
Mini Review
Austin J Endocrinol Diabetes. 2016; 3(2): 1044.
Diabetes Mellitus and its Relation with Ghrelin-A Mini- Review
De A1* and Singh MF2
1Department of Pharmaceutical Technology, Bengal School of Technology, India
2Department of Pharmaceutical Science, Sardar Bhagwan Singh Post Graduate Institute of Biomedical Science and Research, India
*Corresponding author: AAbhijit De, Department of Pharmaceutical Technology, Bengal School of Technology, Sugandha, Hooghly, Westbengal, India
Received: June 29, 2016; Accepted: July 29, 2016; Published: August 05, 2016l
Abstract
Diabetes Mellitus is a metabolic disorder associated with abnormal glucose and insulin resistance. In type 1 diabetes, insulin cannot be synthesized by the pancreas to maintain glycemic condition and in type 2 normal production of insulin hormone occurs but the body cells are resistant to insulin, a condition in which cells fail to use insulin properly or sometimes combined with an absolute insulin deficiency. Diabetic condition is directly related to endocrinological parameters. Elevated level of various hormones has been accounted in this disorder. The recent area of interest has been grown behind the relationship between ghrelin and diabetes mellitus. Ghrelin hormone is synthesized mainly in the gastric oxyntic mucosa in the X/A cells and of intestine, pancreas, kidney, placenta, lymphatics, gonads, adrenal, thyroid gland, heart, lung, pituitary, hypothalamus, eye, human B- and T-lymphocytes, neutrophils, morula, blastocyts and embryos. Ghrelin acts on the Growth Hormone Secretogogue Receptor (GHSR) activate phospholipase C to generate IP3 and diacylglycerol, resulting in an increase of intracellular calcium ion. Various researches have been recorded that ghrelin is diabetogenic as it possesses a negative effect on the insulin secretion from islet β cells. Ghrelin stimulates glucose synthesis andoutput from hepatocytes and hampers insulin’s capacity to inhibit endogenous glucose production. Moreover the recently discovered Uncoupling Protein-2 (UCP2) expression contributes a new concept behind the role of ghrelin in type 2 diabetes. Ghrelin also induces the expression of IA- 2β, a β cell auto antigen for type 1 diabetes, which is an integral membrane glycoprotein expressed in neuro-endocrinetissues.
Keywords: Diabetes; Ghrelin; Oxyntic mucosa; Growth Hormone Secretagogue Receptor; Uncoupling protein-2
Background of Diabetes Mellitus
Diabetes Mellitus is associated with altered carbohydrate metabolism and insulin resistance. It is a group of metabolic disorders in which a person gains high blood glucose either due to the less production of insulin by body or due to the unresponsiveness of the cells for the insulin that is produced by the pancreas. This result in high blood sugar with some classical symptoms like polyuria i.e, frequent urination, polydipsia (increased thirst) and polyphagia (increased hunger) [1]. Type 1 diabetes results from the body’s incapability to produce insulin either due to autoimmune or idiopathic destruction of cells. In type 1 diabetes, insulin cannot be synthesized by the pancreas to maintain glycemic condition. It is more common among children and young adults. To counteract this problem, insulininjections are used for treatment, hence type 1 diabetes is also termed as Insulin Dependent Diabetes Mellitus (IDDM) or Juvenile Diabetes [1,2]. In case of type II diabetes, there is normal production of insulin hormone but the body cells are resistant to insulin, a condition in which cells fail to use insulin properly, or sometimes combined with an absolute insulin deficiency. Cells and tissues are not responsive to insulin, so glucose remains elevated in the bloodstream. Type 2 diabetes is commonly manifested by middle-to-late-aged adults (40 years); however, its prevalence is increasing in younger populations. As insulin was initially not considered necessary for treatment of type 2 diabetes, it is known as Noninsulin Dependent Diabetes Mellitus (NIDDM) or Adult Onset Diabetes [1,3].
A diabetic patient cannot metabolize carbohydrates, proteins or fats due to improper production of insulin, a blood glucose regulator, or resistance to insulin. Insulin helps cells use glucose as a main energy source. However, diabetic patients’ cells do not make use of glucose from the blood due to abnormal insulin metabolism, resulting in elevated blood glucose levels or hyperglycemia. Over time, high glucose levels in the bloodstream can lead to severe complications such as vision loss, cardiovascular diseases, kidney disorder, and nerve damage [1-3].
The adipose tissue, however, is crucial in the normal regulation of the insulin action all over the body. Adipocytes can store excess lipids in obesity but when they become saturated, lipids begin to accumulate inside other organs, and tissues making them insulin resistant. Adipocytes also can produce adipokines such as leptin and adiponectin which have been proved as insulin sensitizers due to their ability to decrease Triglycerides (TG) synthesis, to stimulate beta oxidation of fatty acids, and thus to enhance insulin action in both skeletal muscle and liver [4-6]. Genetically modified animals deficient in white adipose tissue usually have severe insulin resistance in liver and muscle [7]. Transplantation of normal fat tissue into white adipose tissue deficient mice restores the insulin sensitivity. Mice with a knockout of the insulin receptor in muscle have normal glucose tolerance [8], whereas those with a knockout of the insulinsensitive GLUT4 glucose transporter in adipose tissue have impaired glucose tolerance, apparently due to insulin resistance being induced in muscle and liver [9].
Ghrelin and its receptors
In mammals, ghrelin homologs have been identified in human, rhesus monkey, rat, mouse and mongolian gerbil [10,11]. Ghrelin is an acylated, 28-amino-acid peptide that promotesthe release of GH in the hypothalamus. It is the naturalligand of the GHSR-1a receptor [12]. The ghrelin gene is located in chromosome 3 (3p25-26), contains four preproghrelin-coding exons, and encodes aprecursor of 117aa (preproghrelin) with 82% of homologybetween species [13]. Another form of ghrelin, des-acyl ghrelin, exists at significant levels in both stomach and blood. This variant lacks the octanoyl chain in serine 3 and represents more than 90% of the circulating peptide - [14-16]. In plasma, acyl ghrelin levels are 10–20 fmol/ml while total ghrelin levels are 100–150 fmol/ml (including both acyl and non-acyl forms) [17]. Ghrelin is the natural ligand of the Growth Hormone Secretagogue Receptor (GHS-R), and was first found to induce GH secretion in various species [15,16,18]. Ghrelin has been primarily detected in the A-cells of stomach, however, both ghrelin and the GHS-R are also expressed in a large spectrum of tissues including brain, kidney, pancreas, uterus and testis [19,20]. It was found that ghrelin is then only peripheral hormone which stimulates food intake by acting in hypothalamus and is involved in multiple endocrine and non-endocrine processes such as metabolic and cardiovascular effects, regulation of gastric motility and acid secretion, regulation of glucose metabolism and insulin secretion and modulation of cell proliferation [21,22]. During adult life ghrelin is synthesized mainly in the gastric oxyntic mucosa in the X/A cells. Ghrelin is also produced in the X/A cells of the intestine and in some others tissues such as the pancreas, kidney, placenta, lymphatics, gonads, adrenal, thyroid gland, heart, lung, pituitary, hypothalamus, eye [23], human B- and T-lymphocytes, neutrophils [23-25], morula, blastocyts and embryos [26]. In fetal life, ghrelin is mainly produced in the pancreas and lung [27]. The pancreas expresses ghrelin mRNA atmid-gestation being its mRNA levels six to seven times higher than in the fetal stomach [28]. In this period ghrelin’s production in the stomach is very low and only increases after birth [28]. In the fetal lung, this peptide is highly expressed after the pseudo glandular stage of the development [29,30].
Ghrelin receptor, or GHS-R, is a typical GPCR with seven transmembrane domains (7-TM) [31-33].Two distinct ghrelin receptor cDNAs have been isolated [31]. The first, GHS-R type 1a, encodes a 7-TM GPCR with binding and functional properties consistent with its role as ghrelin’s receptor. This type 1a receptor has features characteristic of a typical GPCR, including conservedcysteine residues in the first two extracellular loops, several potential sites for posttranslational modifications (N-linked glycosylation and phosphorylation), andan aromatic triplet sequence (E/DRY) located immediately after TM-3 in the second intracellular loop. Another GHS-R cDNA, type 1b, is produced by an alternative splicing mechanism [31]. The GHS-R geneconsists of two exons; the first exon encodes TM-1 to TM-5, and the second exon encodes TM-6 to TM-7. Type1b is derived from only the first exon and encodes only five of the seven predicted TM domains. The type 1breceptor is thus a COOH-terminal truncated form of thetype 1a receptor and is pharmacologically inactive. The GHS-R has several homologs, whose endogenousligands are gastrointestinal peptides or neuropeptides. The ghrelin receptor is most homologous to the motilin receptor; the human forms share 52% identical amino acids [34-36]. Ghrelin acts on the GHS-R and activates phospholipase C to generate IP3 and diacylglycerol, resulting in an increase of intracellular calcium ion, indicating that the ghrelin receptor is coupled to a Gq subunit as shown in Figure 1.
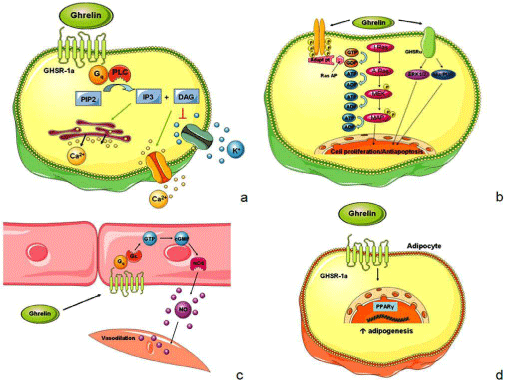
Figure 1: Intracellular pathways involved in ghrelin’s effects.
a. Ghrelin binds to the GHSR-1a which is coupled to a Gq protein, leading to the activation of Phospholipase C (PLC). This enzyme converts phosphatidylinositol
bisphosphate (PIP2) into Inositol triphosphate (IP3) and Diacylglycerol (DAG). IP3 induces Ca2+ release from the sarcoplasmic reticulum while DAG inhibits K+
channels, thereby inducing the opening of voltage-dependent L-type Ca2+ channels in the cellular membrane. The increase of intracellular Ca2+ results in membrane
depolarization.
b. Ghrelin activates a tyrosine kinase receptor, which leads to the activation of the Ras protein. The double phosphorylation of the Ras protein results in the
Mitogen-Activated Protein Kinase (MAPK), which needs another phosphorylation to enter the nucleus and regulate cell proliferation. Ghrelin also binds to an
unknown receptor which activates ERK1 and ERK2 and Akt/PI3K, culminating in an antiapoptotic effect.
c. In the endothelial cells ghrelin stimulates a G-protein-coupled system which activates the Guanylate Cyclase (GC). This enzyme transforms Guanosine
Triphosphate (GTP) into cyclic Guanosine Monophosphate (cGMP). cGMP leads to the activation of Nitric Oxide Synthase (NOS) which increases Nitric Oxide’s
(NO) levels. NO enters the smooth muscle cell and promotes relaxation.
d. In adipocytes ghrelin binds to the GHSR-1a and stimulates the Peroxisome Proliferator-Activated Receptor gamma (PPARγ). PPARγ is a transcription factor
regulating genetic transcription and thereby promoting adipogenesis.
Ghrelin concentration in diabetes
Current evidence suggests that ghrelin could contribute to the metabolic syndrome [37]. It has been shown that ghrelin concentrations are reduced in different pathophysiological conditions including obesity, type 2 diabetes, and other conditions with metabolic disturbances [38,39]. Circulating ghrelin concentrations are also reduced in healthy offspring of type 2 diabetic patients [40] indicating the presence of possible genetic component in the regulation of ghrelin plasma levels. Insulin is shown to inhibit ghrelin secretion in healthy normal-weight and overweight persons [41-43], and both oral and intravenous glucose loads are also shown to regulate ghrelin secretion in humans [42,44-46]. Systemic action of exogenous ghrelin to elevate blood glucose levels has been well documented in humans and rodents. When ghrelin was simultaneously injected into mice with glucose in Glucose Tolerance Test (GTT), the insulin responses were markedly attenuated and the glucose responses were larger in comparison to the control without ghrelin. It has recently been shown that in healthy humans ghrelin suppresses insulin secretion and elevates blood glucose in intravenous GTT. Conversely, GTT performed in mice showed that insulin responses were markedly enhanced and increases in plasma glucose were markedly attenuated by simultaneous injection of GHS-R antagonist [D-Lys3]- GHRP-6. In insulin tolerance tests, hypoglycemic effect of insulin was indistinguishable between the ghrelin-injected, GHS-R antagonistinjected and control mice. Ghrelin injection in humans increases plasma glucose levels [47]. Ghrelin levels are positively correlated to blood glucose levels [48,49] and negatively to plasma insulin levels [50,51]. Although the stomach is the major site of ghrelin production [16,46] it is the pancreatic ghrelin, through an autocrine/paracrine way, that regulates pancreatic insulin release. Ghrelin inhibits insulin secretion from islet β cells [49], an effect which is secretion from islet β cells [52], an effect which is dependent on GHSR-1a [16]. Therefore, the hyperglycemic effect is due to that pancreatic action, but also to other effects attributed to ghrelin. Ghrelin stimulates glucose synthesis and output from hepatocytes [52,53] and hampers insulin’s capacity to inhibit endogenous glucose production [54,55]. Therefore, inhibition of insulin release and other mechanisms are involved in the increase of blood glucose levels induced by ghrelin. Dezaki et al. have described the signaling mechanisms involved in ghrelin’s effect and proposed a pathway to explain how ghrelin inhibits insulin secretion which is shown in Figure 2. Ghrelin acts on the GHS-R expressed in β cells. The Gɑi2 protein leads to the activation of voltage-dependent Kv channels causing a rapid repolarization and shortening the bursts of the action potentials. This attenuates the increase in intracellular Ca2+ induced by glucose and thereby insulin secretion [50,51]. Ghrelin may also interfere with insulin secretion in achronic pattern. The deletion of ghrelin is associated with a reduction in Uncoupling Protein-2 (UCP2) expression [56]. UCP2 is a mitochondrial protein that modulates the efficiency of ATP production by dissociating the substrates of oxidation from ATP synthesis. This effect of UCP2 has a negative impact on insulin secretion [57]. Hence, chronic deprivation of ghrelin augments glucose dependent insulin secretion from the pancreatic β cells by diminishing UCP2 expression [56]. Ghrelin also induces the expression of IA-2β, a βcell auto antigen for type 1 diabetes, which is an integral membrane glycoprotein expressed in neuro-endocrine tissues and is localized to secretory granules [58]. IA-2βinhibits glucose stimulated insulin secretion. The inhibitory effect of ghrelin in insulin secretion, after glucose stimulation, is at least in part due to an increase in IA-2β [58]. Thus, ghrelin influences negatively pancreatic insulin release. In contrast Glucagon stimulates ghrelin secretion through the activation of MAPK. It is hypothesized that physiological background for the metabolic advantages (specifically, the glucose-lowering effects) after bariatric surgery include changed release of GI hormones (increased secretion of hormones with antidiabetes properties and reduced secretion of “diabetogenic” hormones) and surgery-induced restriction of food intake. Various researchers have done investigations on the correlation between diabetes and ghrelin concentration which are listed in Table 1.
Author
Year of Publication
Research outcome
Po¨ykko¨ et al
2003
The population-based study showed that low ghrelin is independently associated with both insulin concentrations and the prevalence of type 2 diabetes and insulin resistance. Moreover, ghrelin might have some role in the etiology of type 2 diabetes and the negative association between ghrelin and BP, which implicates that ghrelin could participate in the regulation of BP, especially in the hypertensive state. Furthermore, the study also supports the hypothesis that the ghrelin Arg51Gln mutation is associated with ghrelin concentrations [38].
Yildirim et al
2007
Shortterm rosiglitazone pretreatment neither prevented beta cell destruction nor altered ghrelin gene expression in diabetic rats, markedly. Study also demonstrated for the first time that ghrelinimmunoreactive cells are frequent in ducts where they could be a local source of the peptide of importance for e.g. islet regeneration [59].
Sharifi et al
2013
This study focused that obesity has a strong association with the reduced level of ghrelin concentration. It seems that the process of ghrelin reduction is initiated in earlier stages of insulin resistance prior to the onset of overt DM [60].
Siddique et al
2014
Study suggested that firstly glucose level and insulin resistance decrease significantly in obese and type 2 diabetic mice after ghrelin antagonist (D-Lys3) administration and secondly administration of ghrelin antagonist to obese and diabetic groups significantly improves the levels of testosterone and luteinizing hormone [61].
Sönmez at al
2015
Study suggested that diabetes causes morphological damage to testicular tissue and serum levels of gonadotropins. The study also demonstrated that both ghrelin and GHSR-1a gene expression are present in control and diabetic rat testes. In this study, both serum gonadotropins and also negative energy balance caused by diabetes probably affect the ghrelin level in the testis [62].
Yildirim et al
2016
This study showed that ghrelin immunoreactivity was increased at the beginning of diabetes. However, with increase in the duration of diabetes, ghrelin immunoreactivity approached to the control values. In addition, expression of GHSR-1a mRNA was decreased with increase in the duration of diabetes. It seemed that down-regulation of GHSR-1a contributed to the renal damage induced by longterm diabetes [63].
Abbreviations: BP: Blood Pressure; DM: Diabetes Mellitus; GHS-R1: Growth Hormone Secretogogue Receptor 1; mRNA: Messenger Ribonucleic Acid.
Table 1: Literature Review of inter-relationship between diabetes and ghrelin.
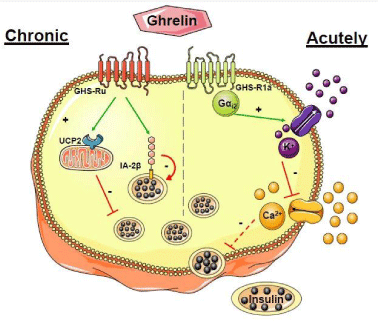
Figure 2: Subcellular pathways involved in the effects of ghrelin in the
pancreatic β cell. Ghrelin may affect pancreatic βcell function and insulin
secretion in an acute and chronic pattern.
Acutely Ghrelin binds to the GHSR-1a which is coupled to a Gɑi2 protein
leading to the opening of K+ channels sensitive to voltage. This repolarizes
the plasma membrane and closes Ca2+ channels. As a consequence
insulin secretion is inhibited. Chronic: Ghrelin increases the expression
of Uncoupling Protein-2 (UCP2), a mitochondrial protein that dissociates
substrates of oxidation from ATP synthesis and IA-2β, a βcell auto-antigen
for type 1 diabetes. These two molecules inhibit insulin secretion.
Conclusion and Future Perspectives
From this review, it is quite understood about ghrelin and its relation with diabetes. The pathophysiology behind this is well depicted. Still there are various aspects which is still to be unfolded. Recently ghrelin agonists and antagonists have been designed to implicate them as therapeutics in various disorders. Capromorelin and JMV 1843, ghrelin agonists are there but with no clinical pharmacotherapeutic effect. GSK1614343, a novel ghrelin receptor antagonist, increase appetite but not clinically used. Besides the traditional antidiabetic drugs, ghrelin antagonist can also be accounted as a good pharmaco-therapeutic approach in case of increased postprandial glucose level and decreased insulin level.
References
- McCance DR, Hanson RL, Pettitt DJ, Bennett PH, Hadden DR, Knowler WC. Diagnosing diabetes mellitus-do we need new criteria? Diabetologia. 1997; 40: 247–255.
- Qader SS, Hakanson R, Rehfeld JF, Lundquist I, Salehi A. Proghrelin-derived peptides influence the secretion of insulin, glucagon, pancreatic polypeptide and somatostatin: a study on isolated islets from mouse and rat pancreas. Regul Pept. 2008; 146: 230-237.
- Hviid A, Stellfeld M, Wohlfahrt J, Melbye M. Childhood vaccination and type 1 diabetes. The New Engl J Med. 2004; 350: 1398–1404.
- Shimomura I, Hammer RE, Ikemoto S, Brown MS, Goldstein JL. Leptin reverses insulin resistance and diabetes mellitus in mice with congenital lipodystrophy. Nature. 1999; 401: 73–76.
- Ebihara K, Ogawa Y, Masuzaki H, Shintani M, Miyanaga F, Aizawa‐Abe M, et al. Transgenic overexpression of leptin rescues insulin resistance and diabetes in a mouse model of lipoatrophic diabetes. Diabetes. 2001; 50: 1440–1448.
- Hotta K, Funahashi T, Arita Y, Takahashi M, Matsuda M, Okamoto Y, et al. Plasma concentrations of a novel, adipose‐specific protein, adiponectin, in type 2 diabetic patients. Arterioscler Thromb Vasc Biol. 2000; 20: 1595–1599.
- Gavrilova O, Marcus‐Samuels B, Graham D, Kim JK, Shulman GI, Castle AL, et al. Surgical implantation of adipose tissue reverses diabetes in lipoatrophic mice. J Clin Investig. 2000; 105: 271–278.
- Bruning JC, Michael MD, Winnay JN, Hayashi T, Horsch D, Accili D, et al. A muscle‐specific insulin receptor knockout exhibits features of the metabolic syndrome of NIDDM without altering glucose tolerance. Mol Cell. 1998; 2: 559–569.
- Abel ED, Peroni O, Kim JK, Kim YB, Boss O, Hadro E, et al. Adipose‐selective targeting of the GLUT4 gene impairs insulin action in muscle and liver. Nature. 2001; 409: 729–733.
- Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999; 402: 656–660.
- Tanaka M, Hayashida Y, Iguchi T, Nakao N, Nakai N, Nakashima K. Organization of the mouse ghrelin gene and promoter: occurrence of a short noncoding first exon. Endocrinol. 2001; 142: 3697–3700.
- Flier JS. Obesity wars: molecular progress confronts an expanding epidemic. Cell. 2004; 116: 337–350.
- Bednarek MA, Feighner SD, Pong SS, McKee KK, Hreniuk DL, Silva MV, et al. Structure-function studies on the new growth hormone-releasing peptide, ghrelin: minimal sequence of ghrelin necessary for activation of growth hormone secretagogue receptor 1a. J Med Chem. 2000; 43: 4370-4376.
- Hosoda H, Kojima M, Matsuo H, Kangawa K. Purification and characterization of rat des-Gln14-Ghrelin, a second endogenous ligand for the growth hormone secretagogue receptor. J BiolChem. 2000; 275: 21995-22000.
- Kojima M, Kangawa K. Ghrelin: structure and function. Physiol Rev. 2005; 85: 495-522.
- Ariyasu H, Takaya K, Tagami T, Ogawa Y, Hosoda K, Akamizu T, et al. Stomach is a major source of circulating ghrelin, and feeding state determines plasma ghrelinlike immunoreactivity levels in humans. J Clin Endocrinol Metab. 2001; 86: 4753-4758.
- Patterson M, Murphy KG, le Roux CW, Ghatei MA, Bloom SR. Characterization of ghrelin-like immunoreactivity in human plasma. J Clin Endocrinol Metab. 2005; 90: 2205-2211.
- Arvat E, Maccario M, Di Vito L. Endocrine activities of ghrelin, a natural growth hormone secretagogue (GHS), in humans: comparison and interactions with hexarelin, a nonnaturalpeptidyl GHS, and GH-releasing hormone. J Clin Endocrinol Metab. 2001; 86: 1169–1174.
- Tschop M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature. 2000; 407: 908–913.
- Unsal F, Sönmez MF. The effects of ovariectomy on ghrelin expression in the rat uterus. Adv Clin Exp Med. 2014; 23: 363–370.
- Sönmez MF, Ozan E. Determination of ghrelin immunore¬activity in the rat stomach after fasting and refeeding. Acta Histochem. 2007; 109: 193–199.
- Jeffery PL, Herington AC, Chopin LK. The potential autocrine/paracrine roles of ghrelin and its receptor in hormone-dependent cancer. Cytokine Growth Factor Rev. 2003; 14: 113–122.
- Rocha-Sousa A, Saraiva J, Henriques-Coelho T, Falcão-Reis F, Correia-Pinto J, Leite-Moreira AF. Ghrelin as a novel locally produced relaxing peptide of the iris sphincter and dilator muscles. Exp Eye Res. 2006; 83: 1179-1187.
- Dixit VD, Schaffer EM, Pyle RS, Collins GD, Sakthivel SK, Palaniappan R, et al. Ghrelin inhibits leptin- and activation-induced proinflammatory cytokine expression by human monocytes and T cells. J Clin Invest. 2004; 114: 57-66.
- Kawamura K, Sato N, Fukuda J, Kodama H, Kumagai J, Tanikawa H, at al. Ghrelin inhibits the development of mouse preimplantation embryos in vitro. Endocrinol. 2003; 144: 2623-2633.
- Rindi G, Savio A, Torsello A, Zoli M, Locatelli V, Cocchi D, et al. Ghrelin expression in gut endocrine growths. Histochem Cell Biol. 2002; 117: 521-525.
- Chanoine JP, Wong AC. Ghrelin gene expression is markedly higher in fetal pancreas compared with fetal stomach: effect of maternal fasting. Endocrinol. 2004; 145: 3813-3820.
- Hayashida T, Nakahara K, Mondal MS, Date Y, Nakazato M, Kojima M, et al. Ghrelin in neonatal rats: distribution in stomach and its possible role. J Endocrinol. 2002; 173: 239-245.
- Volante M, Fulcheri E, Allia E, Cerrato M, Pucci A, Papotti M. Ghrelin expression in fetal, infant, and adult human lung. J Histochem Cytochem. 2002; 50: 1013-1021.
- Santos M, Bastos P, Gonzaga S, Roriz JM, Baptista MJ, Nogueira-Silva C, et al. Ghrelin expression in human and rat fetal lungs and the effect of ghrelin administration in nitrofen-induced congenital diaphragmatic hernia. Pediatr Res. 2006; 59: 531-537.
- Howard AD, Feighner SD, Cully DF, Arena JP, Liberator PA, Rosenblum CI, et al. A receptor in pituitary and hypothalamus that functions in growth hormone release. Science. 1996; 273: 974–977.
- McKee KK, Palyha OC, Feighner SD, Hreniuk DL, Tan CP, Phillips MS, et al. Molecular analysis of rat pituitary and hypothalamic growth hormone secretagogue receptors. Mol Endocrinol. 1997; 11: 415–423.
- Smith RG, Feighner S, Prendergast K, Guan X, Howard A. A new orphan receptor involved in pulsatile growth hormone release. Trends Endocrinol Metab. 1999; 10: 128–135.
- Smith RG, Leonard R, Bailey AR, Palyha O, Feighner S, Tan C, et al. Growth hormone secretagogue receptor family members and ligands. Endocrine. 2001; 14: 9–14.
- Inui A. Ghrelin: an orexigenic and somatotrophic signal from the stomach. Nat Rev Neurosci. 2001; 2: 551–560.
- Feighner SD, Tan CP, McKee KK, Palyha OC, Hreniuk DL, Pong SS, et al. Receptor for motilin identified in the human gastrointestinal system. Science. 1999; 284: 2184–2188.
- O Ukkola. Ghrelin and metabolic disorders. Curr Protein Pept Sci. 2009; 10: 2–7.
- Poykko SM, Kellokoski E, Horkkoe S, Kauma H, Kesaniemi YA, Ukkola O. Low plasma ghrelin is associated with insulin resistance, hypertension, and the prevalence of type 2 diabetes. Diabetes. 2003; 52: 2546–2553.
- Barazzoni R, Zanetti M, Ferreira C, Vinci P, Pirulli A, Mucci M, et al. Relationships between desacylated and acylated ghrelin and insulin sensitivity in the metabolic syndrome. J Clin Endocrinol Metab. 2007; 92: 3935–3940.
- Ostergard T, Hansen TK, Nyholm B, Gravholt CH, Djurhuus CB, Hosoda H, et al. Circulating ghrelin concentrations are reduced in healthy offspring of type 2 diabetic subjects, and are increased in women independent of a family history of type 2 diabetes. Diabetologia. 2003; 46: 134–136.
- St-Pierre DH, Karelis AD, Coderre L, Malita F, Fontaine J, Mignault D, et al. Association of acylated and nonacylated ghrelin with insulin sensitivity in overweight and obese postmenopausal women. J Clin Endocrinol Metab. 2007; 92: 264–269.
- Saad NF, Bernaba B, Hwu CM, Jinagouda S, Fahmi S, Kogosov E, et al. Insulin regulates plasma ghrelin concentration. J Clin Endocrinol Metab. 2002; 87: 3997–4000.
- Weickert MO, Loeffelholz CV, Arafat AM, Schöfl C, Otto B, Spranger J, et al. Euglycemic hyperinsulinemia differentially modulates circulating total and acylated-ghrelin in humans. J Endocrinol Invest. 2008; 31: 119-124.
- Broglio F, Gottero C, Prodam F, Destefanis S, Gauna C, Me E, et al. Ghrelin secretion is inhibited by glucose load and insulin-induced hypoglycaemia but unaffected by glucagon and arginine in humans. Clin Endocrinol. 2004; 61: 503-509.
- Briatore L, Andraghetti G, Cordera R. Acute plasma glucose increase, but not early insulin response, regulates plasma ghrelin. Eur J Endocrinol. 2003; 149: 403-406.
- Flanagan DE, Evans ML, Monsod TP, Rife F, Heptulla RA, Tamborlane WV, et al. The influence of insulin on circulating ghrelin. Am J Physiol. 2003; 284: 313–316.
- Broglio F, Gottero C, Benso A, Prodam F, Destefanis S, Gauna C, et al. Effects of ghrelin on the insulin and glycemic responses to glucose, arginine, or free fatty acids load in humans. J Clin Endocrinol Metab. 2003; 88: 4268-4272.
- Dezaki K, Hosoda H, Kakei M, Hashiguchi S, Watanabe M, Kangawa K, et al. Endogenous ghrelin in pancreatic islets restricts insulin release by attenuating Ca2+ signaling in beta-cells: implication in the glycemic control in rodents. Diabetes. 2004; 53: 3142-3151.
- Broglio F, Arvat E, Benso A, Gottero C, Muccioli G, Papotti M, et al. Ghrelin, a natural GH secretagogue produced by the stomach, induces hyperglycemia and reduces insulin secretion in humans. J Clin Endocrinol Metab. 2001; 86: 5083-5086.
- Dezaki K, Sone H, Koizumi M, Nakata M, Kakei M, Nagai H, et al. Blockade of pancreatic islet-derived ghrelin enhances insulin secretion to prevent high-fat diet-induced glucose intolerance. Diabetes. 2006; 55: 3486-3493.
- Dezaki K, Kakei M, Yada T. Ghrelin uses Galphai2 and activates voltage-dependent K+ channels to attenuate glucose induced Ca2+ signaling and insulin release in islet beta-cells: novel signal transduction of ghrelin. Diabetes. 2007; 56: 2319-2327.
- Gauna C, Delhanty PJ, Hofland LJ, Janssen JA, Broglio F, Ross RJ, et al. Ghrelin stimulates, whereas des-octanoyl ghrelin inhibits, glucose output by primary hepatocytes. J Clin Endocrinol Metab. 2005; 90: 1055-1060.
- Murata M, Okimura Y, Iida K, Matsumoto M, Sowa H, Kaji H, et al. Ghrelin modulates the downstream molecules of insulin signaling in hepatoma cells. J Biol Chem. 2002; 277: 5667-5674.
- Heijboer AC, van den Hoek AM, Parlevliet ET, Havekes LM, Romijn JA, Pijl H, et al. Ghrelin differentially affects hepatic and peripheral insulin sensitivity in mice. Diabetologia. 2006; 49: 732-738.
- Gauna C, Meyler FM, Janssen JA, Delhanty PJ, Abribat T, van Koetsveld P, et al. Administration of acylated ghrelin reduces insulin sensitivity, whereas the combination of acylated plus unacylated ghrelin strongly improves insulin sensitivity. J Clin Endocrinol Metab. 2004; 89: 5035-5042.
- Sun Y, Asnicar M, Saha PK, Chan L, Smith RG. Ablation of ghrelin improves the diabetic but not obese phenotype of ob/ob mice. Cell Metab. 2006; 3: 379-386.
- Joseph JW, Koshkin V, Zhang CY, Wang J, Lowell BB, Chan CB, et al. Uncoupling protein 2 knockout mice have enhanced insulin secretory capacity after a high-fat diet. Diabetes. 2002; 51: 3211-3219.
- Doi A, Shono T, Nishi M, Furuta H, Sasaki H, Nanjo K. IA- 2beta, but not IA-2, is induced by ghrelin and inhibits glucose stimulated insulin secretion. Proc Natl Acad Sci USA. 2006; 103: 885-890.
- Yildirim S, Sundler F, Bolkent S. Ghrelin and insulin gene expression changes in streptozotocin-induceddiabetic rats after rosiglitazone pretreatment. Eur J Histochem. 2007; 51: 11-17.
- Sharifi F, Yamini M, Esmaeilzadeh A, Mousavinasab N, Shajari Z. Acylated ghrelin and leptin concentrations in patients with type 2 diabetes mellitus, people with prediabetes and first degree relatives of patients with diabetes, a comparative study. J Diabetes & Metabolic Disorders. 2013; 12: 51-56.
- Siddique L, Ahmed U, Rajput TA. Effect of Ghrelin Antagonist on Serum Testosterone and Luteinizing Hormone in Obese Type 2 Diabetic Mice. JRMC. 2014; 18: 32-36.
- Sönmez MF, Karabulut D, Kilic E, Akalin H, Sakalar C, Gunduz Y, et al. Folia Histochem Cyto. 2015; 53: 26–34.
- Yildirim AB, Karabulut D, Dundar M, Ulusoy HB, Sonmez MF. Expression of Ghrelin and GHSR-1a in Long Term Diabetic Rat’s Kidney. Braz Arch Biol Technol. 2016; 59: 1-7.