
Case Report
J Endocr Disord. 2014;1(1): 1004.
Intravascular Volemic Assessment of Elderly Hyponatremic Patient Using Non-Invasively Cardiac Echograms
Kyuzi Kamoi*
Center of Diabetes, Niigata Rosai Hospital, Japan
*Corresponding author: Kyuzi Kamoi, Center of Diabetes & Endocrinology Metabolism, Niigata Rosai Hospital, Joetsu, Niigata 942-8502, Japan
Received: Aug 06, 2014; Accepted: Aug 17, 2014; Published: Aug 21, 2014
Abstract
Background: Hyponatremia is a serious, but often overlooked disorder with an increasing incidence in developed countries. In 2007, Expert Panel Recommendations defined strategies for clinicians caring for patients with hyponatremia. Hyponatremia has two types, pseudo and true. The true hyponatremia is due to many causes. The main causes are hypovolemia, euvolemia and hypervolemia. They may reflex the status of intravascular volume. However, it is difficult to grasp the different causes before treatment in patients with true hyponatremia, because that it is difficult to determine status of intravascular volume.
Methods: We examined the usefulness of the Central Venous Pressure index (CVP Index) using cardiac echograms with popular to determine the status of intravascular volume before and after treatments in various patients.
Results: In preliminary result, the CVP index was lower in patients with hypovolemia before treatment than after treatment, whereas it was higher in patients with hypervolemia before treatment than after treatment.
Conclusion: These findings indicate that the CVP Index using noninvasively and popularly cardiac echogram may reflect intravascular volume status in patients with true hyponatremia.
Keywords: Hyponatremia; CVP index; CSWS; Drug-induced hyponatremia; SIADH
Abbreviations
p-Ald: Plasma Levels Of Aldosterone; p-ANP: Atrial Natriuretic Peptide; p-AVP: Plasma Levels of Antidiuretic Hormone; BIA: Bioelectrical Impedance Analysis; BMI: Body Mass Index; p-BNP: Plasma Levels of Brain Natriuretic Peptide; BW: Body Weight; CDI: Central Diabetes Insipidus; CHF: Chronic Heart Failure; CSWS: Cerebral Sodium Wasting Syndrome; CVP: Central Venous Pressure; DIH: Drug-Induced Hyponatremia; Hct: Blood Hematocrit; IVC: Inferior Vena Cava; MRHE: Mineralocorticoid-Responsive Hyponatremia of the Elderly; PP: Primary Polydipsia; RA: Right Atrium; p-Renin: Plasma Levels of Renin; RV: Right Ventricle; SIAD: Syndrome of Inappropriate Antidiuresis; SIADH: Syndrome Of Inappropriate Antidiuretic Hormone; TBW: Total Body Water; s-UA: Serum Uric Acid
Introduction
An incidence of hyponatremia has been increasing in developed countries, particularly intense medical facilities, although the accurate time and cause are unclear [1,2]. In Japanese hospitals, the incidence of patients with hyponatremia from August 2013 to February 2014 also was shown to be higher in intense medical facilities (30%) than in general medical facilities (13-17%). Hyponatremia is a serious, but often overlooked disorder. Untreated acute hyponatremia can cause substantial morbidity and mortality as a result of osmotically induced cerebral edema [1-3], and excessively rapid correction of chronic hyponatremia can cause severe neurologic impairment and death as a result of osmotic demyelination. The diverse etiologies and comorbidities associated with hyponatremia pose substantial challenges in managing this disorder. In 2007, a panel of experts in hyponatremia convened to develop the Hyponatremia Treatment Guidelines 2007 [3]. The Expert Panel Recommendations defined strategies for clinicians caring for patients with hyponatremia [3]. The pathophysiology of hyponatremia is complex. Hyponatremia is mainly divided into two types, pseudo and true. Therefore, measurement of plasma osmolality is essential [4] as the Hyponatremia Treatment Guidelines [3]. When there is normal or high plasma osmolality, the patient has pseudo type, whereas when there is low plasma osmolality, the patient has true type. The true hyponatremia is divided into three causes based on the status of total body water (TBW) [3,4]. The hypovolemia is mainly due to Cerebral Sodium Wasting Syndrome with brain disease (CSWS) reported by Peters et al in 1950 [5]. Furthermore, in 2009, Ishikawa et al. proposed that there were true hyponatremic patients without brain diseases, who were referred to as Mineral Corticoid-Responsive Hyponatremia of the Elderly (MRHE) [6]. Further, some cases have Drug-Induced Hyponatremia (DIH) [7]. The euvolemia is mainly due to Syndrome of Inappropriate Antidiuretic Hormone (SIADH) [8] or Syndrome of Inappropriate Antidiuresis (SIAD) [9]. The hypervolemia is mainly due to cardiac, hepatic and renal failures [10]. The pathophysiology of true hyponatremia should be understood before treatment, but the exact cause cannot be easily determined by any specific variables before treatment. Skin turgor can be used to differentiate states of intravascular volume, but is not able to differentiate the cause in elderly patients with true hyponatremia [11]. Plasma levels of Antidiuretic hormone (p-AVP) have been shown to be high in all patients with the true hyponatremia [12]. Therefore, p-AVP measurements cannot be used to differentiate the path physiologies of true hyponatremia. Similarly, other variables, including blood Hematocrit (Hct), Body Weight (BW), Serum Uric Acid (s-UA) and plasma concentrations of Renin (p-Renin) and Aldosterone (p-Ald) [13] are not suitable for differentiating the causes of the true hyponatremia [14]. Levels of Atria Natriuretic Peptide (p-ANP) related to plasma volume [12]; however, vary in patients with the true hyponatremia [11]. In general, patients with SIADH or SIAD have normal skin turgor [11] and low levels of Hct, p-Renin, and p-Ald, whereas they have high values of BW and p-AVP before treatment [9]. However, as some patients with SIADH have malignancy, Hct may be decreased [14]. Low levels of s-UA in patients with CSWS or MHRE before treatment continue after treatment, whereas patients with SIADH have normal s-UA levels after being recovering from true hyponatremia. Therefore, s-UA level may be able to differentiate CSWS or MHRE from SIADH, but the difference is based on the recovered hyponatremia after treatment [11,15]. Therefore, no variables measured seem adequate to differentiate the various path physiologies of true hyponatremia before treatment [14]. We examined studies whether when patients with the true hyponatremia had serum sodium levels greater than128 mEq/L for at least for 1 week, the water loading test (20 ml/kg for 4 hours) is able to differentiate SIADH or MRHE and CSWS. In a result, serum sodium levels greater than 128 mEq/L continue for at least 1 week, it is safe to perform the water loading test [13], although conventionally, the water loading test should not be performed in patients with true hyponatremia. Urine excretion after 4 hours of the water loading test was shown to be lower in patients with SIADH or MRHE than in control patients, indicating that patients have antidiuresis [10,12]; findings in patients with CSWS were similar to control subjects [13]. Levels of s-UA were shown to be lower in some patients with CSWS or MRHE, whereas in patients with DIH, they were similar to controls [11], despite the presence of depleted plasma volume [11]. Again, however, no test was able to differentiate all causes of true hyponatremia. Todays, the cause of true hyponatremia with brain disease may be mainly divided into two types. Wijdicks et al. in 1985 [16] and others [17-19] reported that most patients with brain diseases had true hyponatremia owing to CSWS [3], whereas, recently, Hannon et al. [20] reported that most patients with brain diseases showed true hyponatremia owing to SIADH [8] or SIAD [9], and did not represent CSWS [3]. As discussed, standard physical and laboratory findings cannot differentiate the causes of true hyponatremia. It may be due to have difficulty of grasping the state of intravascular volume before treatment, although the different pathophysiology is due to the status of TBW.
The Central Venous Pressure (CVP) with an invasive procedure reported by Damarju et al, in 1997 [21] is useful to grasp the state of intravascular volume before treatment. Further, Hoyle et al. [22] reported that non-invasive Bioelectrical Impedance Analysis (BIA) may be possible volemic assessment of TBW in the elderly hyponatraemic patient, although this has small numbers of patients and the BIA is not used in general hospital and not popular in Japan.
We researched whether status of intravascular using cardiac echograms, a non-invasive test, could be used to differentiate the pathophysiology in patients with true hyponatremia before treatment [23]. This paper reports on the preliminary and incomplete results from a single study using cardiac echograms in patients with the different TBW.
Methods
Study Design
This is an observational study with assessment of intravascular volume before and after treatment in patients with true hyponatremia. We examined whether non-invasive cardiac echograms could be used to determine the status of intravascular volume [23]. The method used for cardiac echograms was originally based on a previous report [24]. The apparatus in the original report by Marceline et al. was using the Aloka SSD 2200 (Hitachi Aloka Medical, Ltd, Lisbon, Portugal), but this study used Yokogawa VIVID-7, LOGIQ S 6 (Yokogawa Electronics, Tokyo, Japan), in which cardiac echogram is used in general hospital. Since it was reported that the status of intravascular volume was highly related to the value of central venous pressure using Aloka SSD 2200 [24], the value calculated from the variable using cardiac echogram was represented as Central Venous Pressure index (CVP index) [24]. CVP index = (tricuspid E deceleration) x 0.11 + (RV/RA gradient) x 0.16 - (variation in IVC), E; the E wave of tricuspid inflow, RV; right ventricle, RA; right atrium, IVC; inferior vena cava was calculated by the formula as previously described [24]. Our technicians tried to avoid the changes of respiration phases and respiration movement. They measured the area across the intravenous cava during deep breathing using the M mode at a position separated from the right atrium by 2 cm. The position to measure the CVP Index was unified at the end of expiration under an angle of less than 30 degrees.
We also examined confounding factors, including gender, age, height, Body Mass Index (BMI), plasma concentrations of sodium, osmolality, and creatinine, arterial hypertension, p-Ald, p-ANP, p-AVP, p-renin, and Plasma Levels of Brain Natriuretic Peptide (p-BNP), ventricular hypertrophy owing to the echocardiography and urine concentrations of sodium, potassium, chloride, osmolality, uric acid, and creatinine in patients with various TBW.
This study was performed in accordance with the Declaration of Helsinki and with the approval of our hospital ethics committees, and the trial was registered on ClinialTrials.gov (No. NCT 01568125).
Methods
Results were expressed as means ± SD. Differences between mean of basal clinical variables before and after treatment in each group evaluated statistically by chi square or unpaired t tests with or without Welch’s correction, if numbers of sample were more than 2.
Results
Clinical characteristics of patients with various TBW before and after treatment are shown in Table 1 [23]. This CVP index was measured in 5 patients with DIH, 4 with SIADH, 1 with CSWS, 1 with Primary Polydipsia (PP), 1 with Chronic Heart Failure (CHF), and 1 with Central Diabetes Insipidus (CDI). There was no patient with MRHE.
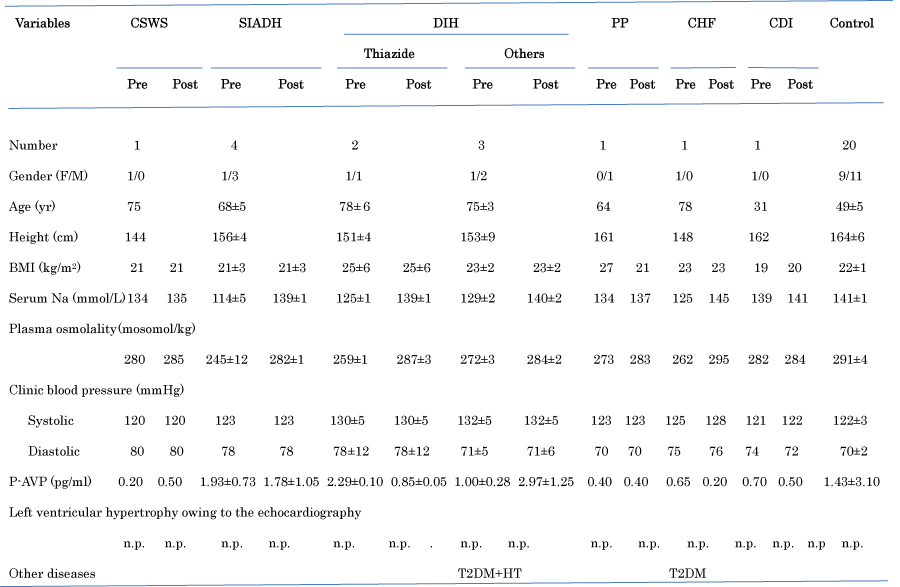
Figure 1: Clinical characteristics of patients with hypovolemia, euvolemia, or hypervolemia before and after treatment.
As shown in Table 1, patients with depleted TBW (n = 5; 66 ± 6 years), with euvolemia with TBW (n= 4, 68 ± 5 years) and with increased TBW (n = 4; 75 ± 6 years) were significantly (P = 0.0001) older than controls (n = 20; 49 ± 5 years), respectively. There were no significant differences in BMI in patients with various TBW except the patient with PP. BMI in the patient with PP after treatment with water restriction was decreased. Plasma sodium and osmolality concentration in patients after treatments recovered to be normal, but, p-AVP did not significantly change after treatments. CVP index based on the original report [24] showed a positively high correlation of values in measured CVP [24]. There was no left ventricular hypertrophy owing to the echocardiography in patients participated, which influenced CVP Index. The original report represented CVP index as indices of Vena Cava Inferior (VCI) [24]. CVP index using this echograms in patients with hypovolemia before treatment was increased after treatment with fludrocortisones or 1-deamino- 8-d-arginine vasopressin, and after stopping treatment with spironolactone A, angiotensin- converting enzymes, and angiotensin receptor blockers, whereas CVP index in patients with euvolemia or hypervolemia before treatment was decreased after treatment with water restriction or V2 receptor antagonist (Table 2). The patient with CSWS was treated with 15g of NaCl and water (20 g/kg body weight) following administration of fludrocortisones, the patients with SIADH were treated with administration of demethylchlortetracycline combined with water restriction, the patients with DIH were treated with discontinuation of administration as anti-hypertensive drugs in thiazide, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, calcium channel blocker, or spironolactone, the patent with PP was treated with water restriction in intake, the patient with CHF was treated with infusion of ANP (Carperitide®) and the patient with CDI was treated with administration of 1-deamino-8- d-arginine vasopressin. There was no alteration of arterial blood pressure before and after examination of the echocardiography in all patients
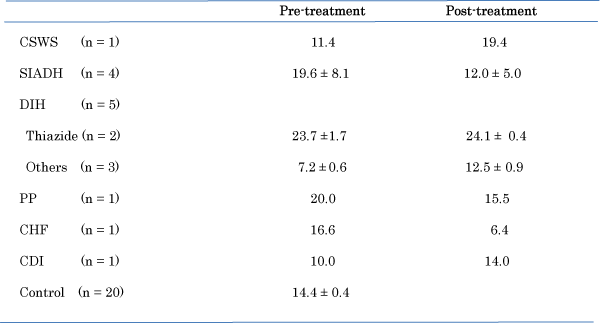
Figure 2: Changes in central venous pressure index, as measured by the cardiac Yokogawa VIVID-7 apparatus in patients with hypovolemia, euvolemia, or hypervolemia before and after treatment.
Discussion
This is a preliminary and short paper. The pathophysiology of true hyponatremia is due to the status of TBW rather than intravascular volume. But, the status of intravascular volume before treatment may be important to grasp the pathophysiology of true hyponatremia as report of Hoyle et al. [22]. Namely, assessment of intravascular volume of the patient is, therefore, a crucial step in many diagnostic algorithms to determine cause of true hyponatraemia. Although this result is preliminary, CVP index using cardiac echogram may be related to status of intravascular volume. Patients with thiazide induced-hyponatremia had a high CVP index before and after treatment. Thiazide induced-hyponatremia has been shown to be represented as euvolemia or hypervolemia [25]. The result is supported this view. However, the values found in this study are higher than the values reported by Damaraju et al. [21]. They showed CVP levels of less than 5.0 cm H2O in patients with hypovolemia, 5-11 cm H2O in control subjects with euvolemia, and greater than 11 cm H2O in patients with hypervolemia. The apparatus in the original report by Marcelino et al. (Figure 1) [24] was using the Aloka SSD 2200, but this study used Yokogawa VIVID-7. The different findings may be due to the use of different apparatus, although the apparatus of cardiac echograms is used in general hospital and is popular. Unfortunately, we were not able to measure the CVP value in each patient directly, and the status of TBW was not determined by another method, for example as the method of BIA [22]. It is difficult to measure the CVP index using the ultrasonic echocardiography in some persons accurately. In particular, a left ventricular hypertrophy may provoke left ventricular outflow tract gradient, which is influenced CVP Index. Further, there was no patient with tricuspid regurgitation or pulmonary hypertension. If the problems are solved, this non-invasive method using cardiac echograms may be a good way to reflect status of intravascular volumes. The apparatus using cardiac echogram is not invasive technique and popular with simple method, whereas CVP measured directly is invasive method and BIA method is not popular.
Although the pathophysiology of true hyponatremia is due to the state of TBW rather than intravascular volume, status of intravascular volume using cardiac echogram as report of Hoyle et al. [22] with associated with water loading test may be helpful to grasp the pathophysiology of true hyponatremia.
Limitations
As because that the number of participants was small, age of control group was low with non-comparability, the analytical method examined confounding factors was not able to determine the values of clinical characteristics and CVP index in patient with some different plasma volume using statistical method was questionable, and also, as this method of using a cardiac echogram to assess intravascular volume has not become well established and its reproducibility may be is problematical, some researchers may question the reliability of our findings.
Conclusion
It is difficult to determine status of intravascular volume in patients with true hyponatremia before treatment. However, if the CVP index using cardiac echogram may be shown to be reliable, it can offer a non-invasive, simple and popular method to determine whether true hyponatremia before treatment is associated with different status of intravascular volume.
Acknowledgment
I am grateful to the clinical laboratory technicians of the Nagaoka Red Cross Hospital (Mr. Y. Toju and Ms. T. Maruyama), the Ojiya General Hospital (Ms. M. Hoshino and Mr. K. Kurosaki), and the Mitsuke Hospital (Mr. M. Makamura) for their valuable technical assistance.
References
- Baran D, Hutchinson TA. The outcome of hyponatremia in a general hospital population. Clin Nephrol. 1984; 22: 72-76.
- Upadhyay A, Jaber BL, Madias NE. Incidence and prevalence of hyponatremia. Am J Med. 2006; 119: 30-35.
- Verbalis JG, Goldsmith SR, Greenberg A, Korzelius C, Schrier RW, Sterns RH, et al. Diagnosis, evaluation, and treatment of hyponatremia: expert panel recommendations. Am J Med. 2013; 126: 1-42.
- Robertson, GL. Posterior pituitary. Felig P, Baxter JD, Frohman LA. editors. In: Endocrinology and Metabolism. 3rd ed. New York, McGraw-Hill Inc. 1995; 385-432.
- peters Jp, Welt Lg, Sims Ea, Orloff J, Needham J. A salt-wasting syndrome associated with cerebral disease. See comment in PubMed Commons below Trans Assoc Am Physicians. 1950; 63: 57-64.
- Ishikawa S, Saito T, Fukagawa A, Higashiyama M, Nakamura T, Kusaka I, et al. Close association of urinary excretion of aquaporin-2 with appropriate and inappropriate arginine vasopressin-dependent antdiuresis in hyponatremia in elderly subjects. J Clin Endocrinol Metab. 2001; 86: 1665-1671.
- Sharabi Y, Illan R, Kamari Y, Cohen H, Nadler M, Messerli FH, et al. Diuretic induced hyponatraemia in elderly hypertensive women. J Hum Hypertens. 2002; 16: 631-635.
- Bartter FC, Schwartz WB. The syndrome of inappropriate secretion of antidiuretic hormone. Am J Med. 1967; 42: 790-806.
- Robertson, GL. Athar, S.; Shelton, RL. Osmotic control of vasopressin function. Andreoli E, Granthan JJ, Rectar FC, Jr. editors. In Disturbance in body fluid osmolality. American Physiological Society, Bethesda, Maryland, Waverly Press Inc. 1977; 125-148.
- Noreen FR, Robert W, Schrier RW. Hyponatremic states. Maxwell M, Kleeman CR, Narins RG. editors. In Clinical Disorders if Fluid and Electrolyte Metabolism. 4th ed. Tokyo, McGraw-Hill Inc. 1987; 461-480.
- Kamoi K. SIADH. Today Diagnosis. In Kanazawa I, Nagai R, eds. 6th ed. Tokyo, Igaku-Shoin. March 2010; 1160-1162.
- Kamoi K, Sato F, Arai O, Ishibashi M, Yamaji T. Effects of plasma volume and osmolality on secretion of atrial natriuretic peptide and vasopressin in man. Acta Endocrinol (Copenh). 1988; 118: 51-58.
- Zadik Z, Kowarski AA. Normal integrated concentration of aldosterone and plasma renin activity: effect of age. J Clin Endocrinol Metab. 1980; 50: 867-869.
- Kamoi K, Ebe T, Kobayashi O, Ishida M, Sato F, Arai O, et al. Atrial natriuretic peptide in patients with the syndrome of inappropriate antidiuretic hormone secretion and with diabetes insipidus. J Clin Endocrinol Metab. 1990; 70: 1385-1390.
- Kamoi K, Toyama M, Ishibashi M, Yamaji T. Hyponatremia and osmoregulation of vasopressin secretion in patients with intracranial bleeding. J Clin Endocrinol Metab. 1995; 80: 2906-2911.
- Wijdicks EF, Vermeulen M, Hijdra A, van Gijn J. Hyponatremia and cerebral infarction in patients with ruptured intracranial aneurysms: is fluid restriction harmful? Ann Neurol. 1985; 17: 137-140.
- Lolin Y, Jackowski A. Hyponatraemia in neurosurgical patients: diagnosis using derived parameters of sodium and water homeostasis. Br J Neurosurg. 1992; 6: 457-466.
- Sherlock M, O'Sullivan E, Agha A, Behan LA, Rawluk D, Brennan P, et al. The incidence and pathophysiology of hyponatraemia after subarachnoid haemorrhage. Clin Endocrinol (Oxf). 2006; 64: 250-254.
- Hardesty DA, Kilbaugh TJ, Storm PB. Cerebral salt wasting syndrome in post-operative pediatric brain tumor patients. Neurocrit Care. 2012; 17: 382-387.
- Hannon MJ, Behan LA, O'Brien MM, Tormey W, Ball SG, Javadpour M, et al. Hyponatremia following mild/moderate subarachnoid hemorrhage is due to SIAD and glucocorticoid deficiency and not cerebral salt wasting. J Clin Endocrinol Metab. 2014; 99: 291-298.
- Damaraju SC, Rajshekhar V, Chandy MJ. Validation study of a central venous pressure-based protocol for the management of neurosurgical patients with hyponatremia and natriuresis. Neurosurgery. 1997; 40: 312-316.
- Hoyle GE, Chua M, Soiza RL. Volaemic assessment of the elderly hyponatraemic patient: reliability of clinical assessment and validation of bioelectrical impedance analysis. QJM. 2011; 104: 35-39.
- Kamoi K, Toju H, Maruyama C. Hyponatremia. J R C H. 2013; 1: 27-32 (In Japanese).
- Marcelino P, Fernandes AP, Marum S, Ribeiro JP. Non-invasive evaluation of central venous pressure by echocardiography. Rev Port Cardiol. 2002; 21: 125-133.
- Hwang KS, Kim GH. Thiazide-induced hyponatremia. Electrolyte Blood Press. 2010; 8: 51-57.