
Special Article - Oral Administration of Leptin
Austin J Endocrinol Diabetes. 2016; 3(3): 1048.
Leptin Secretion by Adipose Tissue and Gastric Mucosa for the Control of Food Intake: A Review
Bendayan M* and Cammisotto PG
Department of Pathology and Cell biology, University of Montreal, Canada
*Corresponding author: Bendayan M, Department of Pathology and Cell biology, University of Montreal, C.P. 6128 Succ. Centre Ville 2900 Edouard MontPetit Montreal, Quebec, H3C 3J7, Canada
Received: July 05, 2016; Accepted: August 23, 2016; Published: August 26, 2016
Abstract
Endocrine and exocrine secretion of leptin by adipose tissue and gastric mucosa respectively is reviewed. Both the adipocytes and the gastric chief cells synthesise leptin and process it through the RER-Golgi-granule secretory pathways. However, while adipocytes process leptin through a slow constitutive endocrine secretion, the gastric cells release leptin through a rapid regulated exocrine secretion into the gastric juice. Both types of cells secrete a peptide bound to the soluble isoform of its receptor. The leptin-leptin receptor complex released by the chief gastric cells is able to resist the harsh hydrolytic conditions of the gastric cavity. It is then vehiculated by the gastric juice to the duodenal lumen in an intact active form. Leptin then interacts with its own transmembrane receptor present on the luminal plasma membrane of the intestinal cells. Upon internalization in the endocytotic compartment, leptin is channelled towards the Golgi apparatus of the intestinal cell where it binds again to its soluble receptor. The leptin-leptin receptor complex is then released by the epithelial cells towards the connective tissue and reaches the blood circulation. It reaches the central nervous system for the control of food intake. Molecular and cellular mechanisms involved in secretion of leptin by the gastric chief cells, its transfer to the duodenal lumen, its internalization by the intestinal enterocytes and its release towards circulation are reviewed and summarized.
Keywords: Leptin; Gastric mucosa; Intestinal mucosa; Adipose tissue
Introduction
Many factors such as abundance of food, easy availability, media advertising, large variety of processed ready-made meals, lack of physical exercise and sedentary social life style associated with psychological issues, is contributing since at least 50 years, to an epidemic problem of obesity in our society [1]. Understanding the regulation of food intake has become increasingly complex not only because of the many hormones both orexigenic and anorexigenic that play key roles in the control of appetite, but also because intervention of emotional more complex aspects. One area in the brain appears to be the main conductor in this field. The hypothalamic gland is the center of control for food intake responding to signals emerging from various origins for the maintenance of a delicate balance between hunger and satiety.
One of the key players in this ensemble appears to be leptin, a hormone that regulates our appetite and restrain the amount of food ingested. Predicted in the 1950s by Kennedy [2] and Hevrey [3] in their lipostatic theory, this hormone and its gene were only identified much later in the 1990s by Friedman [4]. The hormone was named after the Greek word «leptos» meaning «thin» [4-8]. Indeed, lack of the leptin gene, as it occurs in a particular strain of mice, the ob/ob mice, leads to an inability to have satiety sensations with hyperphagy and obesity.
Leptin from adipocytes
The primary source of leptin and the first organ having been identified as secreting this hormone is the white adipose tissue [9,10].
Leptin immunolabeling of adipose tissue revealed the presence of the hormone within the small cytoplasmic ring that surrounds the large lipid droplet of the adipocytes [11] (Figure 1). The hormone was localized by immunoelectron microscopy along the RER-Golgigranules secretory pathway [11] (Figure 2). In fact, leptin has been the first hormone detected in adipose tissue which is now recognized as an endocrine gland [12]. Leptin is being secreted by the adipose tissue in a slow constitutive fashion which means that it is synthesized and secreted continuously [13]. Leptin cellular content and amounts released remain constant at all times. Adipocytes maintained in cell culture which secrete steady amounts of leptin require an hour of stimulation of secretion to increase their leptin synthesis and release (Figure 3) [11,13].
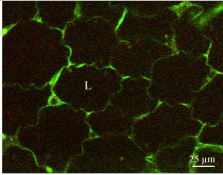
Figure 1: Immunostaining of leptin in white adipose tissue. Tissue section of
rat subcutaneous fat pad incubated with an anti-leptin antibody followed by
a FITC-tagged secondary antibody. The thin cytoplasmic frame surrounding
the lipid droplet (L) displays a strong positive reaction.
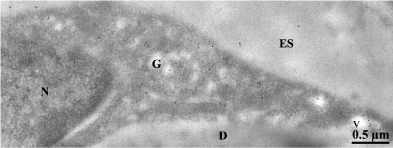
Figure 2: Immunocytochemical protein A-gold labeling of rat epididymal white
adipocyte. The ultra-thin section was incubated with an anti-leptin antibody
followed by protein A-gold. The 10nm gold particles reveal the presence of
leptin at the level of the endoplasmic reticulum, the Golgi apparatus (G) and
some secretory vesicles (v). ES: Extracellular Space; D: Lipid Droplet.
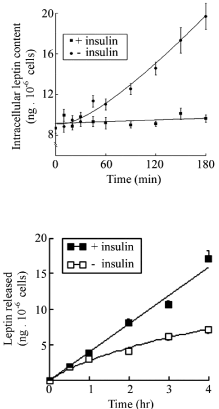
Figure 3: Isolated white adipocytes from rat epididymal adipose tissue in
culture. Cells incubated in the presence of 10nM of insulin were stimulated to
increase leptin secretion. We can see that compared to base line, increase in
secretion occurs only after one hour of stimulation.
Gastric leptin
Later on, it was found that leptin is also secreted by other organs, namely the gastric mucosa [14-17]. Immunocytochemistry has identified the chief cells as the ones secreting leptin. These cells also secrete gastric enzymes such as pepsinogen and lipase (Figures 4 & 5). Leptin secretion by gastric chief cells differs from that of adipose tissue. It does take place along the RER-Golgi-granules secretory pathway (Figure 6) [17] but it is a regulated secretion. This means that gastric cells secretion is triggered by specific stimuli and occurs for short periods of time; the cells return to their basal state quite rapidly. Differences in secretion between white adipose tissue and gastric mucosa reflect variation in leptin action. Leptin from adipose tissue acts in a long-term, targeting the central nervous system for energy expenditure and glucose metabolism in peripheral tissues [18-20] while gastric leptin is secreted only at time of meals, acts rapidly but transiently on the central nervous system to control food intake and to influence by paracrine action the intestinal mucosa and its various functions [21]. Indeed, leptin receptors are present on intestinal luminal and basal epithelial cell membranes [17,22,23]. They are expressed all along the intestinal track from the duodenum to the colon [24] allowing leptin to influence intestinal activities.
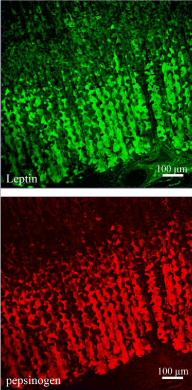
Figure 4: Double labeling of leptin (upper panel) and pepsinogen (lower panel)
of rat gastric mucosa. The tissue section was processed for the simultaneous
detection of pepsinogen and leptin, using a goat anti-pepsinogen antibody
followed by TRITC-conjugated anti-goat IgG and a rabbit anti-leptin antibody
followed by FITC-conjugated anti-rabbit IgG. The labelings revealed the
presence of pepsinogen and leptin in the cells located in the lower half of the
gastric mucosa. The same cells express both antigens.
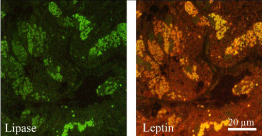
Figure 5: Double labeling of lipase (left panel) and leptin (right panel) of human
gastric mucosa. The tissue section was processed for the simultaneous
detection of lipase and leptin, using a goat anti-lipase antibody followed by
TRITC-conjugated anti-goat IgG and the rabbit anti-leptin antibody followed
by TRITC anti-goat IgG and the FITC-conjugated anti-rabbit IgG. Results
reveal the simultaneous presence of lipase and leptin in the same epithelial
cells.
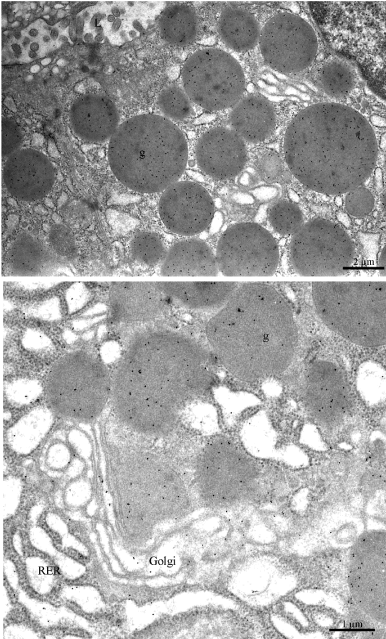
Figure 6: Ultrastructural localization of leptin in rat gastric chief cells. Ultrathin
sections were incubated with the anti-leptin antibody followed by the
protein A-gold complex (10nm gold particles). Labeling by gold particles is
present over the Rough Endoplasmic Reticulum (RER), the Golgi cisternae
(Golgi) and the immature and mature secretory granules (g). In the upper
panel we can see that the secretory granules (g) are located close to the
apical cell membrane lining the lumen (L) of the gastric cavity.
Cytochemical studies have demonstrated that gastric chief cells secrete leptin in an exocrine fashion [17]. By western blot, leptin is indeed detected in the gastric mucosa homogenates as well as in the gastric juice [17,21]. It displays a remarkable resistance to the proteolytic conditions prevailing in the gastric juice which is unusual for such a small peptide. We have demonstrated that in order to resist the conditions of the gastric juice, leptin is protected by a chaperon. Studies using immuno-precipitation and immunoblot have demonstrated that leptin is not free in the gastric juice but rather complexed to a large protein (Figure 7) [21]. This chaperon is in fact a soluble isoform of the leptin receptor [21]. Chief cells synthesise and process a soluble isoform of the leptin receptor (Figure 8). This soluble isoform first synthesised as the complete transmembrane leptin receptor, is then processed from the RER to the Golgi apparatus and packaged into the secretory granules [25]. Proteolytic cleavage of this transmembrane leptin receptor occurs within the secretory granule by sequential action of proteases such as furin and proprotein convertase 7 [25] (Figure 9). The transmembrane leptin receptor molecule carries several PC7-specific cleavage sites making it a target for this proteolytic action. The end product of this processing is a soluble isoform of the leptin receptor that finds itself in the same secretory granules of the chief cells as leptin (Figure 10). Once released from the membrane the soluble receptor binds leptin inside the secretory granule and the complex leptin-leptin receptor is released by the cells at time of exocytosis. We have demonstrated that the leptin-leptin receptor complex is able to resist the harsh conditions found in the gastric juice [26]. Once in the gastric juice, the leptin-leptin receptor complex is vehiculated towards the duodenum where it plays several endocrine and paracrine actions. Indeed, leptin receptors are expressed at the luminal and basal membrane of the enterocytes all along the intestinal track (Figures 11) [26]. Internalized leptin is then processed by the intestinal cells and transcytosed towards the basal connective tissue to reach blood circulation [21,26].

Figure 7: Immuno-precipitation carried out on Gastric Juice (G.J.)
using an anti-leptin antibody demonstrates the presence of one band at
16kDa corresponding to the leptin molecule and another band at 80kDa
corresponding to the soluble isoform of the leptin receptor.
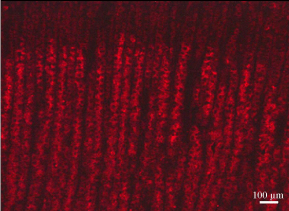
Figure 8: Immunostaining of the leptin receptor on a section of rat gastric
mucosa using the specific antibody followed by the corresponding secondary
antibody tagged with rhodamine. The leptin receptor is present within the
chief cells of the lower half of the gastric mucosa.
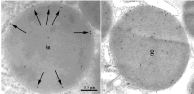
Figure 9: Immunogold labeling of the leptin receptor in secretory granules of
the chief cells. In immature granules (left panel) the labeling appears rather
peripheral in the secretory granule (arrows) while in the mature granule
(right panel) the labeling is rather homogeneously distributed throughout the
granule content.
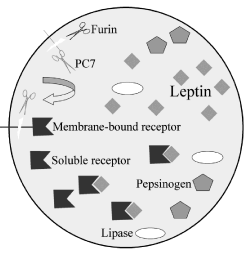
Figure 10: Illustration summarizing the maturation process of the leptin
receptor within the secretory granule of the gastric chief cell and the formation
of the leptin-leptin receptor complex. Membrane-bound leptin receptors are
present on the limiting membrane of the secretory granule. The protease furin
cleaves and activates the membrane-bound convertase 7 (PC7) releasing it
into the granule content. In turn this PC7 cleaves the extracellular domain
of the leptin receptor generating and releasing into the granule content the
soluble isoform of the leptin-receptor. Once in the granule matrix, this soluble
receptor binds to the leptin molecule to generate the leptin-leptin receptor
complex. Other secretory proteins such as lipase and pepsinogen are present
in the same secretory granule.
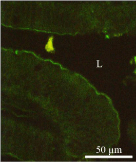
Figure 11a: Immunostaining revealing the presence of the leptin receptor in
the intestinal mucosa using an anti-leptin receptor antibody followed by the
FITC-tagged secondary antibody. The positive staining appears at the brushborder
of the duodenal epithelial cells.
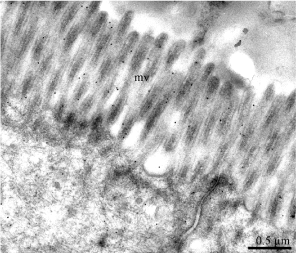
Figure 11b: Protein A-gold labeling of the leptin receptor at the level of the
microvilli (mv) of the duodenal epithelial cells.
Actions of leptin on intestinal function are multiple. Intestinal transport, processing and delivery of nutrients to the systemic circulation are directly or indirectly modulated by endocrine and paracrine factors. Leptin is one of them; gastric and systemic leptins are involved in the regulation of nutrient absorption and enterocyte metabolism [21]. Binding of gastric leptin to the luminal or basal membranes of intestinal cells activates several intracellular pathways [27,28]. Leptin decreases sodium-glucose-transporter 1 (SGLT-1) expression on the apical plasma membrane of enterocytes along with activation of several pathways such as those mediated by PKC [27], PI3K and MAPK [28]. It induces nuclear factors such as STAT3 and STAT5 [22,29]. It enhances absorption of several nutrients but represses many others (Table 1).
Glucose
↓
Galactose
↓
Amino acids
↑
Triglycerides
↓
Lipoproteins B100, B48
↓
Lipoproteins AI, AIV, E
↑
Cholesterol
↓
Table 1: Effect of leptin on nutrient absorption by intestinal cells.
In order to trigger sensation of satiety and to repress food intake, leptin needs to reach its hypothalamic target cells. Since gastric leptin is secreted in an exocrine fashion it must cross the intestinal mucosa to get to blood circulation and the hypothalamic cells. This is carried out by crossing the intestinal wall. An experiment was performed to demonstrate that exocrine-secreted gastric leptin reaches circulation very efficiently [21,26]. Upon cholinergic stimulation of the gastric- exocrine-secreted leptin, circulating levels of leptin were measured in two different conditions. In the first, the pyloric sphincter was maintained open while in the second condition the pylorus was closed. We found that by closing the pylorus, increases in circulating levels of leptin were prevented indicating that a significant part of circulating leptin originates from the gastric mucosa through transcytotic transport across the intestinal wall (Figure 12) [26]. To further confirm this fact and to unveil the path followed by gastric leptin to reach circulation, we tagged leptin with a marker, FITC. Upon inserting the leptin-FITC in the duodenal lumen, the tagged molecule appeared in blood in a time-dependent and saturable manner (Figure 13) [21,26]. Morphological studies using immunocytochemistry allowed demonstrating the pathway taken by the tagged leptin to cross the intestinal mucosa [21,26]. Upon binding to its transmembrane receptor located on the apical membrane of the enterocyte, gastric leptin is internalized through receptormediated clathrin-coated apical vesicles. Within the early endosomal compartment, the leptin-leptin receptor complex is dissociated. The receptor is recycled toward the apical membrane while the free leptin is channelled towards the Golgi apparatus. Within the Golgi, free leptin get associated again to the soluble isoform of its receptor synthesized by the intestinal cell, and the complex leptin-leptin receptor is then transferred towards the basolateral cell membrane to be released into the connective tissue (Figure 14). It then reaches circulation across small vessels vascular wall [26]. Leptin circulates in its bound state, namely the leptin-leptin receptor complex [21,26]. We have demonstrated that even the adipose tissue secretes leptin in its complexed form [21,26]. Figure 15 summarizes the intricate path taken by gastric leptin to reach circulation. It also shows the more direct endocrine secretion of the leptin-leptin receptor complex carried out by the adipose tissue.
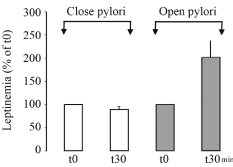
Figure 12: Exocrine secretion of leptin by the gastric mucosa was stimulated
by the cholinergic agonist carbachol. In the situation where the pyloric
sphincter remained open, stimulation f leptin secretion by the gastric mucosa
is followed by a rapid increase in circulating levels of leptin. On the other hand
if the pyloric sphincter is closed, the stimulated secretion of gastric leptin does
not induce any changes in circulating level of leptin. This strongly suggests
that a mechanism of leptin transfer from the duodenal lumen towards blood
circulation is present.
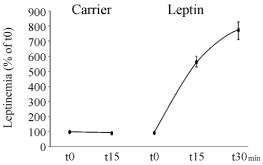
Figure 13: Insertion of leptin-FITC into the duodenal lumen. This insertion is
followed by a rapid and saturable increase in circulating leptin-FITC. On the
other hand insertion of the carrier bicarbonate buffer, the carrier, is inserted
without leptin it did not induce any increase in leptinemia.
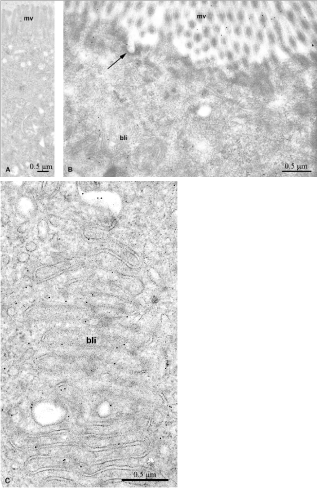
Figure 14: Immunogold labeling of leptin-FITC 30 minutes after insertion of
leptin-FITC into the duodenal lumen. The labeling by gold particles is found
at the level of the microvilli (mv), in endocytotic vesicles (long arrow) (Figure
14B) and associated with the basolateral interdigitations (bli) (Figure 14C).
Control experiment (Figure 14A) shows absence of labeling.
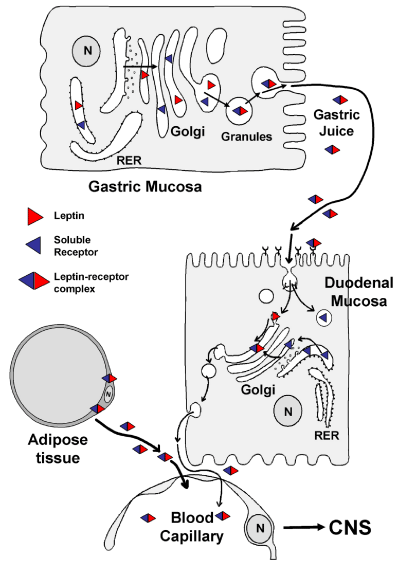
Figure 15: Drawing illustrating the pathway of leptin secretion by adipose cells
as well as by gastric mucosa chief cells. Upon secretion of the leptin-leptin
receptor complex by the gastric cells, this is vehiculated by the gastric juice
towards the lumen of the duodenum. Gastric leptin interacts with its receptor
located on the apical membrane of the intestinal cells and is then internalized
by active receptor-mediated endocytosis. Leptin is then channeled towards
the Golgi apparatus where it binds again to its soluble receptor. The leptinleptin
receptor is then secreted on the baso-lateral side of the epithelial cells
to join the connective tissue and blood circulation. In parallel to this secretion,
leptin is also released by the adipose tissue. Within the adipocyte, leptin is
synthetised and processed along the adipocyte secretory pathway and the
leptin–leptin receptor is released into the connective tissue to reach the blood
circulation. Once in circulation gastric leptin as well as adipose tissue leptin
reach the hypothalamic target cells to trigger their physiological actions.
Exocrine secretion of a hormone is still considered as a rare event. However leptin is not the only hormone to be secreted in an exocrine fashion. Many peptides and even large proteins secreted in an exocrine fashion have been shown to cross the intestinal wall to reach circulation. Blood levels of amylin, cholecystokinin and GLP1 also increase shortly upon food intake [30,31]. Pancreatic exocrine enzymes such as BSDL, amylase and lipase secreted by the pancreas in an exocrine fashion are commonly found in circulation upon crossing the intestinal mucosa [32, 33].
Since leptin secretion by the gastric mucosa and its transfer to blood circulation at time of meals occurs very efficiently and since leptin is a major anorexic hormone that manages food intake, it was primordial to shed some light on the mechanism that allows for the transfer of leptin from the intestinal lumen to circulation. Experiments were performed using the Caco-2 cell culture as a model. Monolayer cultures of Caco-2 cells display many characteristics of intestinal cells in situ. Most significant are the facts that they express membrane leptin receptors and they display highly developed tight junctional complexes [21,34]. These tight junctional complexes among neighboring cells, delineate two independent extracellular compartments, the upper or luminal chamber and the lower or basal chamber [21,34]. Once introduced into the upper chamber, leptin is internalized by the Caco-2 cells, transferred to the basolateral pole of the cells and secreted into the lower chamber [21,34]. Since this transport takes place through the cell and not paracellular, it is time- and temperature-dependent and saturable [21,34]. Extremely efficient at 37°C, the transcellular transport of leptin is inhibited at 4°C reflecting energy-dependent mechanisms [21, 34]. In addition and most significant, although free leptin was introduced into the apical chamber, the leptin harvested in the basal chamber upon transcytosis, was bound to its soluble leptin-receptor [34]. This indicates again that the Caco-2 cells in vitro as well as enterocytes in situ, synthesize the soluble isoform of the leptin receptor and the binding of the leptin to its soluble receptor occurs along the transcellualr pathway, namely in the Golgi apparatus. Leptin released by the Caco-2 cells as the one released by enterocytes in situ is bound to its soluble receptor. In fact, leptin circulates bound to its own receptor [21].
Leptin therapy
Upon discovering leptin and its potent action in suppressing food intake, this hormone presented itself as a powerful avenue for the treatment of some eating disorders and certain types of obesity condition. Sub-cutaneous injections of leptin in humans showed a certain degree of success for the treatment of several pathologies. Efficiency of injected leptin has however deceived anticipated optimism. Leptin resistance as developed by obese patients represents one major matter of frustration. However even in cases where leptin resistance is absent, results have been less successful than expected. One issue that was not taken into account and that certainly influences the efficiency of injected leptin consists in the fact that the circulating hormone is the leptin-leptin receptor complex and not free leptin. All organs secreting leptin do secrete the leptin-leptin receptor complex. Injecting free leptin leads to very low efficiencies. The soluble receptor plays major roles in protecting the peptide and increasing its half-life in circulation. What has been used up to now in the hormone replacement therapy consists in injecting the free leptin. Administration of the leptin-leptin receptor complex instead of the free peptide should increase drastically the efficiency of the treatment.
We put forward a second proposition that may enhance the success of any leptin therapy. Since leptin is secreted by the gastric mucosa and is vehiculated by the gastric juice to reach blood circulation, we can envision administrating leptin orally. Oral leptin will get to the gastric juice and will then be vehiculated along the normal physiological path from the stomach towards the duodenal lumen to be internalized by the enterocytes and transferred to blood circulation using the existing physiological path. Results from such experiments are reported in the following three articles.
Acknowledgment
This study was supported along the years by grants from the Canadian Institutes of Health Research, les Fonds de Recherche du Québec – Santé, Diabète Québec and I-Med Pharma Inc.
References
- Friedman JM. Obesity in the new millennium. Nature. 2000; 404: 632-634.
- Kennedy GC. The role of fat depot in the hypothalamic control of food intake in the rat. Proc R Soc. Lond. B Biol Sci. 1953; 140: 578-596.
- Hervey GR. The effects of lesions in the hypothalamus in parabiotic rats. J Physiol. 1959; 145: 336-352.
- Friedman JM, Leibel RL, Siegel DS, Walsh J, Bahary N. Molecular mapping of the mouse ob mutation. Genomics. 1991; 11: 1054-1062.
- Friedman JM. Leptin, leptin receptors and the control of body weight. Eur J Med Res. 1997; 2: 7-13.
- Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature. 1998; 22: 763-770.
- Cohen SL, Halaas JL, Friedman JM, Chait BT, Bennett L, Chang D, et al. Human leptin characterization. Nature. 1996; 382: 589.
- Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994; 372: 425-432.
- Zheng D, Wooter MH, Zhou Q, Dohm GL. The effects of exercice on ob gene expression. Biochem Biophys Res Comm. 1996; 225: 747-750.
- Zhang Y, Guo KY, Diaz PA, Heo M, Leibel RL. Determinants of leptin gene expression in fat depots of lean mice. Am J Physiol Regul Integr Comp Physiol. 2002; 282: 226-234.
- Cammisotto PG, Bukowiecki LJ, Deshaies Y, Bendayan M. Leptin biosynthetic pathway in white adipocytes. Biochemistry Cell Biology. 2006; 84: 207-214.
- Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab. 2004; 89: 2548-2556.
- Cammisotto PG, Bukowiecki LJ. Mechanisms of leptin secretion from white adipocytes. Am J Physiol Cell Physiol. 2002; 283: 244-250.
- Bado A, Levasseur S, Attoub S, Kermorgant S, Laigneau JP, Bortoluzzi MN, et al. The stomach is a source of leptin. Nature. 1998; 394: 790-793.
- Cinti S, Matteis RD, Picó C, Ceresi E, Obrador A, Maffeis C, et al. Secretory granules of endocrine and chief cells of human stomach mucosa contain leptin. Int J Obes Relat Metab Disord. 2000; 24: 789-793.
- Sobhani I, Bado A, Vissuzaine C, Buyse M, Kermorgant S, Laigneau JP, et al. Leptin secretion and leptin receptor in the human stomach. Gut. 2000; 47: 178-183.
- Cammisotto PG, Renaud C, Gingras D, Delvin E, Levy E, Bendayan M. Endocrine and exocrine secretion of leptin by the gastric mucosa. J Histochem Cytochem. 2005; 53: 851-860.
- Campfield LA, Smith FJ, Guisez Y, Devos R, Burn P. Recombinant mouse OB protein: evidence for a peripheral signal linking adiposity and central neural networks. Science. 1995; 269: 546-549.
- Pelleymounter MA, Cullen MJ, Baker MB, Hecht R, Winters D, Boone T, et al. Effects of the obese gene product on body weight regulation in ob/ob mice. Science. 1995; 269: 540-543.
- Ceddia RB, William WN Jr, Curi R. Leptin increases glucose transport and utilization in skeletal muscle in vitro. Gen Pharmacol. 1998; 31: 799-801.
- Cammisotto PG, Levy E, Bukowiecki LJ, Bendayan M. Cross-talk between adipose and gastric leptins for the control of food intake and energy metabolism. Prog Histochem Cytochem. 2010; 45: 143-200.
- Morton NM, Emilsson V, Liu YL, Cawthorne MA. Leptin action in intestinal cells. J Biol Chem. 1998; 273: 26194-26201.
- Breidert S, Miehlke A, Glasow A, Orban Z, Stolte M, Ehninger G, et al. Leptin and its receptor in normal human gastric mucosa and in Helicobacter pylori-associated gastritis. Scand J Gastroenterol. 1999; 34: 954-961.
- Buyse M, Berlioz F, Guilmeau S, Tsocas A, Voisin T, Péranzi G, et al. PepT1-mediated epithelial transport of dipeptides and cephalexin is enhanced by luminal leptin in the small intestine. J Clin Invest. 2001; 108: 1483-1494.
- Cammisotto PG, Gingras D, Renaud C, Levy E, Bendayan M. Secretion of soluble leptin receptors by exocrine and endocrine cells of the gastric mucosa. Am J Physiol. Gastrointest Liver Physiol. 2006; 290: 242-249.
- Cammisotto PG, Gingras D, Bendayan M. Transcytosis of gastric leptin through the rat duodenal mucosa. Am J Physiol Gastrointest Liver Physiol. 2007; 293: 773-779.
- Ducroc R, Guilmeau S, Akasbi K, Devaud H, Buyse M, Bado A. Luminal leptin induces rapid inhibition of active intestinal absorption of glucose mediated by sodium-glucose cotransporter 1. Diabetes. 2005; 54: 348-354.
- El Homsi M, Ducroc R, Claustre J, Jourdan G, Gertler A, Estienne M, et al. Leptin modulates the expression of secreted and membrane-associated mucins in colonic epithelial cells by targeting PKC, PI3K, and MAPK pathways. Am J Physiol Gastrointest Liver Physiol. 2007; 293: 365-373.
- Goïot H, Attoub S, Kermorgant S, Laigneau JP, Lardeux B, Lehy T, et al. Antral mucosa expresses functional leptin receptors coupled to STAT-3 signaling, which is involved in the control of gastric secretions in the rat. Gastroenterology. 2001; 121: 1417-1427.
- Anini Y, Brubacker PL. Role of Leptin in the Regulation of Glucagon-Like Peptide-1 Secretion. Diabetes 2003; 52: 252-259.
- Moran TH. Gut peptide signaling in the controls of food intake. Obesity (Silver Spring). 2006; 14: 250-253.
- Cloutier M, Gingras D, Bendayan M. Internalization and transcytosis of pancreatic enzymes by the intestinal mucosa. J Histochem Cytochem. 2006; 54: 781-794.
- Bruneau N, Bendayan M, Gingras D, Ghitescu L, Levy E, Lombardo D. Circulating bile salt-dependent lipase originates from the pancreas via intestinal transcytosis. Gastroenterology. 2003; 124: 470-480.
- Cammisotto PG, Bendayan M, Sané A, Dominguez M, Garofalo C, Levy E. Receptor-Mediated Transcytosis of Leptin Through Human Intestinal Cells In Vitro. Int J Cell Biol. 2010; 928169.