
Research Article
Austin J Endocrinol Diabetes. 2016; 3(2): 1045.
Effect of Echinochrome on Body Weight, Musculoskeletal System and Lipid Profile of Male Diabetic Rats
Soliman AM, Mohamed AS* and Marie M-AS
Zoology Department, Cairo University, Egypt
*Corresponding author: AMohamed AS, Zoology Department, Faculty of Science, Cairo University, 12613- Egypt- 01275350954, Egypt
Received: June 13, 2016; Accepted: August 01, 2016; Published: August 08, 2016
Abstract
Background: Diabetes mellitus is associated with several musculoskeletal disorders and functional liver abnormalities that affect protein and lipid metabolism.
Objective: The present study was carried out to evaluate effect of echinochrome on musculoskeletal system and metabolism of lipids and proteins in both types of diabetes mellitus.
Methods: Thirty-six male Wistar albino rats were divided into two main groups, type 1 diabetes and type 2 diabetes groups. Each group divided into 3 subgroups (6 rats /subgroup); control, diabetic and echinochrome groups. Diabetic model was induced by single dose of streptozotocin (60 mg/kg, i.p) for type 1 diabetes and by high fat diet for 4 weeks before the injection with streptozotocin (30 mg/kg, i.p) for type 2 diabetes. Diabetic groups were treated orally with echinochrome extract (1mg/kg body weight in 10% DMSO) daily for 4 weeks.
Results:: Diabetes, Sea urchin- Behavior, Insulin resistant, Obesity, Echinochrome Diabetic groups showed significant decrease in the final body weight, time latencies of hot plate and wire suspension tests, total protein, albumin, A/G ratio and HDL-C. However, globulins, TG, TC and LDL-C concentrations increased significantly. On the other hand Ech groups showed significant increase in time latencies hot plate and wire suspension tests, total protein, albumin and A/G ratio. While, globulins, TG, TC and LDL-C concentrations decrease significantly.
Conclusion: The current study demonstrated the potentials of echinochrome in improvement the musculoskeletal system and the metabolism of lipid and protein metabolism in both types of diabetes mellitus.
Keywords: Diabetes, Sea urchin- Behavior, Insulin resistant, Obesity, Echinochrome
Introduction
Diabetes Mellitus (DM) is a chronic disease characterized by hyperglycemia resulting in insulin resistance and/or insulin deficiency caused by the failure of β-pancreatic cells [1]. DM may affect the musculoskeletal system through many ways including glycosylation of proteins, microvascular abnormalities and collagen accumulation [2]. Musculoskeletal complications are most commonly in both types of diabetes [2]. DM is associated with several structural and functional liver abnormalities that affect on protein and lipid metabolism [3]. It is mainly classified into type 1 diabetes and type 2 diabetes, whereas 90,95% of diabetic patients are type 2 [4]. Type Diabetes mellitus (T1DM) is an autoimmune disease, which characterized by loss of insulin producing β-cells and reliance on exogenous insulin for survival [5]. Type 2 Diabetes Mellitus (T2DM) is increasing in prevalence worldwide [6], and it is strongly associated with obesity and insulin resistance [7], as well as defects in pancreatic beta cells function and mass [8].
Streptozotocin (STZ) is an antibiotic produced by the bacterium Streptomyces achromogens and possesses a broad spectrum of antibacterial activities [9]. It is a widely used chemical for the
induction of experimental diabetes model in rodents [10]. T1DM can be induced in rodents by a single dose of STZ injection [11], while type 2 diabetes can be induced by High Fat Diet (HFD) feeding followed by a low-dose STZ injection [12]. In the HFD/STZ rat models, the state of obesity, insulin resistance and/or glucose intolerance in prediabetesis simulated by a period of a HFD.HFD/STZ model in rats is similar to the metabolic profile of type 2 diabetes in humans [12]. Thus, models of STZ-induced diabetes in animals have been very useful in detecting the mechanisms of diabetic pathogenesis and in screening artificial chemicals, natural products, and pharmacological agents that are potentially capable of lowering blood glucose levels [13].
Despite considerable progress in the treatment of diabetes by oral hypoglycemic agents, search for newer drugs continues because the existing synthetic drugs have several limitations, harmful effects and poor effects in relieving clinical symptoms and controlling diabetic complications [14,15]. Sea urchin (P.lividus) is a widespread species in the Atlantic and the Mediterranean coasts and is subjected to intensive commercial fishing in several countries [16]. It has a number of unique substances, such as quinonoid pigments named spinochromes [17,18]. From these compounds, Echinochrome (Ech) which possesses high antioxidant activity and is the most common dark red pigment of sea urchin shells, spines, and eggs [19]. The hypoglycemic activity and the antioxidant role of Echproved by Mohamed et al. [20]. The present study was carried out to evaluate effect of echinochrome on musculoskeletal system and metabolism of lipids and proteins in both types of diabetes mellitus
Materials and Methods
Chemicals and reagents
Streptozotocin and Dimethyl Sulfoxide (DMSO) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Biochemical kits were purchased from the Biodiagnostic Company (El Moror St, Dokki, EGY).
Sea urchin collection
Sea urchins (P.lividus) were collected from the Mediterranean coast of Alexandria (Egypt) and transported to the laboratory packed in ice. The samples were thoroughly washed with seawater to remove sand and overgrowing organisms at the collection site and transported to the laboratory. The collected specimens were identified by the standard literature of taxonomic guide [21]. The collected specimens were immediately shade dried.
Echinochrome (Ech) extraction
Pigments in the shells and spines were isolated by the Amarowicz method with slight modifications [22,23]. After removal of the internal organs, the shells and spines were washed with a stream of cold water, air-dried at 4°C for 2 days in the dark and then were grounded. The powders (5 g) were dissolved by gradually adding 10 ml of 6 M HCl. The pigments in the solution were extracted 3 times with the same volume of diethyl ether. The ether layer collected was washed with 5% NaCl until the acid was almost removed. The ether solution including the pigments was dried over anhydrous sodium sulfate and the solvent was evaporated under reduced pressure. The extract including the polyhydroxylated naphthoquinone pigment was stored at -30°C in the dark.
Ethical approval
Experimental protocols and procedures used in this study were approved by the Cairo University, Faculty of Science, Institutional Animal Care and Use Committee (IACUC) (Egypt) (CUFS/F/33/14). All the experimental procedures were carried out in accordance with international guidelines for the care and use of laboratory animals.
Experimental animals
Male albino Wistar rats (Rattusnorvegicus) weighing 140 ± 10 gm (6 weeks) for T1DM and 80 ± 10 gm (4 weeks) for T2DMwere used in this study. The rats were obtained from the National Research Center (NRC, Dokki, Giza). Rats were housed in a temperature and humidity controlled environment and given food and water ad libitum.
Induction of type 1 diabetes mellitus (T1DM)
All rats were starved for 12 hrs before the experiment, but were allowed free access to water. T1DM was induced by intraperitoneal injection of 60 mg/kg of Streptozotocin (STZ) dissolved in 0.1mol/l sodium citrate buffer at pH 4.5. Blood glucose levels were measured 72 hr after injection of STZ. Rats were starved, but had access to drinking water for 6 hr before blood glucose measurement. Fasting plasma glucose concentrations ≥ 300mg/100mlwere considered diabetic type 1 in this experiment [24].
Induction of type 2 diabetes mellitus (T2DM)
The rats were fed a high fat diet with energy of 5.3 kcal/g, comprising 60% calories from fat, 35% from protein and 5% from carbohydrate, according to a modification of the protocols of Reed et al. [25-28]. After 4 weeks the rats injected intraperitoneally by a single dose of prepared solution of STZ (30 mg/kg dissolved in 0.1mol/l sodium citrate buffer at pH 4.5). After 72 hours, fasting plasma glucose concentrations ≥ 300mg/100ml were considered diabetic type 2 in this experiment [29].
Experimental design
After one week of acclimatization, 36 rats were assigned into two main groups; T1DM group (18 rats) and T2DM group (18 rats).
T1DM group was divided into 3 subgroups (6rats/subgroup):
Control group: After a single dose of citrate buffer (0.1mol/l, i.p), the rats received 1ml (10% DMSO, orally) daily for 4 weeks.
Diabetes group: After a single dose of STZ (60 mg/kg, i.p), the rats received 1ml (10% DMSO, orally) daily for 4 weeks.
Ech group: After a single dose of STZ (60 mg/kg, i.p), therats received 1mlEch (1mg/kg body weight in 10% DMSO, orally) [30] daily for 4 weeks.
T1DM group was divided also into 3 subgroups (6rats/subgroup):
Control group: After 4 weeks of normal diets feeding, the rats injected with single dose of citrate buffer (0.1mol/l, i.p) then received 1ml of (10% DMSO, orally) daily for 4 weeks.
Diabetes group: After 4 weeks of HFD feeding, the rats injected with single dose of STZ (30 mg/kg, i.p) then received 1ml of (10% DMSO, orally) daily for 4 weeks.
Ech group: After 4 weeks of HFD feeding, the rats injected with single dose of STZ (30 mg/kg, i.p) then received 1mlEch (1mg/kg in 10% DMSO, orally) daily for 4 weeks.
Determination of the physical parameters
Body weight: Body weight measured at the beginning, after administration of HFD and the ending of the experiments.
Hot plate test: The hot plate latency was measured using a modification of the original method of Eddy and Leimbach [31]. Briefly, the modified apparatus consists of an electric cooking plate (Saiso, Japan) with a 1500 Watts stainless steel heating element connected to a thermostat (0-4000C), a thermocouple connects the thermostat to a chrome plated drip pan. The thermocouple together with the thermostat control the temperature of the hot plate within the desired range once set. Pain sensitivity was evaluated by the response latency for paw licking on the hot place. In order to avoid tissue damage, the maximum time the animal could spend on the hot plate was pegged at 60 seconds. Response latencies were measured at 15-minute intervals and the average of the results was taken.
Wire suspension: The wire suspension assay measured muscle strength and the prehensile reflex, an animal’s ability to grasp a taut horizontal wire with its forepaws and to remain suspended. The tail held rats gently and the forepaws were placed on a suspended wire 2 mm in diameter and 62 cm above a cushioned surface. The latency to let go was measured using a stopwatch, with shorter latency to drop indicating reduced strength and/or reflex ability. If a rat did not let go of the wire within 60 s, it was removed from the wire and a latency of 60 s was assigned to that measurement. Each rat was used only once in the wire suspension assay [32].
Animal handling and specimen collection
The rats were fully anesthetized with 3% sodium pentobarbital, and the chest was opened. A needle was inserted through the diaphragm and into the heart. Negative pressure was gently applied once the heart had been punctured, and the needle was repositioned as required until blood flowed into the syringe. The blood collected from the rats was separated by centrifugation (3000 rpm, 15 min) to obtain sera, which was stored at -80˚C for the biochemical measurements.
Biochemical analysis
The serum total protein was estimated by the method of Tietz [33], serum albumin [34], serum total lipids [35], serum triglycerides [36], serum total cholesterol [37], serum low density lipoprotein [38] and serum high density lipoprotein [39] were determined according to the manufacturer’s instructions using Spectrum Diagnostics and Bio-diagnostic kits (Giza, Egypt).
Statistical analysis
Values were expressed as means ± SE. The comparisons within groups were evaluated utilizing one-way Analysis Of Variance (ANOVA) with Duncan post hoc test was used to compare the group means and p < 0.05 was considered statistically significant. SPSS, for Windows (version 15.0) was used for the statistical analysis.
Results
Body weight
Data recorded in Table 1 and Figure 1 demonstrated that, final body weight of diabetic rats decreased significantly (P<0.05) at the end of experiment, as compared to the corresponding control groups. On the other hand, a significant increase (P<0.05) in the final body weight was observed after Ech administration, as compared to the corresponding diabetic groups.
Item
T1DM
Intial
Final
Groups
Control
140.00±2.89a
211.00±11.99c
Diabetes
141.67±1.67a
114.83±4.45a
Ech
143.33±3.33a
154.33±7.95b
Values are means ± se (n = 6 per group).
Each value not sharing a common letter superscript is significantly different (P <0.05).
Table 1: The curative role of echinochrome on the body weight of diabetic rats.
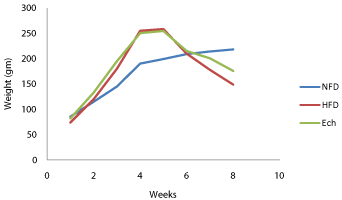
Figure 1: The curative role of Echinochrome (Ech) on body weight of type 2
diabetic rats.
Behavioral tests
The obtained data in Figure 2 revealed that T1DM and T2DM rats showed significant decrease (P < 0.05) in time latencies of hot plate and wire suspension tests as compared to control rats. However, the treated rats with Ech showed significant increase in their time latencies as compared to the diabetic groups.
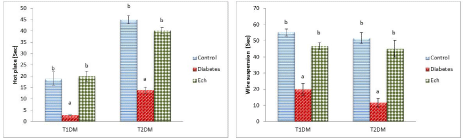
Figure 2: The curative role of Echinochrome (Ech) on musculoskeletal system of diabetic rats. Values are means ± se (n = 6 per group). Each value not sharing a
common letter superscript is significantly different (P <0.05).
Protein profile
The results presented in Table 2 showed that, there was a significant reduction (P <0.05) in the total protein, albumin, and A/G ratio, while globulin concentration increased in the diabetic rats, as compared to control rats. The protein, albumin, and A/G levels was significantly increased, however globulin level decreased significantly after 4 weeks of treatment with Ech, as compared to the corresponding diabetic groups.
Parameter
Diabetes
type
Groups
Control
Diabetes
Ech
Total protein
T1DM
6.92±0.65c
3.20±0.17a
5.75±0.49b
T2DM
5.98±0.27b
3.15±0.21a
5.33±0.80b
Albumin
T1DM
3.24±0.32b
1.87±0.20a
3.19±0.20b
T2DM
2.98±0.37c
0.49±0.06a
1.65±0.24b
Globulins
T1DM
1.27±0.09a
2.37±0.36b
1.56±0.11a
T2DM
2.63±0.28a
5.63±0.27c
4.47±0.26b
A/G ratio
T1DM
3.59±0.37c
0.95±0.12a
2.09±0.16b
T2DM
1.25±0.17c
0.09±0.01a
0.37±0.05b
Values are means ± se (n = 6 per group).
Each value not sharing a common letter superscript is significantly different (P <0.05).
Table 2: The curative role of Echinochrome (Ech) on the protein profile of diabetic rats.
Lipid profile
Table 3 revealed that T1DM and T2DM rats showed a significant increase (P < 0.05) in serum Triglyceride (TG), Total Cholesterol (TC) and LDL-Cholesterol (LDL-C) concentrations, while HDLCholesterol (HDL-C) decreased significantly, as compared to the corresponding control groups. On the other hand, Ech-treated groups showed a significant decrease in serum TG, TC and LDL-C concentrations, while HDL-C showed non-significant change as compared to the corresponding diabetic groups.
Parameter
Diabetes
type
Groups
Control
Diabetes
Ech
TG
T1DM
157.67±1.31a
231±3.72c
183.5±4.14b
T2DM
129.5±3.66a
153±4.63b
133.17±4.75a
TC
T1DM
165.67±8.67a
227.17±16.83b
179.83±5.19a
T2DM
168.83±11.24a
211.50±5.79b
168.33±5.80a
LDL-C
T1DM
130.67±2.33a
189.50±8.48b
132.00±7.36a
T2DM
119.67±7.02a
152.67±2.77b
121.00±4.30a
HDL-C
T1DM
58.83±2.10b
42.41±0.28a
48.90±1.27a
T2DM
58.04±1.98b
45.23±1.44a
49.23±0.79a
Values are means ± se (n = 6 per group).
Each value not sharing a common letter superscript is significantly different (P <0.05).
Table 3: The curative role of Echinochrome (Ech) on the lipids profile of diabetic rats.
Discussion
Diabetes Mellitus (DM) is one of the five leading causes of death in the world and about six deaths per minute are attributable to diabetes complications [40]. It is associated with a reduction in quality of life and an increase in risk factors for comorbidities and mortality [41]. Long-term hyperglycemia is an important factor for the development and progression of microvascular and macrovascular complications [42]. In the present study, the significant decrease in body weight of the diabetic groups was possible due to the defect in glucose metabolism and increased muscle wasting due to excessive breakdown of tissue proteins. Muscle wasting, negative nitrogen balance and enhanced gluconeogenesis are characteristic features of uncontrolled diabetes [43]. The treatment with Ech caused a significant increase in the final body weight of diabetic rats. The restoration of the weight loss which may be due to the reversal of proteolysis, gluconeogenesis and glycogenolysis [43].
It is well known that both neuropathy and myopathy may occur in spontaneous and experimental diabetes mellitus [44]. Diabetic neuropathy is characterized by clinical features like hyperalgesia (exaggerated responses to painful stimuli) due to elevated nociceptive response [45]. Similar symptoms are exhibited by STZ-induced diabetic animals [46]. In the present study, hyperalgesia was observed in the diabetic rats using hot plate test. Diabetic rats clearly demonstrated an increase in the sensitivity to pain. Our findings agreed with previous studies which suggested that acute hyperglycemia may increase musculoskeletal pain [47]. The suggested mechanism is that a hyperglycemic state induces overproduction of intracellular sorbitol in tissues [48], which increases intracellular osmotic pressure that modulates several ionic conductance’s and increased Ca2+ influx and membrane depolarization [49], both of which lead to increase pain sensitivity. On the other hand, the pain sensitivity of Ech-diabetic male rats was decreased in the present study.
The hanging wire test is performed in order to demonstrate a motor neuromuscular impairment and motor coordination [50]. Provided its simplicity, the test was also used in pharmacological studies, for evaluating the neuromuscular tone [50]. The prehensile reflex refers to an animal’s ability to grasp a horizontal wire with its forepaws and to remain suspended, as it is a measure of muscle strength [51]. Muscle strength and the prehensile reflex of the diabetic rats were decreased as shown from wire suspension latencies in the present study.
The decrease in prehensile reflex indicates the morphological destabilization of neuromuscular junction in diabetes such as axonal degeneration, axonal atrophy and demyelination [52]. STZ directly results in a decreased body mass and reduced muscle fiber crosssectional area [53]. Furthermore, STZ negatively affected myoblast proliferative capacities, which result of a G2/M phase cell cycle arrest that was associated with an increase in Reactive Oxygen Species (ROS) production [53]. On the other hand, muscle strength and the prehensile reflex of the diabetic rats were improved after the treatment with Ech. This effect may be through antioxidants properties of Ech, which prevent the decrease of myosin creatine kinase transcription in skeletal muscle cells [54].
The liver is the central metabolic organ in the body, which is responsible for glucose and proteins homeostasis. Albumin is the most abundant circulating protein in the plasma [55] and the most important protein synthesized by the liver [56]. The synthesis of albumin reflects the extent of functioning of liver cell mass [56]. The serum total protein and albumin levels in diabetic rats were reduced in the present study. This decreas has been attributed to inhibition of oxidative phosphorylation which leads to decrease in protein synthesis, increase in catabolic processes and reduction of protein absorption [57]. The treatment with Ech in the present study revealed that, the serum total protein and albumin levels increased in the diabetic rats. The increase in total protein and albumin in the group treated with Ech further supports a possible preservation of liver function [58]. The increase in serum globulins concentration of the diabetic rats in the present study may be due to the more severe impairment of albumin formation in the liver, which attempt by the body to compensate with an increased output of beta and gamma globulins, especially the latter [56]. A/G ratio of diabetic rats is main clinical use when it is reduced as a result of decrease in serum albumin and the sequential increase in serum globulins [59]. On the other hand, Ech-treated rats showed significant reduction in globulin concentration, while A/G ratio increased.
The liver plays an essential role in lipid metabolism, several stages of lipid synthesis and transportation [60]. It is the principal site for the formation and clearance of lipoproteins where it receives fatty acids and cholesterol from peripheral tissues and diet, packages them into lipoprotein complexes and releases these complexes back into the circulation [61]. The liver is insulin dependent tissue that plays a significant role in glucose and lipid homeostasis and is severely affected by diabetes [62]. So, hyperlipidemia has been reported to accompany hyperglycemia states [63]. In the present study, it was found that TG, TC and LDL-C concentrations were significantly higher while HDL-C decreased in diabetic groups. This may be due to lack of insulin [64], where the insulin regulates several of the steps of lipid metabolism [65]. The treatment with Ech in the present showed decreases in concentrations of TG, TC, LDL-C and increase level of HDL-C, which may be due to proper stabilization of glucose level and increase in insulin level. Moreover, this effect may be due to decreased intestinal absorption or decreased cholesterol biosynthesis [66].
Conclusion
The current study demonstrated the potentials of echinochrome in improvement the musculoskeletal system and the metabolism of lipid and protein metabolism in both types of diabetes mellitus.
References
- Damasceno DC, Netto, A O, Iessi, I L, Gallego FQ, Corvino SB, Dallaqua B, et al. Streptozotocin-induced diabetes models: pathophysiological mechanisms and fetal outcomes. Biomed Res Int. 2014; 819065.
- Kim RP, Edelman SV, Kim DD. Musculoskeletal Complications of Diabetes. CLINICAL DIABETES. 2001; 19: 132-135.
- Koyuturk M, Tunali S, Bolkent S, Yanardag R. Effects of vanadyl sulfate on liver of streptozotocin-induced diabetic rats. Biol Trace Elem Res. 2005; 104: 233–247.
- Leng YP, Qiu N, Fang WJ, Zhang M, He ZM, Xiong Y. Involvement of increased endogenous asymmetric dimethylarginine in the hepatic endoplasmic reticulum stress of type 2 diabetic rats. PLoS One. 2014; 97125.
- Simmons KM, Michels AW. Type 1 diabetes: A predictable disease. World J Diabetes. 2015; 6: 380-390.
- Unwin N, Gan D, Whiting D. The IDF Diabetes Atlas: providing evidence, raising awareness and promoting action. Diabetes Res Clin Pract. 2010; 87: 2–3.
- Guilherme A, Virbasius JV, Puri V, Czech MP. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat Rev Mol Cell Biol. 2008; 9: 367-377.
- Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes. 2003; 52: 102–110.
- Vavra JJ, Deboer C, Dietz A, Hanka LJ, Sokolski WT. Streptozotocin, a new antibacterial antibiotic. Antibiot Annu. 1959; 7: 230–235.
- Lenzen S. The mechanisms of alloxan- and streptozotocin-induced diabetes. Diabetologia. 2008; 51: 216–226.
- Yin D, Tao J, Lee DD, Jikun Shen, Manami Hara, James Lopez, et al. Recovery of islet beta-cell function in streptozotocin-induced diabetic mice: an indirect role for the spleen. Diabetes. 2006; 55: 3256–3263.
- Skovsø S. Modeling type 2 diabetes in rats using high fat diet and streptozotocin. J Diabetes Investig. 2014; 5: 349–358.
- Kumar S, Vasudeva N, Sharma S. GC-MS analysis and screening of antidiabetic, antioxidant and hypolipidemic potential of Cinnamomum tamala oil in streptozotocin induced diabetes mellitus in rats. Cardiovasc Diabetol. 2012; 95: 1-11.
- Eurich DT, McAlister FA, Blackburn DF, Majumdar SR, Tsuyuki RT, Varney J, et al. Benefits and harms of antidiabetic agents in patients with diabetes and heart failure: systematic review. BMJ. 2007; 335: 497–501.
- Zhao DD, Yu N, Li XK, Fang X, Mu QQ, Qin PJ, et al. Antidiabetic and antioxidative effect of jiang tang xiao ke granule in high-fat diet and low-dose streptozotocin induced diabetic rats. Evid Based Complement Alternat Med. 2014; 475192.
- Arafa S, Chouaibi M, Sadok S, El Abed A. The influence of season on the gonad index and biochemical composition of the sea urchin Paracentrotus lividus from the Golf of Tunis. Scient. World J. 2012; 815935.
- Thomson RH. Naturally Occurring Quinones. Academic Press, London, UK and New York. 1971.
- Jeong SH, Kim HK, Song IS, Noh SJ, Marquez J, Ko KS, et al. Echinochrome a increases mitochondrial mass and function by modulating mitochondrial biogenesis regulatory genes. Mar Drugs. 2014; 12: 4602-4615.
- Anderson HA, Mathieson JW, Thomson RH. Distribution of spinochrome pigments in echinoids. Comp Biochem Physiol. 1969; 28: 333–345.
- Mohamed AS, Soliman AM, Marie MA. Mechanisms of echinochrome potency in modulating diabetic complications in liver. Life Sciences (In press). 2016.
- Clark AM, Rowe FEW. Monograph of shallow water Indo west pacific echinoderms. London, Trustees of the British Museum (Natural History). 1971.
- Amarowicz R, Synowiecki J, Shahidi F. Sephadex LH-20 separation of pigments from shells of red sea urchin (Strongylocentrotus franciscanus). Food Chem. 1994; 51: 227-229.
- Kuwahara R, Hatatea H, Yukia T, Murata H, Tanakac R, Hamad Y. Antioxidant property of polyhydroxylated naphthoquinone pigments from shells of purple sea urchin Anthocidaris crassispina. LWT Food Science Technol. 2009; 42: 1296-1300.
- Chen X, Fu X, Li C, Zhao H. ER stress and ER stress-induced apoptosis are activated in gastric SMCs in diabetic rats. World J. Gastroenterol. 2014; 20: 8260-8267.
- Reed MJ, Meszaros K, Entes LJ, Claypoo MD, Pinkett JG, Gadbois TM, et al. new rat model of type 2 diabetes: the fat-fed, streptozotocin-treated rat. Metabolism. 2000; 49: 1390–1394.
- Nishi, Ahad A, Kumar P. Protective effect of chlorogenic acid against diabetic nephropathy in high fat diet/streptozotocin induced type-2 diabetic rats. Int J Pharm Pharmaceutl Sci. 2013; 5: 489-495.
- Srinivasan K, Viswanad B, Asrat L, Kaul CL, Ramarao P. Combination of high-fat diet-fed and low-dose streptozotocin-treated rat: a model for type 2 diabetes and pharmacological screening. Pharmacol Res. 2005; 52: 313-320.
- Skovso, S. Modeling type 2 diabetes in rats using high fat diet and streptozotocin. J Diabetes Investig. 2014; 5: 349–358.
- Ebaid H. Promotion of immune and glycaemic functions in streptozotocin-induced diabetic rats treated with un-denatured camel milk whey proteins. Nutr Metabol. 2014; 11: 1-31.
- Lennikov A, Kitaichi N, Noda K, Kazuomi Mizuuchi, Ryo Ando, Zhenyu Dong, et al. Amelioration of endotoxin-induced uveitis treated with the sea urchin pigment echinochrome in rats. Molecular Vision. 2014; 20: 171-177.
- Eddy NB, Leimbach D. Synthetic analgesics 11. Diathianylbutenyl and Dithienyl butylamines. J Pharmac. 1953; 107: 385-393.
- Troen AM, Chao WH, Crivello NA, D'Anci KE, Shukitt-Hale B, Smith DE, et al. Cognitive Impairment in Folate-Deficient Rats Corresponds to Depleted Brain Phosphatidylcholine and Is Prevented by Dietary Methionine without Lowering Plasma Homocysteine. J Nutr. 2008; 138: 2502–2509.
- Tietz NW, Burtis CA, Ashwood ER. Tietz textbook of clinical chemistry. Saunders, Philadelphia. 1994.
- Tietz NW, Finley P, Pruden E Amerson. Clinical Guide to Laboratory Tests Saunders. Philadelphia. 1990; 232-233.
- Zollner N, Kirsch K. Microdetermination of lipids by the sulphophosphovanillin reaction. Z Ges Exp Med. 1962; 135: 545–561.
- Stein, E A and Myers, G. Lipids, lipoproteins and apolipoproteins. In: Burtis CA, Ashwood, E.R. (Eds.). In In Tietz Textbook of Clinical Chemistry. WB Saunders Company. 1994.
- Richmond W. Preparation and properties of a cholesterol oxidase from Nocardia sp. and its application to the enzymatic assay of total cholesterol in serum. Clin Chem. 1973; 19: 1350–1356.
- Wieland H, Seidel D. A simple specific method for precipitation of low density lipoproteins. J Lipid Res. 1983; 24: 904-909.
- Lopes-Virella MF, Stone P, Ellis S, Colwell JA. Cholesterol determination in high-density lipoproteins separated by three different methods. Clin Chem. 1977; 23: 882-884.
- Li K , Wu XD, Davey AK, Wang J. Antihyperglycemic effects of baicalin on streptozotocin-nicotinamide induced diabetic rats. Phytother Res. 2011; 25: 189-194.
- Mascaro MB, França CM, Esquerdo KF, Lara MA, Wadt NS, Bach EE. Effects of Dietary Supplementation with Agaricus sylvaticus Schaeffer on Glycemia and Cholesterol after Streptozotocin-Induced Diabetes in Rats. Evid Based Complement. Alternat Med. 2014; 107629.
- Babio N, Balanza R, Basulto J, Bull´o M, Salas-Salvad´o J. Dietary fibre: influence on body weight, glycemic control and plasma cholesterol profile. Nutricion Hospitalaria. 2010; 25: 327–340.
- West KM, Ahuja MM, Bennett PH, Czyzyk A, De Acosta OM, Fuller JH, et al. The role of circulating glucose and triglyceride concentrations and their interactions with other “risk factors” as determinants of arterial disease in nine diabetic population samples from the WHO multinational study. Diabetes Care. 1983; 6: 361–369.
- Clements RS. Diabetic neuropathy - New concepts of its etiology. Diabetes. 1979; 28: 604-611.
- Ramos KM, Jiang Y, Svensson CI, Calcutt NA. Pathogenesis of spinally mediated hyperalgesia in diabetes. Diabetes. 2007; 56: 1569-1576.
- Calcutt NA, Chaplan SR. Spinal pharmacology of tactile allodynia in diabetic rats. British J Pharmacol.1997; 122: 1478–1482.
- Forman LJ, Estilows S, Lewis M, Vasilenko P. Streptozocin diabetes alters immunoreactive beta-endorphin levels and pain perception after 8 wk in female rats. Diabetes. 1986; 35: 1309-1313.
- Malone JI, Lowitts S. Measurement of tissue sorbitol in diabetes mellitus: enzyme method versus- gas liquid chromatography. Metabol. 1992; 41: 224-227.
- Viana F, Pera EDL, Schmidt RF, Belmonte C. Swelling activated calcium signaling in cultured mouse primary sensory neurons. Eur J neurosci. 2001; 13: 722-734.
- Crestani F, Löw K, Keist R, Mandelli M, Möhler H, Rudolph U. Molecular targets for the myorelaxant action of diazepam. Mol Pharmacol. 2001; 59: 442-445.
- Shukitt-Hale B, Mouzakis G, Joseph JA. Psychomotor and spatial memory performance in aging male Fischer 344 rats. Exp Gerontol. 1998; 33: 615-624.
- Adam PW, Johnston JE, Campbell JG, Found MC, Thomas J. Streptozotocin induces G2 arrest in skeletal muscle myoblasts and impairs muscle growth in vivo. Am J Physio Cell Physiol. 2007; 292: 1033-1040.
- Buck M, Chojkier M. Muscle wasting and dedifferentiation induced by oxidative stress in a murine model of cachexia is prevented by inhibitors of nitric oxide synthesis and antioxidants. EMBO J. 1996; 15: 1753-1765.
- Roche M, Rondeau P, Singh NR, Tarnus E, Bourdon E. The antioxidant properties of serum albumin. FEBS Letters. 2008; 582: 1783-1787.
- Singh K. Evaluation and interpretation of biomarkers of liver diseases. Int J Res Health Sci. 2013; 1: 213-223.
- Yassin MM, Ashour AA, Elyazji NR. Alterations in body weight, protein profile, non-protein nitrogen constituents and kidney structure in diabetic rats under glibenclamide treatment. J Islam Univer Gaza. 2004; 12: 37-54.
- Iweala EEJ, Uhegbu FO, Adesanoye OA. Biochemical effects of leaf extracts of Gongronema latifolium and selenium supplementation in alloxan induced diabetic rats. J Pharmacognosy Phytother. 2013; 5: 91-97.
- Henry JB, Saunders WB. Clinical diagnosis and management by laboratory methods. Saunders WB. Philadelphia. 1996.
- Soliman A, Marie M A-S, Saleh HM, Mohamed AS. Assessment of sepia ink extract role against the kidney dysfunction induced by bile duct ligation. J Bas Appl Zool. 2014; 67: 173–181.
- Mandal SK, Sil K, Chatterjee S, Jacky Ganguly, Koushik Chatterjee, Pankaj Sarkar, et al. A study on lipid profiles in chronic liver diseases. Natl J Med Res. 2013; 3: 70-72.
- Ozturk SA, Aytekin I, Ozsoy HO, Ozturk AN, Ylmaz N. Effects of caffeic acid phenethyl ester on oxidative stress, histopathology and some biochemical parameters in streptozotocin-induced diabetic rats. Turk J Biochem. 2015: 02259.
- Taskinen MR. Lipoprotein and apoproteins in diabetes. In Belfiore, F et al. eds. Current Topics in Diabetes Research. Informa Health Care. 1996.
- Mathur A, Mathur R. Study of association of serum lipids with diabetic retinopathy in type 2 diabetes mellitus. People’s J Scie Res. 2013; 6: 25-28.
- Rivellese AA, Vaccaro O, Patti L. The pathophysiology of lipid metabolism and diabete. Int J Clin Pract. 2004; 58: 32–35.
- Ahmed D, Kumar V, Verm A, Pushpraj S Gupta, Hemant Kumar, Vishal Dhingra, et al. Antidiabetic, renal/hepatic/pancreas/cardiac protective and antioxidant potential of methanol/dichloromethane extract of Albizzia Lebbeck Benth. stem bark (ALEx) on streptozotocin induced diabetic rats. BMC Complement Altern Med. 2014; 14: 214-243.
- Marques MJ, Santo NH. Acetylcholine receptors and nerve terminal distribution at the neuromuscular junction of non-obese diabetic mice. Anat Rec. 2002; 267: 112-119.