
Review Article
Austin J Endocrinol Diabetes. 2021; 8(2): 1083.
Raising the Bar When Assessing Cardiovascular Disease Outcome When Studies with Patients with Type 2 Diabetes Mellitus are Constructed: Revisiting ASCEND
Nashawi M¹*, Ahmed MS¹, Jarrar Y¹, Abualfoul M², Issa O³ and Chilton R¹
1Department of Medicine-Cardiology, UT Health San Antonio, Texas
2Department of Internal Medicine, Methodist Dallas Medical Center, Texas
3Department of Biology, The University of Texas at Arlington, Texas
*Corresponding author: Mouhamed Nashawi, Department of Medicine, MC 7870 UT Health San Antonio 7703 Floyd Curl Dr., San Antonio-78229-3900, Texas
Received: April 03, 2021; Accepted: May 01, 2021; Published: May 08, 2021
Abstract
The emergence of Type 2 Diabetes Mellitus (T2DM) as a clinical syndrome deviating from rudimentary models including “glucose-insulin” disparities to a clinical syndrome impinged upon hyperactivation of inflammatory-mediated signaling pathways, maladaptive pathophysiological changes, and pleiotropic sequelae has not only changed the nature of discussions regarding diabetes in academic circles, but also the treatment of T2DM clinically. The new challenges faced in preventing the advancement of T2DM coupled with the management of associated chronic disease states have impelled avenues for innovative treatments for T2DM sequelae while also ensuring glycemic control. For example, Sodium-Glucose Transport 2 inhibitors (SGLT2i) and Glucagon-Like Peptide-1 Receptor Agonist (GLP-1RA), in addition to passing mandated Food and Drug Administration (FDA) Cardiovascular Outcome Trials (CVOT), have displayed positive outcomes with respect to cardiorenal considerations in patients with T2DM. Such performances have recently elevated these pleiotropic medications to higher tiers of recommendations by medical societies such as the American Diabetes Association and the American College of Cardiology. Moreover, the tandem of the notion that pleiotropic pharmacotherapeutic options with an expanded understanding of T2DM on the epigenetic, molecular, and cellular domains have galvanized researchers to explore pharmacologic mechanisms in the context of our ever-changing model in T2DM. In this communication, we would like to use lessons from the “Effects of aspirin for primary prevention in persons with diabetes mellitus” (ASCEND) trial to implore aspiring researchers and practitioners to explore, or at least consider external factors of the T2DM patient populous when reporting findings of cardiovascular clinical endpoints for proper context.
Keywords: Ascend; Statin; T2dm; Aspirin; Vascular; Mace; Heart; Cardiovascular
Background
The intimate association between uncontrolled T2DM and macrovascular complications culminating in Major Adverse Cardiovascular Events (MACE) has been well studied. Evidence for this can be appreciated in a longitudinal study of 29,863 patients (5,501 diagnosed with T2DM and 24,632 control patients). In this study, a statistically significant relative risk afflicting T2DM patients was shown, elucidating a 1.10, 1.53, 1.58, and 2.12 fold increase for lifetime disease burden of Coronary Artery Disease (CAD), Myocardial Infarction (MI), stroke, and Heart Failure (HF), respectively [1,2]. A substantial amount of disease burden associated with cardiovascular disease morbidity and mortality occurs by why of deficits in vascular biology, which are compromised across multiple axes in T2DM [3]. These deficits in vascular biology have deleterious ramifications such as the mitigation of oxygen flux (with consequences in tissue metabolism and subsequent maladaptive phenotype acquisition), serving the role as the nidus for atherosclerosis with concomitant complications (coronary artery disease or CAD, stroke, etc.), or hypertension [4-8]. All of the latter are implicated in one form in another in the cardiovascular deficits studied by Straka et al. A visual reference to illustrate the importance of the vasculature as the interface of cardiovascular disease can be found in Figure 1.
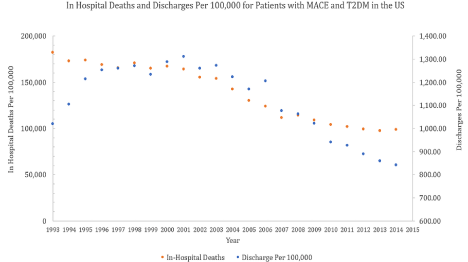
Figure 1: Morbidity of Heart Disease with T2DM in the United States between 1993 and 2015.
If the road between vascular biology deficits and MACE is set by T2DM, then Reactive Oxygen Species (ROS) is the cement that makes such a road traversable. Hyperglycemia from T2DM promotes the formation of ROS via the mitochondria through an array of mechanisms [9]. One method is through the status of insulin resistance, which causes increased serum glucose, leading to the propensity to undergo non-enzymatic condensation reactions between glucose and amine residues of proteins and some other macronutrients, such as lipids and nucleic acids. Such constituents are dubbed Advanced Glycation End Products (AGEs). In one study by Morita et al. it was shown that AGEs activates nuclear factor-κB (NF-κB) in human aortic endothelial cells through the modulation of signaling pathways [10]. NF-κB is a transcription factor which has the propensity to modulate multiple genes. Namely, NF-κB has been demonstrated to be paramount in the proliferation of Smooth Muscle Cells (SMCs) that is crucial in the process of arteriosclerosis within the vascular injury process as well as lesion acquisition resulting in increased thrombosis risk, pathways central to MACE incidents [11,12]. For example, NF-κB is regulates Vascular Cell Adhesion Molecule (VCAM)-1, which plays a role among other proteins in the promotion of cytokine and inflammatory adhesion of leukocyte mediated adhesion to vessels, which has the propensity to degranulate cytotoxic elements on damaged vasculature [13]. The damaged intima is especially susceptible to the accumulation of Low-Density Lipoprotein (LDL) in the subendotheolial space, where circulating ROS promotes pathologic plaque formation [14]. There is a tendency for these lesions to promote induction of inflammatory cytokines and chemokines, which has systemic influence as well as local formation of atherogenic structures that may embolize leading to MI, or en route to such process, promote stenotic phenotypes [15]. Moreover, hyperglycemia that has not undergone the glycosylation process has the propensity to induce ROS in mitochondria by increasing expression of ROS ligands, making the ROS accumulated throughout T2DM more viable in their downstream effects [16]. Non-glycosylated hyperglycemia also induces vascular damage through the production of TNF-a, a cytokine employed by the immune system with vast clout in the domain of human cell signaling pathway induction, and within the interest of this review, vascular tone.
In vitro studies have shown that the immunoreceptor motif CD33, which has been shown to downregulating TNF-a production, statistically significantly increases TNF-a when cultured in higher glucose concentrations via motif downregulation, with the theory that hyperglycemia overloads the electron transport chain of the mitochondria, causing the production of radicals that induce oxidative stress, stimulate cytokine production, and promote CD33 downregulation [17,18]. This process is critical as TNF-a promotes arginase activity, an enzyme that has competitive actions against Endothelial Nitric Oxide Synthase (eNOS) [19]. As the name implies, eNOS produces Nitric Oxide (NO), which leads to the W of vascular tone through vasodilation by cGMP mediated smooth muscle cell relaxation. By indirectly decreasing vascular tone homeostasis, hyperglycemia mediated TNF-a reduces endothelial cell NO and promoting endothelial cell dysfunction with a net contractile phenotype [20]. Studies of the vascular endothelium note that their neuronal innervation are dense in mitochondria, implying a reliance on oxidative phosphorylation for metabolic homeostasis [21-24]. Unfortunately, the same oxidative constituents that promote deleterious tissue metabolism in oxidative stress also lead to autonomic dysfunction in these sensitive neuronal innervation networks [25,26]. The ramifications of oxidative stress and damage of endothelium mediated vascular tone is a constriction that decreases the flow rate of oxygen, causing a vicious cycle of hypoxia and endothelial cell dysfunction [4,27,28]. The resultant hypoxia has the propensity to result in cardiomyocytes death, with the concomitant abnormalities in the myocardium hampers the coupling process of electromechanical physiology with consequences in arrhythmias, thrombus and emboli advancement, and the development of processes central to MACE [29-33].
The following dialogue raises an inquiry as to the viability of prophylactic pharmacotherapeutic protection of the vascular architecture in patients with T2DM, given their tendency to be prolific producers of ROS, have higher risks of MACE at baseline, and generally carry comorbidities that are problematic for the maintenance of long-term adequate cardiovascular history [34,35]. Such a solution would have severe ramifications in mitigating hospitalizations and deaths in patients with T2DM for MACE, of which such burden at least in the United States can be visualized in Figure 1. While there is a slight downtrend in the magnitude of the patient flux in admissions according to the National Inpatient Sample, the degree of morbidity remains high [36]. In reference to the proposition raised at the beginning of this paragraph, such a relationship and the viability of such an option has been pursued, and we will appraise the design and lessons learned from such trials. Moreover, these trials have relative distinctions in their constructs and mainstream adoption of their results. Revisiting such distinctions, especially when discussed in tandem with the severity of vascular complications in patients with T2DM as described above, will culminate in recommendations for the communications of proceedings regarding therapeutic approaches for the clinical cardiovascular sequalae of T2DM. We hope such a commentary will not only expand our knowledge of T2DM, vascular biology, and the translational biology of cardiovascular health, but will help push for contextual annotations of prospective works of literature discussing cardiology in patients with T2DM. In this commentary, we will discuss the ramifications of the results of the ASCEND t trial and the consequences it has in reporting results in T2DM and MACE concordance.
ASCEND Trial
The ASCEND (Effects of aspirin for primary prevention in persons with diabetes mellitus) trial was a trial conducted in 2018 that sought to answer the question, “In patients with T2DM without atherosclerotic disease, can low dose aspirin be used as a prophylactic compound to mitigate vascular events relative to placebo?”. The interest in this research was spurred considering that T2DM conveys a 2-4 times increase of vascular incidents according to some studies [37] through the array of mechanisms as discussed previously and can be found succinctly in Figure 2.
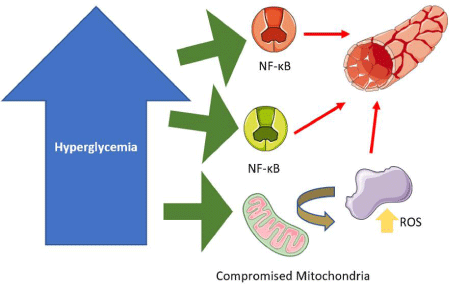
Figure 2: 2: A Review of the Ramifications of Hyperglycemia on Vascular Dynamics Hyperglycemic Patient with Implications for Vascular prophylaxis. Adapted from
Servier Medical Art by Servier, which is licensed under a Creative Commons Attribution 3.0 Unported License.
The ASCEND trial recruited 15,480 patients with T2DM randomized to receive primary prevention prophylaxis with aspirin or placebo, with subsequent assessment of vascular incidents. After a subsequent follow-up of 7.4 years, there was a 1.1% absolute reduction in vascular events in patients randomized to receive aspirin prophylaxis (NNT=91 at 7.4 years). However, one major point to note was over half of the patients recruited (roughly 51%) had a defined “controlled diabetic” status with an HgbA1C of <8% (only 12% had a higher threshold). With this comes a reduction in the sequelae of diabetes as reductions of HgbA1C (or on the converse their increase) have microvascular and macrovascular ramifications as per UKPDS, one a series of trials conducted in the United Kingdom to assess the burden of T2DM on systemic sequelae such as microvascular and macrovascular events [38,39]. Another criticism of ASCEN was that nearly 75% of the patients were on statin therapy, which has been recommended by societies such as the American Diabetes Association (ADA) in mitigating vascular decline, and has molecular evidence backing the pleiotropic effects of statins in not only reducing atherosclerotic disease, but through other signaling pathways, maintaining vascular integrity [40,41]. Some of these are through modulation of the family of rho-associated protein kinases (ROCKs) [42]. Such ramifications include maintenance of endothelial cell integrity through mitigation of harmful reactive oxygen species caused by hyperglycemia and vascular pliability needed to control for an array of blood pressures [43]. Evidence in trials shows that aspirin is associated with an increase risked in bleeding, however, the friability of vessels due to uncontrolled T2DM and its sequelae such as Hypertension (HTN) may mask the use of aspirin as a prophylactic in the early stages of metabolic derangement diagnosis before the latter syndromes become too detrimental to the health of a patient. Due to these findings, USPSFTF guidelines recommend aspirin prophylaxis in a very narrow grow (age 50-50, with estimated 10-year ASCVD risk <10%) [44].
Conclusion
In cardiovascular diabetology and endocrinology, we are entering a new era of therapeutics that target the overlap of sequelae of T2DM and heart disease (for example, sodium-glucose 2 inhibitors and GLP- 1 receptor agonists) [45-47]. However, due to the systemic effects of T2DM, and the sequelae it induces such as HTN and atherosclerosis, it is prudent for researchers who are exploring the pleiotropic effects of pharmacotherapeutics to reduce overall cardiovascular morbidity. The ASCEND trial is an example of which the background populous of a patient can lead to underwhelming results of other prophylaxis to reduce vascular effects associated with diabetes. Reconsiderations may be warranted given the ubiquity of aspirin usage in patients with Cardiovascular Disease (CVD) and the overlap of T2DM, of which there is a close association in the western world. These measures can help communicate the underlying effects of whether or not vascular mitigations are at their true proclivity or are merely masked by other ASCVD risk reduction co-therapeutics taken with patients (i.e, statins or glucose-controlling agents).
References
- Sheikh MSA, Aljohani EM, Alrayes FH, Aldakhil AR. Hypertension and Coronary Heart Disease in Diabetic Patients: A Systematic Review. Archives of Pharmacy Practice. 2020; 1: 130.
- Straka RJ, Liu LZ, Girase PS, DeLorenzo A, Chapman RH. Incremental cardiovascular costs and resource use associated with diabetes: an assessment of 29,863 patients in the US managed-care setting. Cardiovascular Diabetology. 2009.
- Steven S, et al. Vascular inflammation and oxidative stress: major triggers for cardiovascular disease. Oxidative medicine and cellular longevity. 2019.
- Nashawi M, Sheikh O, Battisha A, Mir M, Chilton R. Beyond the myocardium? SGLT2 inhibitors target peripheral components of reduced oxygen flux in the diabetic patient with heart failure with preserved ejection fraction. Heart Failure Reviews. 2020.
- Hansson GK. Inflammation and atherosclerosis: the end of a controversy. Circulation. 2017; 136: 1875-1877.
- Basatemur GL, Jørgensen HF, Clarke MCH, Bennett MR, Mallat Z. Vascular smooth muscle cells in atherosclerosis. Nature reviews cardiology. 2019; 16: 727-744.
- Chen Q, Wang Q, Zhu J, Xiao Q, Zhang L. Reactive oxygen species: key regulators in vascular health and diseases. British journal of pharmacology. 2018; 175: 1279-1292.
- Moris D, et al. The role of reactive oxygen species in the pathophysiology of cardiovascular diseases and the clinical significance of myocardial redox. Annals of translational medicine. 2017; 5.
- Rendra E, et al. Reactive Oxygen Species (ROS) in macrophage activation and function in diabetes. Immunobiology. 2019; 224: 242-253.
- Morita M, Yano S, Yamaguchi T, Sugimoto T. Advanced glycation end products-induced reactive oxygen species generation is partly through NFkappa B activation in human aortic endothelial cells. Journal of Diabetes and its Complications. 2013; 27: 11-15.
- Mussbacher M, et al. Cell type-specific roles of NF-κB linking inflammation and thrombosis. Frontiers in immunology. 2019; 10: 85.
- Allahverdian S, Chaabane C, Boukais K, Francis GA, Bochaton-Piallat M-L. Smooth muscle cell fate and plasticity in atherosclerosis. Cardiovascular research. 2018; 114: 540-550.
- Milstone DS, et al. Differential role of an NF-κB transcriptional response element in endothelial versus intimal cell VCAM-1 expression. Circulation research. 2015; 117: 166-177.
- Khatana C, et al. Mechanistic Insights into the Oxidized Low-Density Lipoprotein-Induced Atherosclerosis. Oxidative Medicine and Cellular Longevity. 2020.
- Katakami N. Mechanism of development of atherosclerosis and cardiovascular disease in diabetes mellitus. Journal of atherosclerosis and thrombosis. 2018.
- Yao D, Brownlee M. Hyperglycemia-induced reactive oxygen species increase expression of the Receptor for Advanced Glycation End Products (RAGE) and RAGE ligands. Diabetes. 2010; 59: 249-255.
- Gonzalez Y, et al. High glucose concentrations induce TNF-a production through the down-regulation of CD33 in primary human monocytes. BMC immunology. 2012; 13: 1-14.
- Yaribeygi H, Atkin SL, Sahebkar A. A review of the molecular mechanisms of hyperglycemia-induced free radical generation leading to oxidative stress. Journal of cellular physiology. 2019; 234: 1300-1312.
- Yao L, et al. Obesity-induced vascular inflammation involves elevated arginase activity. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 2017; 313: R560-R571.
- Virdis A, et al. Microvascular endothelial dysfunction in human obesity: role of TNF-a. The Journal of Clinical Endocrinology & Metabolism. 2019; 104: 341-348.
- Hung CHL, et al. A reciprocal relationship between reactive oxygen species and mitochondrial dynamics in neurodegeneration. Redox biology. 2018; 14: 7-19.
- Nannelli G, et al. ALDH2 activity reduces mitochondrial oxygen reserve capacity in endothelial cells and induces senescence properties. Oxidative medicine and cellular longevity. 2018.
- Busija DW, Rutkai I, Dutta S, Katakam PV. Role of mitochondria in cerebral vascular function: energy production, cellular protection, and regulation of vascular tone. Comprehensive Physiology. 2011; 6: 1529-1548.
- Katakam PVG, et al. Depolarization of mitochondria in neurons promotes activation of nitric oxide synthase and generation of nitric oxide. American Journal of Physiology-Heart and Circulatory Physiology. 2016; 310: H1097-H1106.
- Bhati P, Alam R, Moiz JA, Hussain ME. Subclinical inflammation and endothelial dysfunction are linked to cardiac autonomic neuropathy in type 2 diabetes. Journal of Diabetes & Metabolic Disorders. 2019; 18: 419-428.
- Alem MM. Endothelial dysfunction in chronic heart failure: assessment, findings, significance, and potential therapeutic targets. International journal of molecular sciences. 2003; 20: 3198.
- Miura H, et al. Diabetes mellitus impairs vasodilation to hypoxia in human coronary arterioles: reduced activity of ATP-sensitive potassium channels. Circulation research. 2003; 92: 151-158.
- Sheng Y, Zhu L. The crosstalk between autonomic nervous system and blood vessels. International journal of physiology, pathophysiology and pharmacology. 2018; 10: 17.
- Chapman AR, Adamson PD, Mills NL. Assessment and classification of patients with myocardial injury and infarction in clinical practice. Heart. 2017; 103: 10-18.
- Garbern JC, Lee RT. Mitochondria and metabolic transitions in cardiomyocytes: lessons from development for stem cell-derived cardiomyocytes. Stem Cell Research & Therapy. 2021; 12: 1-25.
- Jeong E-M, et al. Metabolic stress, reactive oxygen species, and arrhythmia. Journal of molecular and cellular cardiology. 2012; 52: 454-463.
- Tse G, Yan BP, Chan YWF, Tian XY, Huang Y. Reactive oxygen species, endoplasmic reticulum stress and mitochondrial dysfunction: the link with cardiac arrhythmogenesis. Frontiers in Physiology. 2016; 7: 313.
- Caplan LR, Wong KS, Gao S, Hennerici MG. Is hypoperfusion an important cause of strokes? If so, how? Cerebrovascular diseases. 2016; 21: 145-153.
- Hussain S, Chowdhury TA. The impact of comorbidities on the pharmacological management of type 2 diabetes mellitus. Drugs. 2019; 79: 231-242.
- Cherney DZI, et al. Impact of cardio-renal-metabolic comorbidities on cardiovascular outcomes and mortality in type 2 diabetes mellitus. American journal of nephrology. 2020; 51: 74-82.
- Khera R, Krumholz HM. With great power comes great responsibility: big data research from the National Inpatient Sample. Circulation: Cardiovascular Quality and Outcomes. 2017; 10: e003846.
- Rawshani A, et al. Risk factors, mortality, and cardiovascular outcomes in patients with type 2 diabetes. New England journal of medicine. 2018.
- Manley S. Haemoglobin A1c–a marker for complications of type 2 diabetes: the experience from the UK Prospective Diabetes Study (UKPDS). Clinical Chemistry and Laboratory Medicine (CCLM). 2003; 41: 1182-1190.
- Craciun CI, et al. The C-peptide correlations and effect on the glycemic variability parameters in patients with type 2 diabetes. Human and Veterinary Medicine. 2018; 10: 111-116.
- American Diabetes A. Standards of medical care in diabetes-2017 abridged for primary care providers. Clinical diabetes: a publication of the American Diabetes Association. 2017; 35: 5-26.
- Chamberlain JJ, et al. Pharmacologic therapy for type 2 diabetes: synopsis of the 2017 American Diabetes Association Standards of Medical Care in Diabetes. Annals of internal medicine. 2017; 166: 572-578.
- Dai Y, Luo W, Chang J. Rho kinase signaling and cardiac physiology. Current opinion in physiology. 2018; 1: 14-20.
- Chandra S, Fulton DJR, Caldwell RB, Caldwell RW, Toque HA. Hyperglycemiaimpaired aortic vasorelaxation mediated through arginase elevation: Role of stress kinase pathways. European journal of pharmacology. 2019; 844: 26- 37.
- Bibbins-Domingo K. Aspirin use for the primary prevention of cardiovascular disease and colorectal cancer: US Preventive Services Task Force recommendation statement. Annals of internal medicine. 2016; 164: 836-845.
- Garg V, Verma S, Connelly K. Mechanistic insights regarding the role of SGLT2 inhibitors and GLP1 agonist drugs on cardiovascular disease in diabetes. Progress in cardiovascular diseases. 2019; 62: 349-357.
- Sheikh O, Nashawi M, Battisha A, Chilton R. A focused review of cardiovascular guideline related recommendations for the primary care physician in the USA. Cardiovascular Endocrinology & Metabolism. 2020; 9: 36.
- Bonnet F, Scheen AJ. Effects of SGLT2 inhibitors on systemic and tissue lowgrade inflammation: the potential contribution to diabetes complications and cardiovascular disease. Diabetes & metabolism. 2018; 44: 457-464.