
Special Article - Oral Administration of Leptin
Austin J Endocrinol Diabetes. 2016; 3(3): 1049.
Control of Food Intake and Body Weight Through Oral Administration of Leptin
Bendayan M* and Cammisotto PG
Department of Pathology and Cell Biology, University of Montreal, Canada
*Corresponding author: Bendayan M, Department of Pathology and Cell Biology, University of Montreal, C.P. 6128 Succ. Centre Ville, 2900 Edouard Mont Petit Montreal, Quebec, H3C 3J7, Canada
Received: May 19, 2016; Accepted: August 11, 2016; Published: August 26, 2016
Abstract
Upon demonstrating that leptin is secreted by gastric chief cells into the gastric juice, vehiculated towards the intestine, internalized by the duodenal epithelial cells to be transferred to circulation, we put forward the hypothesis that oral administration of leptin may be appropriate for the control of food intake and management of body weight. Oral leptin should follow the same path as the endogenous one. Leptin dissolved in an appropriate vehicle, was administered orally to ob/ob mice that lack leptin becoming rapidly obese. Oral leptin reached blood circulation very efficiently. When receiving regularly oral leptin twice a day, the animals experienced a drastic decrease in food intake and consequently in body weight. These changes were correlated to daily amounts of oral leptin. Adjusting these amounts was able to stabilize the body weight of the animals. Stopping leptin treatment led to increase in food intake and body mass. Experiments performed on normal C57 mice revealed that these animals, with normal endogenous circulating leptin, are by far more sensitive to oral leptin than the leptin-lacking ob/ob mice. On the other hand, db/db mice lacking the leptin receptors were non-responsive to the oral leptin. Long-term daily treatment of mice with oral leptin stabilized their body weight and demonstrated no morphological alterations of their gastric and duodenal mucosa and liver tissue. Oral administration of leptin thus appears as a promising avenue for the management of food intake and the control of body weight as well as for pathologies linked to leptin deficiency.
Keywords: Obesity; Leptin; Oral administration; Weight loss
Introduction
Understanding regulation of food intake has become increasingly complex. Several hormones, both orexigenic and anorexigenic, have been identified. Once crossing the blood brain barrier they reach their main hypothalamic target sites to balance satiety and hunger. Among others, leptin plays fundamental roles in the control of appetite and in regulating energy expenditure [1-16]. Originally discovered in white adipose tissue [5,6,9,12], leptin is also expressed by other tissues and mainly by the gastric mucosa that secretes large amounts [2,7,12]. Leptin secretion is controlled by a wide range of factors such as food intake, certain hormones as insulin as well as nutrients like amino acids and glucose [12].
While adipose tissue secretes leptin through a slow constitutive endocrine pathway [5,6,9], the gastric mucosa releases leptin into the gastric juice through the classical RER-Golgi-secretory granule regulated exocrine pathway [7,12]. The gastric mucosa has been shown to contain both endocrine and exocrine leptin secreting cells [7]. Indeed, light and electron microscopy immunocytochemistry has revealed the presence of a number of isolated endocrine leptinsecreting cells within its connective tissue while all chief cells lining the gastric epithelium express and release leptin into the gastric juice [7]. The exocrine secretion of leptin by the gastric chief cells is carried out concomitantly with other products such as lipase and pepsinogen [12]. The question was raised on the capability for leptin, a small peptide of 17kD, to survive the harsh conditions of the gastric juice. This was answered by a series of studies demonstrating that within the chief cell secretory pathway, the small leptin peptide gets associated to a large protective chaperon that corresponds to the soluble isoform of the leptin-receptor [8,10]. This soluble receptor is synthesized by the gastric chief cells in parallel with leptin. From the RER, both molecules get transferred to the Golgi where maturation occurs, generating leptin on the one hand and, through furin and pro-convertase 7 converting enzyme actions, the soluble isoform of the leptin-receptor [12]. The leptin gets associated to this soluble leptin receptor isoform in the trans-Golgi network to form a complex which is packed in large secretory granules and discharged into the gastric lumen by exocytosis. The leptin-leptin receptor complex, highly resistant to hydrolysis from the gastric juice, is channelled to the duodenal lumen [12].
Levels of plasma leptin in rodents, rise within 15 min after the onset of food intake and was demonstrated to be the result of the gastric secretion since adipose tissue secretes leptin in a slow and constitutive manner [12]. We have demonstrated that the increase in postprandial circulating leptin is the result of the rapid transfer of gastric-secreted leptin across the duodenal wall to blood [7,10,12]. A complicated but highly efficient transcytotic pathway takes place within duodenal enterocytes that express transmembrane leptin receptor at both their luminal and baso-lateral membranes [9,10,12].
We have carried out a series of studies to comprehend the mechanism by which leptin is synthesized and secreted by the gastric chief cells, transported by the gastric juice without being degraded and the pathway taken by this gastric leptin to cross the intestinal barrier to reach circulation [12].
Exocrine-secreted gastric leptin thus belongs to a physiological axis, independent in terms of time of secretion and regulation from that of adipose tissue-secreted leptin in order to rapidly adjust food intake and nutrient absorption. Adipocytes and gastric epithelial cells are two cell types the metabolism of which is closely linked to food intake and energy storage. The coordinated secretion of adipose and gastric leptin ensures proper management of food processing and energy storage.
In view of the facts 1- that leptin is normally present in the gastric lumen, 2- that there is an efficient protective configuration to ensure survival of leptin in the gastric lumen 3- and that there is a well-organized transport system responsible for the transfer of leptin from the intestinal lumen to blood circulation, we put forward the hypothesis that exogenous leptin given orally could reach blood circulation and act upon the hypothalamic cells for the control of food intake.
Studies to support such hypothesis were carried out in vivo using various animal models such the normal mouse, the ob/ob mouse, the db/db mouse, other normal rodents and larger animals. The work was carried out in various steps and convey as a series of reports. In this first one, we have designed an optimal vehicle to administer oral leptin and then force-fed ob/ob, db/db and C57 control mice with leptin to evaluate the efficiency of the approach.
Material & Methods and Results
Based on previous studies [3,4,22,23], we designed a vehicle able to dissolve leptin, to protect it while in the gastric juice and to promote its transfer to the blood across the duodenal wall upon its oral administration to animals.
Normal, ob/ob and db/db mice were acquired from Jackson Laboratories (Maine, USA). Normal mice were about 6 weeks old and weight ~20g. Ob/b mice were also young animals, about 6 weeks old and weight 40g while the db/db mice were 8 weeks old and weight about 40g. Work was carried out following the guidelines of the Canadian Committee of Animal Care and those of the Animal Facility of the University of Montreal, Several recipes and concentrations of reagents were tested by force-feeding the animals with a constant amount of leptin and varying the composition of our vehicle. Upon leptin administration, we assessed circulating levels of leptin at different time points. In order to facilitate the experiments, we decided to work with ob/ob mice since they are totally devoid of leptin. Indeed due to genetic mutations, the ob/ob gene, responsible for leptin expression, is not active in these animals and circulating plasma levels of leptin are undetectable. Because of their lack of leptin, these animals have no satiety feelings and upon having food ad libitum, they very rapidly become obese [14,15].
Different recipes of the vehicle were assessed by force-feeding the animals with 50 μg of mouse recombinant leptin (R&D Systems Inc. Minneapolis, MN, USA) dissolved in 0.5 ml of vehicle. Blood (0.1 ml) was sampled at time 0 and at each hour for five hours upon leptin administration. Plasma levels of leptin were determined using the Elisa kit (R&D Systems Inc. Minneapolis, MN, USA). Recipes of vehicle #1 and vehicle #2 used in the present study, are: Vehicle #1 contains leptin mixed with 2000 Kallikrein inhibitor units (KIU) of aprotinin (Trasylol, Bayer, Leverkusen, FRG) dissolved in 0.5ml of bicarbonate buffer pH 9. While in vehicle #2, we added 5 mg of sodium deoxycholate (22mmol/l) (Sigma St-Louis, Mo, USA). Addition of aprotinin was required to reduce leptin degradation, while the addition of sodium deoxycholate was found to enhance duodenal absorption [3,4,22,23]. Circulating levels of leptin were assessed using the Leptin Elisa kit (R&D Systems Inc. Minneapolis, MN, USA). This kit displays <0.5% cross reactivity with available related human leptin molecules and no cross-reactivity with rodent leptin. Detection of human leptin is carried out with a Minimum Detectable Dose (MDD) of less that 7.8pg/mL.
Figure 1 shows the results obtained upon force-feeding the animals with 50μg of leptin dissolved in buffer (PBS), in vehicle #1 and in vehicle #2. Very small amounts of leptin reach circulation when oral leptin was dissolved in buffer (Figure 1). However, upon dissolving it in vehicle #1 or #2 the amounts of leptin detected in plasma during the first hours after its oral administration are quite significant (Figure 1). The levels reach a pick at 60 minutes and decrease gradually thereafter. Vehicle #2 appears to be significantly more efficient, circulating levels of leptin reaching about three times the amounts observed with vehicle #1 (Figure 1).
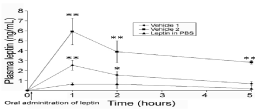
Figure 1: Plasma leptin levels in ob/ob mice after oral administration of leptin
50 μg in buffer (PBS), vehicle #1 or vehicle #2. *P<0.05; **P<0.01.
Upon completion of these studies, we carried out a long term experiment in which ob/ob mice were force-fed with 50μg of leptin twice a day. Food intake and body weight were recorded. We performed the experiment with a group of 15 young ob/ob mice of about 30 g. After a few days of acclimation during which all animals gained weight, 5 animals were selected for the first experiment, carrying the 10 others as the control group. This control group of 10 animals was force-fed with the vehicle alone while the selected 5 animals were force-fed with 50μg of leptin dissolved in vehicle #1 twice a day during 7 days. Compared to the control group the body weight of the leptin-receiving animals remained quite stable at about 32-33g, while that of the control group went from 32 g to 36 g (Figure 2). After these first 7 days of leptin administration, the experiment was modified. The 50μg of leptin were dissolved in vehicle #2 instead of vehicle #1. Changing the nature of the vehicle increased significantly the efficiency of leptin absorption and the animals started to lose weight. Upon the 9 days of treatment, body weight of the animals receiving the leptin went from 33 g to 28 g (Figure 2). The experiment was then stopped. Removing oral leptin administration triggered an immediate change in the animal behavior with an increase in food intake leading to a slow raise in body weight (Figure 2).
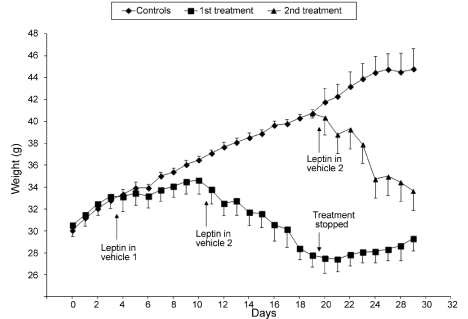
Figure 2: Changes in body weight in ob/ob mice upon administration of leptin
50 μg twice a day.
During this part of the experiment, the control group of 10 animals continued to gain weight reaching by this time 40g (Figure 2). This group of 10 animals was then divided into two groups of 5. One group was force-fed with 50μg of leptin dissolved in vehicle #2 twice a day, while the remaining 5 animals were kept as a control group. The animals receiving the leptin demonstrated an immediate change in their eating behaviour, decreasing drastically their food ingestion and steadily losing weight (Figure 2). Upon 8 days of treatment the animals went from 40g to 34g. On the other hand, the control group continued increasing their body weight to 46g (Figure 2).
Statistical analyses of the data are reported in Figure 3. The average food intake for non-treated ob/ob mice was found to be 8 g a day while oral treatment with 50μg of leptin in vehicle #2, twice a day reduced that amount to an average of 2 g a day, an amount similar to that eaten by C57 control mice. Administration of the vehicle alone did not interfere with food intake (Figure 3). Body weight of the animals followed accordingly. Non-treated animals gained an average of 0.3g a day while the leptin-treated animals lost an average of 1g a day (Figure 3). Again administration of the vehicle alone did not influence significantly the increases in body weight (Figure 3).
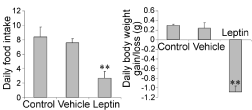
Figure 3: Daily food intake and changes in body weight of ob/ob mice. Nontreated
mice; mice receiving twice a day the vehicle #2 and mice receiving
twice a day 50 μg of oral leptin. P<0.01.
The next experiment intended to investigate the dose-response of the animals to the oral leptin administration (Figure 4). Groups of five ob/ob animals received oral leptin dissolved in vehicle #2, during four days. Amounts of the administered leptin varied from one group to the next. Body weight changes were registered every day. Results reported in Figure 4, show that the control group as well as the group receiving 5μg of leptin gain similar amounts of weight. Body weight of animals in the group receiving 10μg of oral leptin, remained quite stable (Figure 4). On the other hand, animals in the groups receiving 20 or 50μg of oral leptin show significant and steady decreases in body weight (Figure 4).
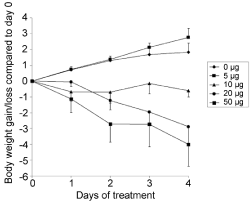
Figure 4: Effect of oral administration of various concentrations of leptin
dissolved in vehicle #2 on body weight of ob/ob mice.
According to these experiments, ob/ob mice appear to have a low sensitivity towards leptin. This was confirmed when similar experiments were carried out with normal (C57BL/6J) mice. These show a much higher sensitivity to circulating leptin.
Indeed, upon carrying the experiments with the ob/ob mice, we proceeded with normal (C57BL/6J) mice. Blood levels of endogenous leptin in control mice fluctuate around 1ng/ml. Upon force-feeding 10μg of recombinant mouse leptin in vehicle #2 to these animals, we observed a significant rise in circulating leptin, up to 5ng/ml, as early as 5 minutes after administration (Figure 5). Levels decreased rapidly to reach basal line by 60 minutes.
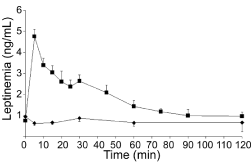
Figure 5: Plasma leptin levels in control C7BL/6J mice upon oral
administration of 10μg of leptin in vehicle #2.
Upon daily administration of 2.5μg or 10μg of leptin to normal mice, food intake decreased by 20% and 60% respectively (Figure 6). Body weigh changes followed a similar pattern (Figure 6). On the other hand, administration of 1μg of leptin seems to have little effect (Figure 6).
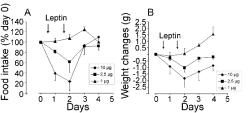
Figure 6: Food intake and body weight changes in normal C57BL/6J mice
after oral administration of leptin.
Thus, efficiency of circulating leptin varies according to the strain of mice. Ob/Ob mice lack of leptin while normal mice display significant levels of circulating leptin. This circulating leptin reacts with the leptin receptors express at the membrane of various cell types among which those of the intestinal lining and the hypothalamic ones. Thus, the leptin-leptin receptor system is present, functional and efficient in normal mice as compared to ob/ob animals that have no leptin [24,25].
Following these results we decided to carry experiments on another strain of obese mice, the db/db. The db/db mouse displays large amounts of circulating leptin but lack leptin receptor at their cell membranes. Indeed, these animals have a genetic mutation on the long isoform of their leptin receptor expressed by hypothalamic cells [14,15,19]. Thus, in spite of large amounts of circulating leptin, these animals have no satiety feelings and demonstrate hyperphagia leading to morbid obesity. Ob/Ob and db/db mice have similar phenotypes while their genotypes are totally different. Figures 7 and 8 report the results obtained on the experiments performed with db/ db mice. Figure 7 illustrates the changes in body weight of the animals during 28 days. We can see that the animals gain weight steadily, before the onset of the experiment, during the entire period of oral leptin administration and after the end of the leptin treatment (Figure 7). Amounts of daily food intake remain quite stable during the entire experiment with little day to day variations (Figure 8) indicating that due to the lack of specific leptin receptors, endogenous as well as oral exogenous leptin have no effect on food intake.
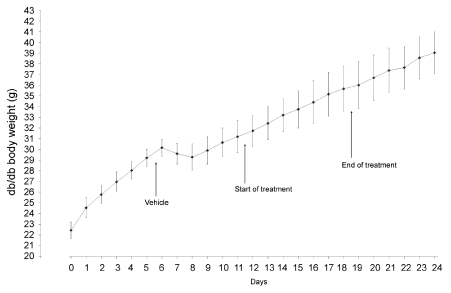
Figure 7: Body weight changes of db/db mice before, during and after daily
administration of 10 μg of leptin.
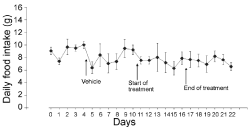
Figure 8: Daily food intake by db/db mice before, during and after daily oral
administration of 10 μg of leptin.
Upon performing these experiments, we proceeded by assessing by histo-pathological examination, certain tissues of control and longterm leptin-treated animals for eventual evidence of leptin toxicity. Ob/Ob mice received 10μg of leptin twice a day for 10, 20 and 30 days in vehicle #2.Two days after the onset of the treatment, body weight of treated animals stabilizes while that of the control group continued to increase. At the end of the experiments and under anaesthesia, stomach, duodenum and liver tissues were sampled and processed for light and electron microscopy according to routine techniques. In addition to our own evaluation, tissues were also assessed by the Head of Molecular Pathology at the University Hospital and Head of the Department of Pathology and Cell Biology of our University. His report confirmed our own observations. As illustrated in Figure 9, all tissues show little to no alteration. Epithelial cells show their classical characteristics, with intact brush borders at the luminal membranes and junctional complexes on their lateral membranes (Figure 9). Cytoplasmic components appear all normal.
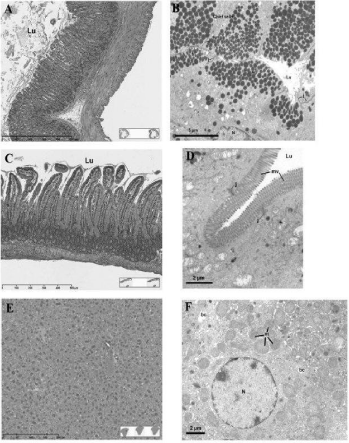
Figure 9: Light (A,C,E) and electron (B,D,F) microscopy of gastric (A,B)
and intestinal (C,D) mucosa, as well as liver (E,F) tissues of animals upon
daily oral leptin administration for 30 days. Tissues and cells display their
normal characteristics without any significant changes. Lu: Luminal space;
j: junctional complexes; mv: microvilli; bc: bile canaliculi; m: mitochondria;
N: Nucleus.
Discussion
In previous studies we have demonstrated that leptin a potent hormone that regulates food intake by acting on the hypothalamus, is secreted by the gastric mucosa at time of feeding [7,8]. The raise in circulating leptin adjusts our food intake preventing in normal conditions, any excesses leading to obesity. Animals lacking leptin, or its corresponding membrane receptor, are unable to have feelings of satiety, which leads to morbid obesity [14,15,20,26]. Gastric leptin is secreted by the gastric mucosa in an exocrine fashion at time of food intake together with other components of the gastric juice such as lipase and pepsinogen [12]. In fact, we have shown that these three secretory products, leptin, pepsinogen and lipase are packaged together in the same secretory granule of the chief cell and are released by exocytosis following the same stimulation [12]. Further, we have shown that leptin being a small peptide, does not survive as such in the gastric juice. To remain intact and biologically active in the gastric juice, leptin is thus secreted in a protected fashion. The gastric chief cells synthesise and process a soluble isoform of the leptin-receptor. This soluble leptin receptor binds to the leptin within the Golgi apparatus of the chief cell and leptin is thus secreted in a complexed form bound to its soluble receptor [8,10,12]. The leptin-leptin receptor complex survives in the gastric juice and is vehiculated to the lumen of the duodenum. Duodenal cells express leptin-receptors on their luminal membrane [12]. Through binding to this transmembrane leptin receptor, gastric leptin is internalized by the intestinal cells and through a complex transcytotic pathway [12], is released into the intestinal connective tissue to reach blood circulation and eventually their target hypothalamic cells to control food intake [12].
Since leptin is normally present in the gastric lumen, the possibility of introducing exogenous leptin by oral administration was raised and tested. However, oral administration of leptin is confronted to the fact that this small peptide needs to survive the harsh conditions of the gastric juice. As mentioned above, we have demonstrated that in situ, leptin is released into the gastric lumen associated to its soluble receptor in order to remain intact and active [12]. Oral administered leptin confronted to the problem of the gastric juice, requires some protective means. The first and most obvious form in which leptin could be given orally would be complexed to its soluble receptor mimicking the physiological situation. The soluble isoform of the leptin receptor should then be synthesised and complexed to leptin prior oral administration.
We decided to bypass this approach and administered leptin in a vehicle containing factors that prevent major degradation of peptides in the gastric juice and facilitate their absorption by the intestinal epithelium. The reported vehicle #2 appears to play such roles quite efficiently. Indeed, upon administration of leptin dissolved in vehicle #2, significant amounts of exogenous leptin reach circulation in very short times. This vehicle #2 contains three important factors: one, a proteases inhibitor to protect the peptide from major degradation in the gastric cavity; second, bicarbonate to neutralize the acidic pH of the gastric lumen; and third, an adjuvant that facilitates absorption of peptides by the intestinal lining [22,23]. When we studied the transport of exogenous leptin in normal C57 mice, we found that blood levels reach a pick as soon as 5 minutes after its oral administration (Figure 5). This demonstrates that we are dealing with a very efficient transport system, vehicle #2 being a very appropriate medium. Furthermore, once in circulation this exogenous leptin acts on hypothalamic cells regulating food intakes also in a very efficient fashion since animals changed drastically their eating habits. Keeping with one of its physiological roles, oral leptin is able by reducing the amounts of food intake, to regulate the animal gain in body weight.
Comparing results obtained on ob/ob mice and C57 normal mice, some interesting data were generated. Indeed, it appears that C57 control mice, being used to respond to leptin are by far more sensitive to oral leptin than the leptin-deficient ob/ob mice. Not only levels of circulating leptin needed to trigger a response are much lower in normal mice but the trans-duodenal transport of leptin is also by far more efficient. The experiments on db/db mice on the other hand confirmed that the satiety effect of leptin is dependent on the existence of a functional full-length receptor at the level of the hypothalamic cell membranes.
Further to demonstrate the efficiency of oral leptin, we have shown that amounts of oral leptin can be adjusted in order to stabilize the animal body weight. This is of major importance when we envision management of food intake in human. Finally, morphological examination at the light and electron microscope levels of various tissues has demonstrated that daily oral administration of leptin in sodium cholate and aprotinin, for a relatively long period of time (considering life span of small rodents) did not trigger any changes on the integrity of the gastric and intestinal lining nor on liver tissue. As previously reported for insulin [3,4,23], we were unable to detect any tissular or cellular toxicity in the different target tissues.
Taken together these results demonstrate that oral administration of leptin can be a powerful avenue to regulate food intake in order to decrease body weight in obesity and mainly, to maintain its losses.
Acknowledgement
The authors would like to express their gratitude to Daniel Hofmann and Doctor Ilan Hofmann for their support and enthusiasm as well as to Doctor Louis Gaboury for examining our histological samples. This work was supported by a grant from I-Med Pharma Inc.
References
- Ahima RS, Antwi DA. Brain regulation of apetite and satiety. Endocrinol Metab Clin North Am. 2008; 37: 811-823.
- Bado A, Levasseur S, Attoub S, Kermogant S, Laigneau JP, Bortoluzzi MN, et al. The stomach is the source of leptin. Nature. 1998; 394: 790-793.
- Bendayan M, Ziv E, Ben-Sasson R, Bar-On H, Kidron M. Morpho-cytochemical and biochemical evidence for insulin absorption by the rat ileal epithelium. Diabetologia. 1990; 33: 197-204.
- Bendayan M, Ziv E, Gingras D, Ben-Sasson R, Bar-On H, Kidron M. Biochemical and morpho-cytochemical evidence for the intestinal absorption of insulin in control and diabetic rats. Comparison between the effectiveness of duodenal and colon mucosa. Diabetologia. 1994; 37: 119-126.
- Cammisotto PG, Bukowiecki LJ. Mechanisms of leptin secretion from white adipocytes. Am J Physiol Cell Physiol. 2002; 283: 244-250.
- Cammisotto PG, Gelinas Y, Deshaies Y, Bukowiecki LJ. Regulation of leptin secretion from white by free fatty acids. Am J Physiol Endocrinol Metab. 2003; 285: 521-526.
- Cammisotto PG, Renaud C, Gingras D, Delvin E, Levy E, Bendayan M. Endocrine and exocrine secretion of leptin by the gastric mucosa. J Histochem Cytochem. 2005; 53: 851-860.
- Cammisotto PG, Gingras D, Renaud C, Levy E, Bendayan M. Secretion of soluble leptin receptors by exocrine and endocrine cells of the gastric mucosa. Am J Physiol Gastrointest Liver Physiol. 2006; 290: 242-249.
- Cammisotto PG, Bukowiecki LJ, Deshaies Y, Bendayan M. Leptin biosynthetic pathway in white adipocytes. Biochemistry Cell Biology. 2006; 84: 207-214.
- Cammisotto PG, Gingras D, Bendayan M. Transcytosis of gastric leptin through the rat duodenal mucosa. Am J Physiol Gastrointest Liver Physiol. 2007; 293: 773-779.
- Cammisotto PG, Bendayan M, Sané A, Dominguez M, Garofalo C, Levy E. Receptor-Mediated Transcytosis of Leptin Through Human Intestinal Cells. Int J Cell Biol. 2010; 2010: 928169.
- Cammisotto PG, Levy E, Bukowiecki LJ, Bendayan M. Cross-talk between adipose and gastric leptins for the control of food intake and energy metabolism. Prog Histochem Cytochem. 2010; 45: 143-200.
- Campfield LA, Smith FJ, Guisez Y, Devos R, Burn P. Recombinant mouse OB protein: evidence for a peripheral signal linking adiposity and central neural networks. Science. 1995; 269: 456-549.
- Coleman DL, Hummel KP. Studies with the mutation diabetes in the mouse. Diabetologia. 1967; 3:238-248.
- Coleman DL. Obese and diabetes: two mutant genes causing diabetes-obesity syndromes in mice. Diabetologia. 1978; 14: 141-148.
- Considine RV. Human leptin: an adipocyte hormone with weight-regulatory and endocrine functions. Semin Vasc Med. 2005; 5: 15-24.
- Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals". Nature. 1998; 395: 763–770.
- Himms-Hagen J. Physiological roles of the leptin endocrine system: differences between mice and humans. Crit Rev Clin Lab Sci. 1999; 36: 575-655.
- Hervey GR. The effects of lesions in the hypothalamus in parabiotic rats. J Physiol. 1959; 145: 336-352.
- Ingalls AM, Dickie MM, Snell GD. Obese, a new mutation in the house mouse. J Hered. 1950; 41: 317-318.
- Kennedy GC. The role of fat depot in the hypothalamic control of food intake in the rat. Proc R Soc Lond B Biol Sci. 1953; 140: 578-596.
- Ziv E, Lior O, Kidron M. Absorption of proteins via the intestinal wall, a quantitative model. Biochem Pharmacol. 1987; 36: 1035-1039.
- Ziv E, Bendayan M. Intestinal absorption of peptides through the enterocytes. Microsc Re.s Techn. 2000; 49: 346-352.
- Tartaglia LA, Dembski M, Weng X, Deng N, Culpepper J, Devos R, et al. Identification and expression cloning of a leptin receptor, OB-R. Cell. 1995; 83: 1263-1271.
- Tartaglia LA. The leptin receptor. J Biol Chem. 1997; 272: 6093-6096.
- Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994; 372: 425-432.