
Case Report
J Endocr Disord. 2014;1(1): 1002.
Ectopic Corticotropin-Releasing Hormone (CRH) Syndrome from a Primary Nerve Ectoderm Tumor in the Perineum: A Case Report and Review of the Literature
Jianping Xu1, Xinhua Xiao1*, Yan Jiang1, Dingrong Zhong2, Xiaoping Xing1
1Department of Endocrinology, Peking Union Medical College Hospital, China
2Department of Pathology, Peking Union Medical College Hospital, China
*Corresponding author: Xinhua Xiao, Department of Endocrinology, Peking Union Medical College Hospital, Peking Union Medical College, Chinese Academy of Medical Sciences, Beijing 100730,China,The Ministry of Health Key Laboratory of Endocrinology
Received: July 12, 2014; Accepted: July 27, 2014; Published: July 28,, 2014
Abstract
Context: Cushing’s syndrome (CS), which is caused by the isolated production of CRH production instead of ACTH, is extremely rare.
Objective: We report the case of a 12-year-old female with a primary nerve ectoderm tumor in the perineum who presented with clinical and biochemical evidence of ACTH-dependent Cushing’s syndrome.
Case Illustration: After an operation on her vulva, her clinical condition deteriorated rapidly, and her blood cortisol levels were significantly elevated. Pituitary imaging was normal, and cortisol was not suppressed with a high-dose dexamethasone test, consistent with a diagnosis of ectopic ACTH. PET-CT confirmed a tumor in the left perineum and multiple lesions in both lungs. To identify the primary tumor, a biopsy of the mass in the labia was performed. Immunohistochemistry was consistent with a neuroendocrine tumor, specifically, a primitive neuroectodermal tumor (PNET).
Results: Immunostaining was negative for ACTH but was strongly positive for CRH.
Conclusion: This case illustrates the importance of considering the ectopic production of CRH in the differential diagnosis of presentations of ACTHdependent Cushing’s syndrome. To the best of our knowledge, there has been no report of ectopic CRH syndrome caused by PNET in children.
Conclusion: This case illustrates the importance of considering the ectopic production of CRH in the differential diagnosis of presentations of ACTHdependent Cushing’s syndrome. To the best of our knowledge, there has been no report of ectopic CRH syndrome caused by PNET in children.
Keywords: Ectopic Cushing’s syndrome; Primitive Neuroectodermal Tumor; Children
Introduction
Endogenous Cushing’s syndrome (CS) is a rare syndrome with an estimated annual incidence of 0.1 to 1.0 new cases per 100,000 [1]. IS classified as ACTH dependent or ACTH independent. The former includes pituitary-dependent Cushing’s disease and ectopic ACTH secretion. CS caused by ectopic ACTH, ACTH/CRH, or CRH secretion may be difficult to distinguish from pituitary- dependent Cushing’s disease [2].
We report the case of a 12-yr-old female who presented to the Peking Union Medical College Hospital 2010 with a history of an operation on the vulva and the recent onset of moderately severe ACTH-dependent CS.
Materials and Methods
A 12-year-old female patient was admitted to our hospital secondary to rapid weight gain (from 45 kg to 58 kg) and fatigue for the past four months and irregular heart rate for the past week. Her height had increased by 7 cm over the past year. The patient seemed depressed, but her appetite was normal. She suffered from insomnia. The patient denied taking any prescription drugs or medications that might contain corticosteroids. Menarche had begun one year prior, with two subsequent periods. The patient had not been menstruating for the past two months.
A 3-cm mass in the vulva was removed surgically nine months prior to admission. The patient’s face became circular, and her belly became enlarged. Her weight increased gradually from 45 kg to 58 kg in four months, central obesity developed, and her skin become slightly darker. Hair on her shoulders and back became more prevalent. One month before being admitted, she found bilateral purple striae on her stomach and the medial side of her leg; she also reported fatigue. Another hospital’s examination revealed a blood ACTH level of 80 pg/ml at 0800 h. Blood cortisol was 500 ng/ml (normal range: 50-280 ng/ml). The patient’s spirit was poor. Her appetite was good; she ate 400 grams of food daily. She was easily woken.
Past medical history: Nine months prior to admission, a 3-cm knot was found in the patient’s vulva, and the patient underwent an operation.
Menstruation history: The patient began menarche in September 2009 and menstruated in September, October and November before ceasing menstruation.
Family history: The patient’s grandfather has stomach cancer, her grandmother and grandfather have high blood pressure and cerebral infarction, and her grandmother has diabetes.
Physical examination revealed signs typical of Cushing syndrome, including “moon face”, hirsutism, and purple striae on the trunk and legs. The patient’s body temperature was 36.8°C, and her pulse rate was 89/minute. Her blood pressure was 160/100 mmHg (right arm) and 165/110 mmHg (left arm). The patient weighed 58 kg and was 164 cm tall, with a BMI of 21.6 kg/m2 and a waistline measuring 85.5 cm. The patient exhibited thin skin, a full moon face and buffalo hump, upper clavicular fat, and bilateral purple striae on her abdomen and medial leg. Auscultation of the heart and lungs was unremarkable. Both breasts were Tanner Stage IV; the mammary areola color was partially black. The heart and lung examination was normal. Pubic hair suggested Tanner Stage III, and the clitoris was normal. The left labia majora was slightly enlarged, and a 0.5-cm knot could be palpated with good mobility and without pain. The lower limbs were not swollen.
Routine urinalysis was normal. The laboratory values obtained at the hospital are listed in Table 1, and the endocrine values are listed in Table 2. Tumor markers, such as AFP and CEA, were negative. An electrocardiogram revealed depressed ST on II, III, AVF, and V4-V6. Abdominal ultrasound revealed fatty liver and the possibility of a small stone calculus in the right kidney. . Both kidney arteries appeared normal.
Arterial blood pH
7.514
Arterial blood pO2 (mmHg)
48.8
Arterial blood pCO2 (mmHg)
46.1
HCO3- (mEq/l)
36.9
White blood cell count (cells/mmc)
9.29
Hb (g/l)
124
Glucose (mmol/l)
7.3
Sodium (mmol/l)
150
Potassium (mmol/l)
2.1
Chloride (mEq/l)
92
Creatinine (µmol/l)
53
Urea nitrogen (mmol/l)
5.53
ALT (U/L)
51
AST (U/L)
29
Serum total bilirubin (µmol/L)
19.2
Unconjugated bilirubin (µmol/L)
5.6
Urine Ca (mmol/d)
7.09
Urine P (mmol/d)
11.55
Table 1: Laboratory values at hospital admission.
Values
Reference range
GH (ng/ml)
0.1
<2.0
IGF-1 (ng /ml )
179
183-850
0800 h cortisol (µg/dl)
53.87
4-22.3
ACTH (pg/ml)
218
0-46
Urinary-free cortisol (µg/24 h)
2707
3294
12.3-103.5
LDDST (2nd day)
2810
HDDST (2nd day)
1983
FT3 (pg/ml)
2.02
1.8-4.1
FT4 (ng/dl)
1.36
0.81-1.89
T3 (ng/ml)
0.76
0.66-1.92
T4 (µg/dl)
6
4.4-12.5
TSH (uiu/ml)
0.1
0.38-4.34
FSH (mIu/ml)
0.0
0-20.3
LH (mIu/ml)
0.0
0-11.1
E2 (pg/ml)
28.3
19.9-47.9
T (ng/dl)
57.2
358-1217
PRL (ng/ml)
4.47
21-11.7
Table 2: Endocrine laboratory values.
The diagnosis and localization of Cushing’s disease: The overnight dexamethasone suppression test (DST) revealed unchanged blood F prior to and after dexamethasone (53.87 vs. 55.99 μg/dl). ACTH was 218 and 235 pg/ml prior to and after dexamethasone, respectively. Twenty-four-hour UFC was 2707 and 3294 μg/d. The urinary excretion of cortisol was 2810 μg/d on the second day after low-dose DST and 1983 μg/d on the second day after high-dose DST.
The pituitary MRI did not reveal any abnormalities. A chest CT revealed multiple lesions, possibly metastatic tumors, in both lungs, enlarged lymph nodes in the mediastinum and the hilum, and an enlarged heart. The lumbar vertebrae were normal on CT.
Results
The abdominal MRI suggested a tumor in the left perineum. PETCT confirmed the presence of a tumor in the left perineum (standard uptake value, SUV: 8) (Figure1) and multiple lesions in both lungs (SUV: 7). Percutaneous pneumocentesis failed to reveal the cell type/ source because of the limited tissue sample. A biopsy of the mass in the labia revealed a primitive neuroectodermal tumor (PNET). Material from the biopsy that was also examined suing immunohistochemistry revealed strong reactivity for CRH (Abcam ab8901), synaptophysin, and CD99, whereas immunostains were negative for ACTH (Dako, Figure 2). Ki-67 staining was approximately 40%. A simple explanation for the lack of ACTH immunostaining is that ACTH was not expressed in the tumor cells. Ectopic CRH syndrome was confirmed. We did not measure the plasma CRH, which is elevated in cases of ectopic CRH secretion, as the means to do so was not available at our hospital.
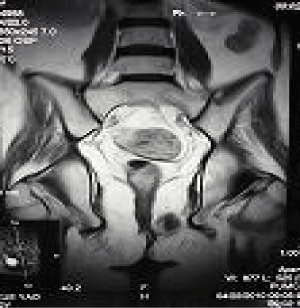
Figure 1: A tumor in the left vagina.
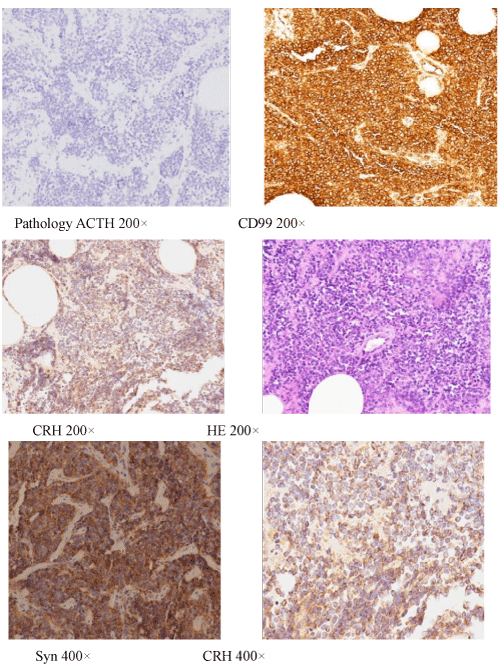
Figure 2: Immunohistochemistry of biopsy samples from perineal tumors. The cells stained positively for synaptophysin and CD-99. The cells stained negatively for ACTH but were strongly positive for CRH.
The patient received anti-hypertensives and insulin to control blood pressure and glucose. VDCA/IE chemotherapy was planned, but the patient decided to transfer to a local hospital closer to her home for treatment. She never returned. Several months later, she died of respiratory failure.
Discussion
Chronic exposure to excess endogenous glucocorticoids in adults, children, and adolescents with Cushing syndrome (CS) is associated with detrimental health effects, including truncal obesity, hypertension, insulin resistance, hyperglycemia, impaired wound healing, hypercoagulability, osteoporosis, and gonadal dysfunction. In children, chronic exposure to excess endogenous glucocorticoids is also associated with the suppression of linear growth [3]. It is very important to accurately diagnose and treat Cushing syndrome to improve the quality of life of affected individuals.
The diagnosis of Cushing’s syndrome typically includes serum cortisol measurements, low-dose DST, and the urinary excretion of free cortisol. UFC excretion after 2 days of low-dose DST is the most accurate test for the diagnosis of CS; excretion of UFC after 2 days of low-dose DST is more sensitive and specific as a screening tool than testing serum cortisol at the same point [4]. In the current case, 24-h UFC was increased without repression with low-dose DST treatment, and circadian variations of serum cortisol were not observed. ACTH was elevated. A diagnosis of ACTH-dependent Cushing’s syndrome was therefore established. In this case, high-dose DST was not repressed, and the pituitary MRI was normal. Ectopic Cushing’s syndrome was therefore diagnosed. Inferior petrosal sinus sampling (IPSS), a procedure typically used to distinguish pituitary Cushing’s disease from ectopic ACTH syndrome with occult origins [5], was not conducted. A chest X-ray suggested metastatic tumors in both lungs. The lesions were confirmed by chest CT. Enhanced MRI examination of the abdomen revealed a tumor in the left perineum. Several methods of diagnosis were discussed. First, a percutaneous lung biopsy could be performed after the patient’s blood pressure was controlled and infection was prevented, but the biopsy tissue was insufficient to identify the cell type. A biopsy with thoracoscopy was not attempted because the patient faced the significant risk of lung infection and bronchopleural fistula. Second, biopsy tissue could be taken from the perineum, but whether this tumor was the source of the Cushing’s syndrome was uncertain. Because of this tumor’s specific location, infection was likely. Third, it was possible to remove the adrenal glands to alleviate the hypercortisol blood syndrome and reduce the chance of infection. However, it was not known whether the patient could tolerate the operation and whether a biopsy was possible. Furthermore, if the tumor were malignant, it would be necessary to take a biopsy immediately to avoid delaying her chemotherapy. After consultation with several departments, including the departments of thoracic surgery, urinary surgery, infection and the department of obstetrics and gynecology, we decided to biopsy the mass in the labial perineum, before which we gave the patient meropenem to prevent infection. Before the operation, a PET-CT revealed a tumor in the left portion of the vagina and multiple tumors in the lungs. On April 29th, 2010, the patient underwent a biopsy operation of the perineum tumor under intravenous anesthesia. Approximately 15 grams of tissue were taken, and pathology revealed a small-cell malignant tumor diagnosed as PNET. Immunohistochemistry indicated strong reactivity for CRH, whereas immunostaining was negative for ACTH.
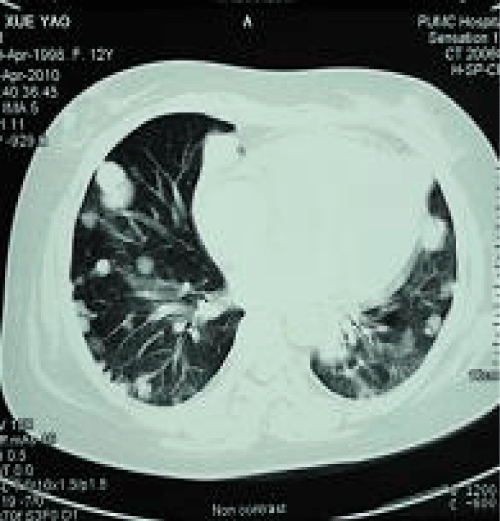
Figure 3: Multiple nodes were found in the lung.
The association between Cushing’s syndrome and cancer first became evident in 1928 [6]. More than three decades later, certain non-pituitary neoplasms, mostly of neuroendocrine origin [7], were discovered to induce ACTH-dependent Cushing’s syndrome [8]. An extensive search of the literature revealed isolated CRH in 21 cases [9-19]. Medullary thyroid carcinoma (33%) and pheochromocytoma (19%) are the most prevalent cancers among the cases of isolated ectopic CRH. Carcinoid (5%) and small-cell lung carcinoma (9.5%) are less common in ectopic CRH, unlike in ectopic ACTH cases, for which those cancers are the most prevalent causes [20]. For ectopic ACTH syndrome, the primary tumor could not be identified in approximately 12.5% of the patients 10 years after the first visit [21]. The rate of infection in ectopic ACTH is estimated to be 50% [2]. Our patient exhibited multiple infections, including an upper respiratory infection and PTCA wound infection, a boil around the anus, and a wound in the perineum. The infection was controlled with aggressive anti-infection treatment. To summarize, the successful diagnosis of this patient required interdepartmental cooperation, PET-CT to determine the fixed position diagnosis, and proper antibiotic therapy to manage infections during the diagnostic procedures. This case illustrates the importance of considering the ectopic production of CRH in the differential diagnosis of presentations of ACTHdependent Cushing’s syndrome. To the best of our knowledge, there has been no report of ectopic CRH syndrome caused by PNET in children.
Funding: financial support: National Natural Science Foundation of China (81170736), National Key
Program of Clinical Science.
References
- Vassiliadi D, Tsagarakis S. Unusual causes of Cushing's syndrome. See comment in PubMed Commons below Arq Bras Endocrinol Metabol. 2007; 51: 1245-1252.
- Ilias I, Torpy DJ, Pacak K, Mullen N, Wesley RA, Nieman LK. Cushing's syndrome due to ectopic corticotropin secretion: twenty years' experience at the National Institutes of Health. See comment in PubMed Commons below J Clin Endocrinol Metab. 2005; 90: 4955-4962.
- Keil MF, Merke DP, Gandhi R, Wiggs EA, Obunse K, Stratakis CA. Quality of life in children and adolescents 1-year after cure of Cushing syndrome: a prospective study. See comment in PubMed Commons below Clin Endocrinol (Oxf). 2009; 71: 326-333.
- Batista DL, Courcoutsakis N, Riar J, Keil MF, Stratakis CA. Severe obesity confounds the interpretation of low-dose dexamethasone test combined with the administration of ovine corticotrophin-releasing hormone in childhood Cushing syndrome. See comment in PubMed Commons below J Clin Endocrinol Metab. 2008; 93: 4323-4330.
- Graham KE, Samuels MH, Nesbit GM, Cook DM, O'Neill OR, Barnwell SL. Cavernous sinus sampling is highly accurate in distinguishing Cushing's disease from the ectopic adrenocorticotropin syndrome and in predicting intrapituitary tumor location. See comment in PubMed Commons below J Clin Endocrinol Metab. 1999; 84: 1602-1610.
- Becker M, Aron DC. Ectopic ACTH syndrome and CRH-mediated Cushing's syndrome. See comment in PubMed Commons below Endocrinol Metab Clin North Am. 1994; 23: 585-606.
- Odell WD. Ectopic ACTH secretion. A misnomer. See comment in PubMed Commons below Endocrinol Metab Clin North Am. 1991; 20: 371-379.
- Vrezas I, Willenberg HS, Mansmann G, Hiroi N, Fritzen R, Bornstein SR. Ectopic adrenocorticotropin (ACTH) and corticotropin-releasing hormone (CRH) production in the adrenal gland: basic and clinical aspects. See comment in PubMed Commons below Microsc Res Tech. 2003; 61: 308-314.
- Saeger W, Reincke M, Scholz GH, Lüdecke DK. [Ectopic ACTH- or CRH-secreting tumors in Cushing's syndrome]. See comment in PubMed Commons below Zentralbl Pathol. 1993; 139: 157-163.
- Oates SK, Roth SI, Molitch ME 2000 Corticotropin-releasing hormone-producing medullary thyroid carcinoma causing Cushing’s syndrome: clinical and pathological findings. Endocr Pathol 11: 277–285
- Wajchenberg BL, Mendonça B, Liberman B, Adelaide M, Pereira A, Kirschner MA. Ectopic ACTH syndrome. See comment in PubMed Commons below J Steroid Biochem Mol Biol. 1995; 53: 139-151.
- Eng PH, Tan LH, Wong KS, Cheng CW, Fok AC, Khoo DH. Cushing's syndrome in a patient with a corticotropin-releasing hormone-producing pheochromocytoma. See comment in PubMed Commons below Endocr Pract. 1999; 5: 84-87.
- Jessop DS, Cunnah D, Millar JG, Neville E, Coates P, Doniach I, et al. A phaeochromocytoma presenting with Cushing's syndrome associated with increased concentrations of circulating corticotrophin-releasing factor. J Endocrinol. 1987; 113: 133-138.
- Chrisoulidou A, Pazaitou-Panayiotou K, Georgiou E, Boudina M, Kontogeorgos G, Iakovou I, et al. Ectopic Cushing's syndrome due to CRH secreting liver metastasis in a patient with medullary thyroid carcinoma. Hormones (Athens). 2008; 7: 259-262.
- Bayraktar F, Kebapcilar L, Kocdor MA, Asa SL, Yesil S, Canda S. Cushing's syndrome due to ectopic CRH secretion by adrenal pheochromocytoma accompanied by renal infarction. See comment in PubMed Commons below Exp Clin Endocrinol Diabetes. 2006; 114: 444-447.
- Kristiansen MT, Rasmussen LM, Olsen N, Asa SL, Jørgensen JO. Ectopic ACTH syndrome: discrepancy between somatostatin receptor status in vivo and ex vivo, and between immunostaining and gene transcription for POMC and CRH. See comment in PubMed Commons below Horm Res. 2002; 57: 200-204.
- Parenti G, Nassi R, Silvestri S, Bianchi S, Valeri A, Manca G. Multi-step approach in a complex case of Cushing's syndrome and medullary thyroid carcinoma. See comment in PubMed Commons below J Endocrinol Invest. 2006; 29: 177-181.
- Ruggeri RM, Ferraù F, Campennì A, Simone A, Barresi V, Giuffrè G, et al. Immunohistochemical localization and functional characterization of somatostatin receptor subtypes in a corticotropin releasing hormone- secreting adrenal phaeochromocytoma: review of the literature and report of a case. Eur J Histochem. 2009; 53: 1-6.
- Auchus RJ, Mastorakos G, Friedman TC, Chrousos GP. Corticotropin-releasing hormone production by a small cell carcinoma in a patient with ACTH-dependent Cushing's syndrome. See comment in PubMed Commons below J Endocrinol Invest. 1994; 17: 447-452.
- Shahani S, Nudelman RJ, Nalini R, Kim HS, Samson SL. Ectopic corticotropin-releasing hormone (CRH) syndrome from metastatic small cell carcinoma: a case report and review of the literature. See comment in PubMed Commons below Diagn Pathol. 2010; 5: 56.
- Isidori AM, Lenzi A. Ectopic ACTH syndrome. See comment in PubMed Commons below Arq Bras Endocrinol Metabol. 2007; 51: 1217-1225.