Role of cellular zinc in programmed cell death: temporal relationship between zinc depletion, activation of caspases, and cleavage of Sp family transcription factors. Biochem Pharmacol. 2001; 62: 51-62.
- Quan W, Jo EK, Lee MS. Role of pancreatic β-cell death and inflammation in diabetes. Diabetes Obes Metab. 2013; 15: 141-151.
- Tamaki M, Fujitani Y, Uchida T, Hirose T, Kawamori R, Watada H. Downregulation of ZnT8 expression in pancreatic β-cells of diabetic mice. Islets. 2009; 1: 124-128.
- Diabetes Genetics Initiative of Broad Institute of Harvard and MIT, Lund University, and Novartis Institutes of BioMedical Research1, Saxena R, Voight BF, Lyssenko V, Burtt NP. Genome-wide association analysis identifies loci for type 2 diabetes and triglyceride levels. Science. 2007; 316: 1331-1336.
- Scott LJ, Mohlke KL, Bonnycastle LL, Willer CJ, Li Y, Duren WL, et al. A genome-wide association study of type 2 diabetes in Finns detects multiple susceptibility variants. Science. 2007; 316: 1341-1345.
- Sladek R, Rocheleau G, Rung J, Dina C, Shen L, Serre D, et al. A genome-wide association study identifies novel risk loci for type 2 diabetes. Nature. 2007; 445: 881-885.
- Davidson HW, Wenzlau JM, O'Brien RM. Zinc transporter 8 (ZnT8) and β cell function. Trends Endocrinol Metab. 2014; 25: 415-424.
- Tamaki M, Fujitani Y, Hara A, Uchida T, Tamura Y, Takeno K, et al. The diabetes-susceptible gene SLC30A8/ZnT8 regulates hepatic insulin clearance. J Clin Invest. 2013; 123: 4513-4524.
- Hardy AB, Wijesekara N, Genkin I, Prentice KJ, Bhattacharjee A, Kong D, et al. Effects of high-fat diet feeding on Znt8-null mice: differences between beta-cell and global knockout of Znt8. Am J Physiol Endocrinol Metab. 2012; 302: 1084-1096.
- Lemaire K, Ravier MA, Schraenen A, Creemers JW, Van de Plas R, Granvik M, et al. Insulin crystallization depends on zinc transporter ZnT8 expression, but is not required for normal glucose homeostasis in mice. Proc Natl Acad Sci USA. 2009; 106: 14872-14877.
- Huang L. Zinc and its transporters, pancreatic b-cells, and insulin metabolism. The pancreatic beta cell, ed. Litwack G. USA: Elsevier Inc. 2014.
- Egefjord L, Jensen JL, Bang-Berthelsen CH, Petersen AB, Smidt K, Schmitz O, et al. Zinc transporter gene expression is regulated by pro-inflammatory cytokines: a potential role for zinc transporters in beta-cell apoptosis? BMC Endocr Disord. 2009; 9: 7.
- El Muayed M, Billings LK, Raja MR, Zhang X, Park PJ, Newman MV, et al. Acute cytokine-mediated downregulation of the zinc transporter ZnT8 alters pancreatic beta-cell function. J Endocrinol. 2010; 206: 159-169.