
Research Article
Austin J Endocrinol Diabetes. 2021; 8(2): 1085.
Effects of Calcium Source on Calcium-Metabolic Parameters in Healthy Women and Men
Hitz MF1,2*, Dahl M2,3 and Jørgensen NR2,4
1National Research Center for Bone Health, Medical Department, Zealand University Hospital, Denmark
2Department of Clinical Medicine, University of Copenhagen, Denmark
3Department of Clinical Biochemistry, Zealand University Hospital, Denmark
4Department of Clinical Biochemistry, Copenhagen University Hospital Rigshospitalet, Denmark
*Corresponding author: Mette Friberg Hitz, Associated Professor, National Research Center for Bone Health, Medical Department, Zealand University Hospital, Lykkebaekvej 1, 4600 Koege, Denmark
Received: May 20, 2021; Accepted: June 22, 2021; Published: June 29, 2021
Abstract
Background/Objectives: Calcium and vitamin D are important for bone health. We compared 24-hour urinary calcium-excretion (Uca/24hrs), during dietary calcium steady-state condition, for different calcium-sources and effects of vitamin D, age and sex.
Subjects/Methods: Fifty-two healthy pre- and postmenopausal women and men completed the regimens: placebo, calcium carbonate (400mg) +18μg vitamin D, calcium carbonate (400mg) +38μg vitamin D and 400mg calcium phosphate (milk). Uca/24hrs was measured during dietary calcium steady state as a surrogate measure of calcium-absorption. Serum-calcium, parathyroid hormone (PTH), 25-hydroxy-vitamin D, Procollagen Type 1 N-terminal Pro- Peptide (P1NP) and C-terminal Telopeptide of type 1 collagen (CTX) were measured.
Results: Mean daily intake of calcium for the study group ± SD was 1105 ±396 mg. Mean-Uca ± SD: placebo 5.19 ± 2.04 mmol/24hrs, milk 5.88 ± 2.39 mmol/24hrs, (CaCO3+D) 6.19 ± 2.34 mmol/24hrs and (CaCO3 + DD) 6.26 ± 2.32 mmol/day. Uca were higher for all regimens compared to placebo (p <0.001), no difference was found between regimens. CTX was lower during all regimens compared to placebo (p <0.001): placebo 450 ± 243 μg/L, Milk 377 ± 248 μg/L, (CaCO3+D) 392 ± 266 μg/L and (CaCO3+DD) 361 ± 232 μg/L.
Conclusions: Uca was higher during supplementation with calcium compared to placebo. Supplementation with calcium reduced bone resorption significantly without effecting PTH. Menopausal status, sex and supplement with vitamin d demonstrated no effect on calcium excretion.
Keywords: Calcium absorption; Calcium carbonate; Biochemical markers of bone turnover; Bone health
Abbreviations
CaCO3+D: Calcium Carbonate with 18 Microgram Vitamin D; CaCO3+DD: Calcium Carbonate with 38 Microgram Vitamin D; CTX: C-Terminal Telepeptide of Type 1 Collagen; CV: Coefficient of Variation; IGF-1: Insulin Growth Factor 1; P1NP: Procollagen Type 1 N-Termial Propeptide; PTH: Parathyroid Hormone; SD: Standard Deviation; Uca: Urinary Calcium; Uca/24hrs: Urinary Calcium Excretion over 24 hour
Introduction
Calcium is largely absorbed in the proximal part of the small intestine via both an active transcellular mechanism and a passive para-cellular route [1].
The ability to absorb calcium may decline with age, postmenopausal status and vitamin D deficiency, since both activated vitamin D and estrogen stimulate the synthesis of important calcium binding proteins responsible for active calcium absorption [2,3].
A sufficient intake of calcium and vitamin D prevents secondary hyperparathyroidism, reduce bone turnover, bone loss, and is the first choice of prophylactic treatment of osteopenia and the basis of medical treatment for osteoporosis [4,5]. Calcium may originate from the diet or be taken as a supplement. There are different calcium salts available on the market; calcium carbonate, -citrate, -citrate-malate and lactate-gluconate. There is a small-demonstrated difference in fractional calcium absorption between the different salts, which is of little practical significance for individuals with normal gastric acidic environment. Thus choice of supplement may in large depend on patient preference [6,7].
The main source of calcium in the diet originates from dairy products but other food substances contribute as well, such as vegetables [8]. The bioavailability of calcium constitutes the fraction of calcium available for systemic circulation after absorption. For calcium supplementation, the bioavailability of calcium from food depends largely on the presence of other factors such as oxalic acid, phytic acid, lactose, fibers and oligosaccharides [8,9].
The milk protein casein is known to form Caseinophosphopeptides (CPP) during digestion, which have been shown to improve bioavailability of calcium from milk. Furthermore, casein acts as a calcium binding protein, which also facilitates calcium absorption [11]. The results are conflicting and most studies are animal studies [12]. In clinical studies, CPP has been demonstrated to inhibit bone turnover in postmenopausal women and elderly men and increase Insulin Growth Fractor 1 (IGF-1).
Activated vitamin D stimulates the synthesis of calcium binding transport proteins in the intestine. It has previously been speculated whether ingested vitamin D (cholecalciferol) has a direct effect on calcium absorption from the luminal side [13].
The aim of this study was to compare the urinary excretion of calcium from the sources calcium carbonate and milk as a surrogate measure of calcium absorption. To investigate if supplementation with vitamin D in an increasing dose could reduce bone turnover markers and to investigate if ingestion of calcium from milk could demonstrate an improved bioavailability of calcium compared to calcium carbonate evaluated by the ability to suppress bone turnover markers as well as PTH.
The study was conducted in a group of healthy pre- and postmenopausal women and men.
Materials and Methods
Participants were 52 healthy men and women invited to participate in the study after advertisements in the local press in Zealand County, Denmark. One hundred-fourth-eight participants were eligible, of these 31 did not meet inclusion criteria and 65 chose not to participate due to study design; before (61) or during the study (4) (Figure 1).
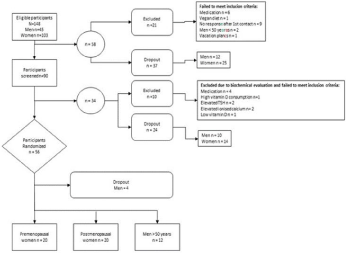
Figure 1: Flow Chart.
Inclusion criteria were no presence of diseases or drug consumption that could affect bone metabolism or gastrointestinal absorption, including hormonal replacement therapy. Normal biochemical screening included 25-hydroxy- vitamin D level >50nmol/L as well as parathyroid hormone within the normal reference. Additionally none of the included subjects had a daily intake of calcium supplements, which exceeded 1000mg/day. The participants were divided into three groups based upon sex and menopausal state: premenopausal women, postmenopausal women and men over the age of 50.
We used a randomized, participant partly blinded, crossover placebo controlled design. The study lasted 4 weeks and participants were assigned to four treatment regimens:
• Three placebo tablets a day for 3 days each given with a meal (Placebo).
• One liter extra cow-milk a day for three days, equivalent to 1200mg elemental calcium (not Vitamin D enriched milk) divided in three portions given with a meal (Milk).
• 400mg Calcium Carbonate combined with 19 mcg Vitamin D (Unikalk Forte ) three times a day for 3 days, equivalent to a total intake of 1200mg elemental calcium and 57 mcg (2280IU) cholecalciferol - given with a meal (CaCO3+D).
• 400mg Calcium Carbonate combined with 38 mcg Vitamin D (Unikalk Mega) three times a day for 3 days, equivalent to a total intake of 1200mg elemental calcium and 114 mcg (4560IU) cholecalciferol - given with a meal (CaCO3+DD).
Treatment regimens 1, 3 and 4 were blinded for the participants but not for the investigator. Treatment regimen 2 was not blinded.
The regimens 3 and 4 were given to all participants in study weeks 3 and 4 for all participants due to a long-term effect of vitamin D on calcium absorption. The regimen 4 was given after regimen 3 in order to investigate the effect of an escalated dose of vitamin D on calcium absorption.
Regimens 1 and 2 were given randomly.
On the basis of a 7-day dietary registration, an experienced dietitian conducted a dietary analysis using the DANKOST 2000 computer program, (Danish Catering Center, Herlev Denmark).
The participants were instructed by a dietitian to follow a balanced diet, in order to maintain a constant intake of calcium and vitamin D during the study. They were especially instructed to consume the same amount of dairy products every day throughout the study. They followed the diet 4 days every week, starting the treatment regimen according to their randomization number and collection of 24-hour urine from day 2 to 4.
Urine was collected in plastic containers (acidified).
All tablets looked identical and had the same neutral flavor and the tablets were taken with a meal three times a day.
If a participant failed to follow the treatment regimen on a particular day, that day was excluded from the analysis. The total content of creatinine was measured in all collected urine samples to investigate compliance. Remaining tablets were returned after the 4-week study period.
Concentrations of hemoglobin, potassium, sodium, creatinine, urea, albumin, alanine aminotransferase, lactate dehydrogenase, thyroid-stimulating hormone, phosphate, magnesium, and alkaline phosphatase and were measured in blood at baseline using commercially available CE-IVD marked assays.
Serum concentrations of calcium ion, parathyroid hormone, vitamin D, Procollagen Type 1 N-Terminal Propeptide (P1NP), and C-Terminal Telopeptide of Type 1 Collagen (CTX) were measured at baseline and after each treatment regimen.
Serum intact Parathyroid Hormone (PTH) was measured with a commercially available two-step immunometric sandwich method using a Chemiluminescent Immunoassay (CLIA) detection technique (Siemens Healthcare, Ballerup, Denmark) with an intermediate precision Coefficient of Variation (CV) of 6.4% (3.5pmol/L) and 6.4% (29.9pmol/L).
Vitamin D (25-hydroxycholecalciferol) was measured in serum using a commercially available competitive chemiluminescent immunoassay from Siemens Healthcare (Ballerup, Denmark) with intermediate precision CVs of 8.5% (60nmol/L) and 4.7% (250nmol/L).
Serum/plasma CTX was measured using the IDS-iSYS CTX (CrossLaps®) assay (Immunodiagnostic Systems, plc, Tyne and Wear, UK), and serum/plasma P1NP was measured using the IDS-iSYS intact P1NP assay (Immunodiagnostic Systems). Both assays were carried out on a dedicated automated analyzer, iSYS (Immunodiagnostic Systems) according to the manufacturer’s instructions. Both assays are chemiluminescence immunoassays. For each assay, the sample aliquots were kept frozen at - 80 degrees Celsius until the day of analysis. None of the samples had previously been thawed, and all analyses were performed immediately after thawing the samples. All samples were analyzed using one single batch of each assay. Assay performance was verified using the manufacturers’ control specimens. The intermediary precision CVs for CTX were 5.3% (at CTX concentration 213ng/L), 3.4% (869ng/L), and 3.5% (2,113 ng/L) for iSYS. For P1NP the intermediary precision CVs were 5.4% (18.96μg/L), 6.5% (48.48μg/L), and 6.1% (122.10μg/L) for iSYS.
Serum levels of vitamin D <50nmol/L were considered insufficient for this population.
The content of calcium, creatinine, and phosphate (mmol/day) was measured in all collected urine samples. The concentration of calcium was measured using the urease indicator method reflection photometry (MP2-9; Vitros Chemistry Products, Ortho-Clinical Diagnostic, Strasburg, France), and the total concentration was calculated from the total urine volume. The intermediate precision CV was 5.0% (2.81mmol/L).
The mean calcium excretion for the 3 days in each treatment period was calculated and used as an indirect measure of daily calcium absorption (Uca/mmol) on a balanced calcium diet (ref).
The study was designed according to the Helsinki II declaration and approved by local scientific ethical committee.
Informed consent was obtained from all participants before inclusion.
Statistical measures
With a power of 80% and a level of significance of 5% (p <0.05) an increase in calcium-excretion from 5.0 to 5.5 mmol/24 hours ± 0.5 (mean ± SD) could be detected with 16 participants in each group.
Data were analyzed using the computer program SPSS 11.5 for Windows (SPSS, Chicago, IL). P <0.05 was considered a statistically significant difference. Normally distributed data was expressed in mean (standard deviation) and non-normally distributed data was expressed in median and range. A paired t-test was used to compare parameters for the same individual, and p-values were corrected for multiple testing (Bonferroni).
Independent sample t-test was used for comparison between study groups and data was corrected for multiple testing.
The effect of age, sex, Vitamin D and calcium intake on calcium excretion were analyzed using regression analysis.
Results
Mean age (SD) was 42 (9), 62 (8) and 62 (7) years in pre-, postmenopausal women and men respectively. Premenopausal women were significantly younger (p <0.001). All study participants had a baseline biochemical evaluation within the normal reference range (Table 1). Overall baseline level of vitamin D was 73nmol/L and PTH 5.4pmol/L. No difference was found between study groups. Baseline biochemical markers of bone turnover, P1NP and CTX were higher in post-menopausal women (P1NP 75.1 ± 24.9 μg/L, CTX 603 ± 257 ng/L) compared to the group of premenopausal women (P1NP 49.5 ± 15.4 μg/L, CTX 345 ± 179 ng/L) and men over 50 years of age (P1NP 65.1 ± 26.2 μg/L, CTX 495 ± 275 ng/L) (p <0.001).
HB mmol/L
Potassium mmol/L
Sodium mmol/L
Creatinine mcmol/L
Urea mmol/L
Albumin g/L
ALT U/L
LDH U/L
TSH mIU/L
All (mean)
8.79
4
141
72
5.32
38.17
25
177
1.9
STD
0.62
0.3
1
12
1.16
2.54
10
27
0.8
Premenopausal (mean)
8.52
3.9
141
67
4.9
38.32
22
167
2.1
STD
0.52
0.2
1
7
1.11
2.45
6
18
0.8
Postmenopausal (mean)
8.57
3.9
141
67
5.1
37.15
24
181
1.9
STD
0.46
0.2
1
8
1.18
2.3
8
25
0.9
Men (mean)
9.44
4.2
141
85
6.16
39.33
32
184
1.6
STD
0.42
0.3
2
12
0.73
2.55
13
35
0.5
Calcium
Phosphous
Magnesium
ALP
PTH
Vitamin D
P1NP
CTX
Age
All (mean)
1.27
1
0.91
66
5.4
73
59.6
442
55
STD
0.04
0.15
0.06
20
1.8
16
24
254
13
Premenopausal (mean)
1.27
0.97
0.89
54
5.5
75
49.5
345
42
STD
0.05
0.18
0.06
16
1.8
17
15.4
179
9
Postmenopausal (mean)
1.27
1.08
0.91
77
5.1
75
75.1
603+
62*
STD
0.04
0.12
0.06
17
1.5
18
24.9
257
8
Men (mean)
1.27
0.94
0.91
67
5.9
69
51.7
351
62*
STD
0.05
0.1
0.05
20
2
13
22.1
235
7
*P <0.001 compared to the group of premenopausal women.
+P <0.001 compared to the group of premenopausal women and compared to group of men >50 years.
Table 1: Baseline biochemical evaluation of all study participants and for premenopausal, postmenopausal women and men.
The daily intake of calcium for the study group (mean ± SD) was 1105 ± 396 mg. In subgroups, intake of calcium was higher in men; this was only significant compared to the premenopausal women. The dietary analysis of the study participants is shown in Table 2. The group of men consumed a diet with a significantly higher content of most of the investigated parameters. In a regression analysis, none of these parameters demonstrated an effect on calcium excretion.
Energy (KJ)
Fat (g)
Carbohydrate (g)
Protein (g)
Fiber (g)
Calcium (mg)
Magnesium (mg)
Phosphorus (mg)
D-Vitamin (µg)
K-Vitamin (µg)
All (mean)
8,298
74
208
88
24
1,105
306
1,303
7
88
SD
1,904
19
56
17
8
396
91
383
11
110
Men (mean)
10,290
89
263
105
28
1,351
384
1,699
9
43
SD
1,593
18
64
13
7
309
66
307
7
28
Premenopausal (mean)
7,175*
65*
179*
79*
19*
981+
253*
1,073*
5
82
(SD)
1,341
15
38
13
5
408
78
288
13
101
Postmenopausal (mean)
8,116+
73
202+
85+
27
1,067
309
1,274+
7
126
(SD)
1,542
19
47
17
9
379
82
338
10
138
*P <0.001 compared to the group of men above 50 years.
+P <0.01 compared to the group of men above 50 years.
Table 2: Seven-day dietary registration for all study participants and for premenopausal, postmenopausal women and men.
Overall compliance to the treatment regimens was 98%. Urinary excretion of calcium is shown in Figure 2. The excretion of calcium was increased for all treatment regimens compared to placebo. The increase in calcium excretion compared to placebo (median (range)) was 0.74 (-1.84-4.41) mmol/24hrs (milk); 0.97 (-1.80- 4.47) mmol/24hrs (CaCO3+D) and 1.23 (-2.37-3.97) mmol/24hrs (CaCO3+DD). No effect of increasing supplement of vitamin D was demonstrated and no difference in absorption of calcium from milk compared to calcium carbonate was found. Correcting the urinary calcium excretion for creatinine did not alter the results and there were no significant differences between urinary creatinine between the study periods (data not shown).
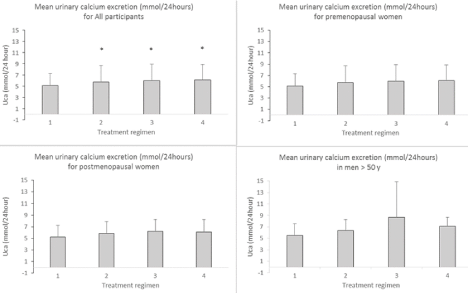
Figure 2: Urinary calcium excretion expressed as mmol per 24 hours for all participants and for the group of premenopausal women, postmenopausal women and
men. *p <0.001 compared to placebo.
Calcium metabolic parameters in serum/plasma (calcium, PTH and 25-hydroxy vitamin D) and bone turnover markers (P1NP and CTX) were measured at the end of each study regimen. There were no differences in calcium, PTH, vitamin D or P1NP for any of the treatment regimens for any of the study groups (Figure 3A and 3B). CTX was lower during all supplementation regimen compared to placebo (mean ± SD) 450 ± 243 ng/L, milk 377 ± 248 ng/L, CaCO3+D 392 ± 266 ng/L and CaCO3+DD 361 ± 232 ng/L. In a subgroup analysis, it remained significant for all regimens compared to placebo for the group of premenopausal women (Mean ± SD); Placebo 339 ± 149 μg/L, milk 258 ± 132 μg/L, CaCO3+D 259 ± 121 μ/L and CaCO3+DD 227 ± 89 μ/L (Figure 3A).
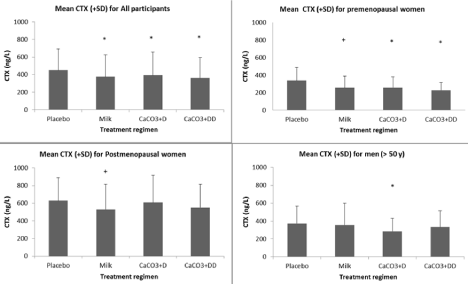
Figure 3A: Biochemical markers of bone resorption (CTX) for all participants and for the subgroups of premenopausal women, postmenopausal women and men.
*P <0.001 compared to placebo; +p <0.05 compared to placebo.
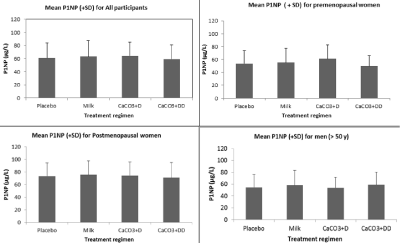
Figure 3B: Biochemical markers of bone formation (P1NP) for all participants and for the subgroups of premenopausal women, postmenopausal women and men.
No significant effect was found compared to placebo.
P1NP during the study are shown in Figure 3B.
Discussion
The urinary excretion of calcium from calcium carbonate and milk was measured in a group of healthy pre- and post-menopausal women and men. We used the method of 24-hours urinary calcium excretion during a calcium balanced diet, in a crossover design, as an indirect measure of calcium absorption. The method has been described previously [14,15]. The urinary calcium excretion was increased significantly during the regimen of calcium carbonate plus 18 mcg and 38 mcg of vitamin D and milk compared to placebo.
We aimed at including 20 participants in each study group but were unable to include 20 healthy men above the age of 50 years. The primary reason was choice of study design with collection of 24-hour urine 3 days per week. For many it was incompatible with work-lift and this resulted in a reduced power in subgroup analysis.
We were unable to demonstrate an improved urinary calcium excretion from milk compared to calcium carbonate. No significant difference in urinary excretion of calcium during the milk regimen compared to calcium carbonate was demonstrated. Calcium in milk is bound to the milk protein casein. It was speculated if binding of calcium to protein, could have resulted in increased active calcium absorption and thus urinary calcium excretion. Casein forms caseinophosphopeptides after ingestion; this prolongs the time available for calcium absorption and involves more distal segments of the intestine. This potentially increases the bioavailability of calcium [11]. It has been demonstrated in both animal and human studies, that combining calcium supplementation with prebiotics such as oligo- and poly-saccharides, indigestible starch or inulin improves bioavailability and bone health [16,17]. A study in rats showed that bioactive peptides from casein stimulate osteoblast differentiation, in ovariectomized rats improved bone mineral density, trabecular bone structure, and reduced bone turnover [18]. We did not find an improved bioavailability of calcium from milk compared to calcium carbonate in this group of healthy women and men. All participants had a high intake of calcium in the diet and in this setting; the passive para-cellular absorption predominates, making the benefits of casein protein binding less important. Apart from water and proteins, milk contains disaccharides but not oligoor polysaccharides, which in some forms have a prebiotic effect. Whether the casinophosphopeptides act as prebiotics in the large intestine and improve bone health beyond calcium supplementation alone, cannot be concluded from this study.
We could not demonstrate a decline in urinary calcium exertion, by age or menopausal status as expected. In estrogen deficiency, synthesis of calcium binding globulin declines. This route of calcium absorption dominates when calcium intake is low but as calcium intake increases, the para-cellular route becomes more dominating. Therefore, in a state of high calcium intake, the absorption of calcium will be less sensitive to estrogen deficiency and the individual more prone to obtain a positive calcium balance [19].
All participants were vitamin D replete and all parameters of calcium metabolism were within the normal reference range. During the regimens 3 and 4 (calcium carbonate) increasing doses of vitamin D were given, equivalent to 54 mcg and 114 mcg daily. Supplementation with vitamin D for three days during each of these treatment regimens did not increase the serum level of vitamin D nor did it result in a decline in PTH for any of the study groups. This illustrates that the metabolism of cholecalciferol from absorption to activation in the liver, requires more than three days of treatment or higher doses.
Activated vitamin D 1,25-dihydroxycholecalciferol has an important role in intestinal calcium absorption, especially during low-calcium diets. We speculate whether increasing the availability of cholecalciferol in the intestinal lumen could stimulate the absorption via a direct luminal effect. We did not find an increase in urinary calcium excretion after increasing the dose of vitamin D (cholecalciferol) from 54 to 114 mcg for three days [15].
Absorption of calcium may also occur via a non-genomic action, by binding to a membrane receptor after administration of 1,25-dihydroxyvitamin D. In contrast to the calcium absorption stimulated by genomic action, occurring over several hours, the non-genomic action occurs rapidly [2]. One theory could have been, that cholecalciferol has direct effect on this non-genomic action or is activated in the local environment, though this could not be demonstrated.
Levels of biochemical markers of bone turnover were significantly higher for the group of post-menopausal women. Increased osteoclast activity accounts for higher level of CTX in postmenopausal women and since the resorption and formation are coupled, a corresponding increase in bone formation (P1NP) is seen. A bone-loss occurs since resorption exceeds formation. Increasing the intake of calcium from a baseline level of approximately 1000mg to around 2500mg daily resulted in a reduction in CTX in all study groups, in subgroup analysis; the reduction in CTX was only significant in the premenopausal group. This may be due to lack of power or a demonstration of the premenopausal group responding to the increased supplementation with calcium being incorporated into bone (reducing bone turnover). Calcium intake relates to PTH levels, reflecting the homeostatic mechanism, resulting in high fractional calcium absorption when calcium intake is low and low fractional calcium absorption when calcium intake is high. Increasing intake of calcium has been demonstrated to reduce bone-turnover, possibly by filling some of the osteoclastic resorption sites [20]. The risk associated with a high calcium intake has been discussed in the literature. Especially the risk of vascular calcifications and a possible increased risk of cardiovascular events has been evaluated in large studies [21,22].
Therefore, this study should not result in alterations of the recommendations on calcium intake; further studies are needed to confirm the result and the specific importance for bone health. However, the study demonstrates that both milk and calcium carbonate are relevant sources of calcium for both women and men regardless of age, and choice of supplement may be based on personal preferences.
Conclusion
In conclusion, the bioavailability of calcium (Uca/24hrs) from calcium carbonate with varying doses of vitamin D and milk was demonstrated. A superior effect of calcium from milk could not be demonstrated in this study and an acute effect on increasing supplement of vitamin D could not be demonstrated either.
Increasing the intake of calcium from 1g to 2.5g per day reduced bone resorption, without alterations of levels of PTH.
Acknowledgements
The authors thank Orkla Care, Denmark for an unrestricted grant.
Declarations
Funding: The research was supported by an unrestricted grant from Orkla Care, Denmark
Conflict of interest: Mette Friberg Hitz has received grants from Orkla Care, Denmark, UCB, Ellab Fond and Amgen and received personal payment in relation to lectures and advisory board meetings.
Authors’ contribution: Mette Friberg Hitz has conceived and designed the project, collected the data, performed data analysis and contributed to the writing of the paper. Morten Dahl has contributed to analysis of samples, contributed to data analysis and contributed to the writing of the paper. Niklas Rye Jorgensen has contributed to analysis of samples, contributed to the writing of the paper.
References
- Christakos S, Li S, De La Cruz, Shroyer NF, Criss ZK, et al. Vitamin D and the intestine: Review and update. J Steroid Biochem Mol Biol. 2020; 196.
- Wongdee K, Rodrat M, Teerapornpuntakit J, Krishnamra N, Charoenphandhu N. Factors inhibiting intestinal calcium absorption: hormones and luminal factors that prevent excessive calcium uptake. J Physiol Sci. 2019; 69: 683- 696.
- Heaney RP, Recker RR, Stegman MR, Moy AJ. Calcium absorption in women: Relationship to calcium Intake, estrogen status, and age. J Bone Miner Res. 1989; 4: 469-475.
- Nordin BE. Calcium and osteoporosis. Nutrition. 1997: 13: 664-686.
- Malabanan AO, Holick MF. Vitamin D and bone health in postmenopausal women. J Womens Health. 2003; 12: 151-156.
- Sheik MS, Santa Ana CA, Nicar BSM, Schiller LR, Fordtran JS. Gastrointestinal absorption of calcium from milk and calcium salts. N Engl J Med. 1987; 317: 532-536.
- Lamy O, Burckhardt. Calcium revisited: Part II calcium supplements and their effects. BoneKey Rep. 2014; 3: 579.
- Burckhardt P. Calcium revisited, part III: Effect of dietary calcium on BMD and fracture risk. BoneKey rep. 2015; 4: 708.
- Cámara-Martos F, Amaro-López MA. Influence of dietary factors on calcium bioavailability: A brief review. Biol Trace Elem Res. 2002; 89: 43-52.
- Bouhallab S, Bouglé D. Biopeptides of milk: Caseinophosphopeptides and mineral bioavailability. Reprod Nutr Dev. 2004; 44: 493-498.
- Glab TK, Boratynski J. Potential of casein as a carrier for biologically active agents. Top Curr Chem (Cham). 2017; 375: 71.
- Guéguen L, Pointillart A. The bioavailability of dietary calcium. J Am Coll Nutr. 2000; 19: 119S-136S.
- Christakos S, Dhawan P, Porta A, Mady LJ, Seth T. Vitamin D and intestinal calcium absorption. Mol Cell Endocrinol. 2011; 347: 25-29.
- Mortensen L, Charles P. Bioavailability of calcium supplements and the effect of vitamin D: Comparisons between milk, calcium carbonate, and calcium carbonate plus vitamin D. Am J Clin Nutr. 1996; 63: 354-357.
- Hitz MF, Eskildsen PC, Jensen JB. Bioavailability of calcium: Comparison of calcium carbonate and milk and the effect of vitamin D, age, and sex using 24-Hour urine calcium as a method. Calcif Tissue Int. 2005; 77: 361-366.
- Abrams, SA, Griffin IJ, Hawthorne KM, Liang L, Gunn SK, Darlington G, et al. A combination of prebiotic short - and long-chain inulin-type fructans enhances calcium absorption and bone mineralization in young adolescents. Am J Clin Nutr. 2005; 82: 471-476.
- Whisner CM, Castillo LF. Prebiotics, bone and mineral metabolism. Calcif Tissue Int. 2018; 102: 443-479.
- Reddi S, Shanmugam VP, Tanedjeu KS, Kapila D, Kapila R. “Effect of buffalo casein-derived novel bioactive peptides on osteblost differentiation”. Eur J Nutr. 2018; 57: 593-605.
- Nordin BE. Calcium and osteoporosis. Nutrition. 1997; 13: 664-686.
- Reid IR, Bristow SM, Bolland MJ. Calcium supplements: benefits and risks. J Intern Med. 2015; 278: 345-368.
- Bolland MJ, Avenell A, Baron JA, Grey A, MacLennan GS, Gamble GD, et al. Effect of calcium supplements on risk of myocardial infarction and cardiovascular events: Meta-analysis. BMJ. 2010; 341: c3691.
- Bolland MJ, Grey A, Avenell A, Gamble GD, Reid IR. Calcium supplements with or without vitamin D and risk of cardiovascular events: reanalysis of the Women’s Health Initiative limited access dataset and meta-analysis. BMJ. 2011; 342: d2040.