
Case Report
J Endocr Disord. 2014;1(1): 1005.
Androgens have Forgotten and Emerging Roles Outside of their Reproductive Functions, with Implications for Diseases and Disorders
D. Alwyn Dart*
1Department of Surgery & Cancer, Cardiff University – Peking University Cancer Institute, UK
*Corresponding author: D. Alwyn Dart, Department of Surgery & Cancer, Cardiff University – Peking University Cancer Institute, Heath Park, Cardiff CF14 4XN, UK
Received: Aug 01, 2014; Accepted: Aug 22, 2014; Published: Aug 23, 2014
Abstract
Cellular and physiological responses to the androgens are mediated by the Androgen Receptor (AR) - a member of the steroid nuclear receptor superfamily. The AR is a ligand-dependent transcription factor that modulates the expression of androgen target genes. The AR and androgens are mainly thought of as being ‘male’ hormones involved in the regulation of development of male reproductive tissues, anabolic effects and the development of male secondary sexual characteristics at puberty.
However, recent development of mouse reporter models for androgen activity has confirmed that androgens not only have a wide variety of target tissues in the body but also have a variety of targets in both sexes. In males the testes secrete testosterone from metabolic adrenal sourced androgens, and are relatively high. In the female the ovaries and some peripheral tissues secrete androgens at a low level but may fluctuate with the estrous cycle.
The androgen receptor is found to be expressed in many different tissues where its functions are relatively unknown. Given the fact that many domestic and agricultural animals are surgically gonadectomised they appear to be relatively healthy. However, the effects of androgens on non-reproductive organs are subtle and long lasting and may be hidden due to their complexity. Often the effects of androgen loss can only be seen in those undergoing hormone ablations e.g. for cancer therapies, or in the elderly where steroid hormone levels fall. This review reprises, re-evaluates and reminds us of the varied targets of androgens in the body, and how both sexes may be predisposed to certain diseases as a result.
Introduction
The Androgen Receptor (AR) is a member of the steroid nuclear receptor super family-ligand-dependent transcription factors that modulate the expression of their target genes. The AR is mainly involved in the regulation of development of male reproductive tissues, but has diverse functions within the female. The AR mediates the generation of sexually dimorphic characteristics in a wide variety of other body tissues, outside the obvious differences between the reproductive tissues.
The androgen receptor may function in two discrete pathways. The classical genomic response involves ligand binding, nuclear translocation and DNA binding with resultant recruitment of gene transcription machinery (see Figure 1). The second is a non-classical pathway in which the actions of androgens bound to Sex Hormone Binding Globulin (SHBG) leads to G-protein complex activation and intracellular signalling mediated via second messenger molecules (see Figure 2).
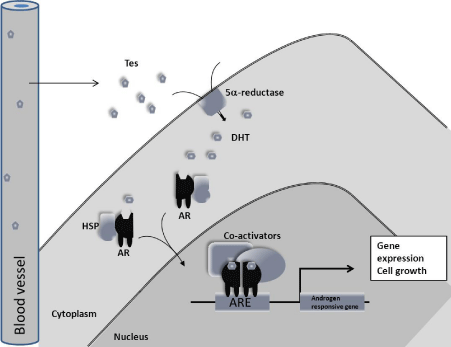
Figure 1: The classical genomic androgen receptor signalling pathway. Testosterone secreted from the testes circulates in the blood where it diffuses into androgen responsive cells. Testosterone may be converted to the more potent Dihydrotestosterone (DHT) by the actions of 5a-reductase enzymes. Testosterone then binds AR causing a dimerisation and translocation into the nucleus where it binds Androgen Response Elements (ARE) on the DNA. The AR then recruits cofactors and gene transcription machinery to activate androgen responsive genes.
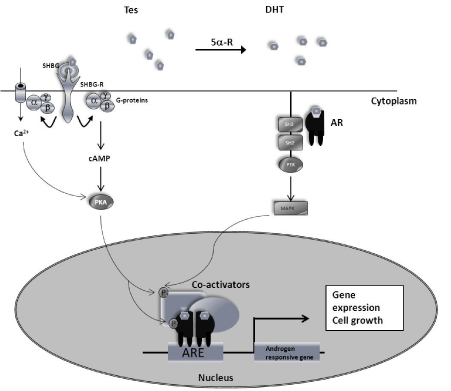
Figure 2: Androgen signalling via the non-classical pathway. Sex Hormone Binding Globulin (SHBG) may carry testosterone and bind to extracellular receptors leading to G-protein complex formation of indeed may bind directly to G-protein coupled receptors. This leads to stimulated intracellular signalling pathways involving second messenger molecules e.g. Ca2+, cAMP and the triggering of the MAP kinase cascade. Transcription factors, including AR, may be phosphorylated in response and trigger gene expression responses.
Recent development of steroid receptor reporter mice, e.g. estrogen receptor (ER) [1,2], AR [3,4] and Farnesoid Receptor (FXR) [5] showed that several tissues are unexpectedly hormonally responsive. To overcome tissue specificity of gene promoter-reporter fusions, novel reporter models are engineered utilising artificially constructed gene promoters- using pure hormone response elements coupled to a TATA box (or similar) for RNA polII binding [1,6]. Such simplified promoters exclude transcription factor cross-talk and confounding influences of enhancers. These promoters are pure assays for liganded activated transcription factors and have resulted in unanticipated results revealing tissues responding in a previously unknown manner.
In mammals the synthesis of sex steroids takes place in the gonads as well as in peripheral tissues such as adipose, prostate and breast tissues. This local synthesis (intracrine synthesis), occurs from adrenal gland derived pro-hormones - dehydroepiandrosterone (DHEA) and androstenedione - by steroidogenic enzymes leading to local synthesis of androgens or estrogens.
AR expression and function in tissues
Outside the realms of the gonadal tissues, many tissue-specific effects of androgens, are very subtle and overlooked. Androgens have strong effects on bone, immune system, CNS, adipose tissue, muscle mass, behavioural/brain, and the gut, but a complete loss of the AR pathway as seen in AR-knock out (ARKO) or testicular feminisation (Tfm) mouse, or indeed commonly found in surgically castrated agricultural and domestic animals leads to a relatively healthy organism with minimal side effects, albeit not reproducibly viable. This makes androgenic effects on the body difficult to study, as their systemic effects are not as profound as their gonadal effects. Only through long term, in depth, clinical studies that sex-linked differences in disease relative risk become clear (see Table 1). This reviewwill highlight these non-gonadal androgen-dependent tissues, and the role androgens have within them.
Disease
Male
Female
References
Brain
Autism
4-5
1
[106]
Parkinson’s
1.5
1
[107]
Alzheimer’s
1
1.6
[108]
Depression
1
2
[109]
Schizophrenia
1.42
1
[110]
Autoimmune
Sjogren syndrome
1
10
[111]
Glomerulonephritis
1
1.78
[112]
Rheumatoid arthritis
1
2-4
[113]
Scleroderma
1
3
[114]
Lupus
1
9
[115]
Cardiovascular
Asthma
3
1
[116]
Pulmonary fibrosis
1.7
1
[117]
Heart disease
1.5-2.5
1
[118]
Bone related
Osteoporosis
1
3
[119]
Craniosynostosis
3.3
1
[120]
Other diseases
Multiple scerlosis
1
3
[121]
Colon cancer
1.3
1
[122]
Keratoconus
1.7
1
[123]
Table 1: A simplified list of some human disease with an apparent gender risk bias, which may be linked to androgen levels or androgen receptor activity.
Non gonadal tissues
The Brain: Brain development due to its extreme complexity is the least well understood organ generally and in terms of steroid signalling. The brain shows sexual dimorphism, with differentiation influenced, during sensitive perinatal periods, by gonadal steroids.
In mammals, the default pattern is female. During gestation, testicular-derived androgens cause an irreversible differentiation of developing neuronal tissues to the ‘male pattern’ with specific neuronal morphology and defeminisation. A large proportion of male specific development is actually ER-mediated. Estrogens are formed locally in the prenatal and early postnatal rat brain, from testicular derived androgens, via cytochrome P-450 [7]. However, certain brain regions show AR expression, and therefore testosterone has effects, independent of its conversion to estrogen.
Androgens exposure early in development masculinises the brain, causing permanent physiological changes. Prenatal and neonatal testosterone surges masculinize neural circuitries leading to a sexually dimorphic behavioural pattern in males e.g. sex, aggression, and mating behaviour. According to the aromatisation theory, testosterone is converted to E2 which then acts via the ER to masculinise and defeminise the brain. Some brain areas are more susceptible to aromatisation and these areas adhere well to the theory e.g. the sexually dimorphic nucleus of the preoptic area and the anteroventral periventricular nucleus. Aromatisation does not account for all sexual differentiation in brain morphology and animal behaviour. Complete Androgen Insensitivity Syndrome (CAIS) in humans presents with dysfunctional AR and a feminine appearance, however, internalised testes secrete normal levels of testosterone [8]. Additionally, the brain in human CAIS develops along the female pattern. Human males with aromatase mutations present as male with a masculinised brain pattern, indicating a limited role for ER [9].
Androgens are believed to provoke an aggressive response or behaviour in males, however, little evidence supports this theory. Aggressive behaviour does not correlate with circulating androgen levels. Androgen removal from certain species, does lower aggressive tendencies and behaviour associated with mating, which is also diminished. The acute neural sensitivity to androgens governs this behaviour and not the actual level of circulating androgens, which is governed by intracellular levels of receptors and cofactors. Mice with tissue specific AR knock-out within the hippocampus, medial amygdala, striaterminalis, preoptic area and the hypothalamus show a virtually complete absence of sexual behaviour even when relative levels of testosterone may be higher [10].
Although controversial, females are more risk averse and less competitive than males, and may have a solid evolutionary basis. Males have a lower obligate investment in reproduction, therefore, if a male out competes another male, he can achieve maximum offspring, potentially with more than one female. Whilst the female brain may be programmed for protective and less risk taking, the male brain may be programmed to be more aggressive, territorial, dominant, and status seeking. Supplementing women (post-menopausal) with testosterone does not increase risk taking, indicative of male brains ’differential ‘wiring’ earlier in life. Steroid receptors are strongly implicated in psychosexual differentiation and dimorphism of the brain, involving gender identity and gender-specific behaviour.
Steroids can affect mood, mental state, and cognition in humans. In hypogonadal men, testosterone replacement can greatly improve mood, as it can increase the expression of serotonin 2A receptor in the frontal cortex. However, the more potent (non-aromatisable) androgen (DHT) cannot, indicating that this particular event is mediated via conversion to estrogen [10].
Neurones containing AR and ER are distributed throughout the vomeronasal projection pathway, which is important for male sexual behaviour in mice and processing of pheromones cues. AR expression in this area is highly sexually dimorphic, with males having more receptors in the amygdala, striaterminalis, periventicular nucleus and the medial preoptic area (MPOA). In castrated males, where their consomatory, copulatory and sexual motivation is inhibited, testosterone replacement is sufficient to restore behaviour, [11]. Naturally occurring non-copulating male mice, are not restored by testosterone, and these mice have much higher AR levels in the MPOA and the posterior dorsal amygdala (MePD) [12]. Tfmmice also have reduced male sexual behaviour [13].
The Cerebral cortex
The cerebral cortex is a sheet of neuronal tissue that surrounds the cerebrum of the brain, with a large area due to a high degree of folding. The cortex is vital for higher brain functions e.g. thought, memory, awareness, perception, consciousness and specifically for humans - language, abstract thought and self-awareness. It receives sensory, motor and associated inputs and is heavily involved in processing these signals and communicates with other regions of the brain.
Dopamine modulates neuronal activity within the cortex, and the dopamine-D2 receptor is alternatively spliced in the brain and pituitary gland in response to steroid hormones such as testosterone and estrogen [14]. The protein isoforms produced differentially interact with G-protein coupled receptors, modulating the neuronal response and implies a “fine tuning” response of steroid receptors.
Aging is the commonest risk factor for Alzheimers disease (AD), and decreased levels of steroid hormones (Tes & E2) also increase risk, whose contributions cannot be separated as steroid levels drop in the elderly. AR is found at high levels in those brain areas that mediate cognition e.g. cortex, hippocampus and amygdala, and the gradual reduction of testosterone in men is correlated with cognitive impairment and increased risk of AD [15].
The Hypothalamus
The Hypothalamo-Pituitary-Adrenal (HPA) axis functions to maintain the homeostatic state and help to return to equilibrium following a disturbance. Several neuro-signalling factors, including steroid hormones, can regulate this pathway. Sex differences in the HPA axis response to a variety of stress signals have been demonstrated e.g. female rats’ respond to physical stress with a greater endocrine response than males, and not just due to differences in the adrenal hormone synthesis, but due to sex difference in the HPA neurones themselves [16]. Castration of male rats increases the adrenal response to physical stress, which can be reversed by testosterone injections [17], indicating that testosterone may normally act to inhibit the HPA response. Additionally non-aromatisable DHT could reverse this effect indicating that it is not ER-mediated.
The circadian timing system is coordinated by a master clock located in the hypothalamic Suprachiasmatic Nucleus (SCN), which is synchronised by the light cycles of day and night. The circadian rhythm coordinates several pathways within the body, including sleep-wake cycle, hormone secretion, glucose metabolism, memory formation and changes in body temperature, and several oscillations in many bodily tissues. Endocrine cues can modulate the phase, period and amplitude, but the integration site between these two systems is unknown. One potential locus is the population of AR positive neurones in the SCN, which in mice, are restricted to the region that receives photic cues from the retina [18]. Interestingly, a strong link exists between Alzheimers risk and sleep pattern disruption due to deterioration of the SCN, and may be a contributory factor with diminishing circulating testosterone in the elderly.
Gonadectomy dramatically lengthens the free-running period and increases day to day variability in the start of the active phase. Gonadectomy alters synaptic connectivity by increasing glial fibrillary acidic protein and synaptophys in expression as well as influencing the function and circuitry of the SCN by affecting astrocyte morphology and expression of the “clock” genes Per 1,2. This effect is rescued by both testosterone and DHT, indicating that ER is not responsible for this effect [19,20]. In some mammals day length is important in indicating the time of year and suitability for breeding. In Siberian hamsters, day length regulates gonadotropin levels via control of gonadotropin-releasing hormone release from the hypothalamus [21], with short photoperiod also reducing AR expression in these areas [22]. Short photoperiod can reduce LH and FSH levels, and subsequently reduce gonadal function and steroidogenesis, reducing reproductive function to permissive seasons.
The Amygdala
The amygdala is a highly conserved brain region, associated with two major behavior types: fear/anxiety and social/mating behaviors. The amygdala is implicated in several diseases/disorders related to these behaviors, including autism, depression, schizophrenia, and anxiety. Interestingly, all of these disorders exhibit sex biases in prevalence rates. The posterodorsal division of the amygdala shows marked plasticity in response to gonadal hormones. The volume and size of the neuronal somata are normally greater in adult males than in females. Castration of male mice results in a reduction of dendritic branching in the posterior medial amygdala leading to deficits in social and sexual behavior, a phenomenon reversed by androgen treatment.
Astrocytes in the amygdala default to a “female” pattern in individuals with AR dysfunction e.g. testicular feminization syndrome. Their number and growth complexity depends on androgens and these cells show plasticity even in the adult, however, androgen exert more effects in the males indicating a prior requirement earlier in life [23].
Chemosensory signals (odors) are important in social communication, especially in rodents. They determine and control reproductive behaviors in response to pheromones and airborne odorants. Sensory signals are detected by the accessory olfactory bulb and processed via the medial amygdala, and conveyed to the medial preoptic area, a highly sexually dimorphic region of the brain, sensitive to steroids.
In humans uniquely, neural pathways exist in the amygdala that govern the sense of interpersonal trust. These pathways are responsive to oxytocin, and that the pathway is antagonized by vasopressin, a testosterone-induced peptide hormone [24,25]. One mechanism involves testosterone reducing functional connectivity between the amygdala and the frontal cortex leading to increase social vigilance and promoting untrustworthiness, especially towards facial recognition.
The Brain Stem
AR is expressed in the posterior brain, which connects to the spinal chord, through which the sensory motor neurons pass. It also controls cardiac and respiration functions. The neurodegenerative Kennedy disease (spinal and bulbar muscular atrophy) affects the lower motor neurons, characterised by progressive muscle atrophy, weakness and tremor [26,27]. The abnormality consists of an X-linked recessive mutation of the AR and mainly affects males. The mutation consists of an expansion of the AR gene CAG repeat, producing a toxicpolyglutamine expansion in the protein [28].
The Pituitary gland
The pituitary is an endocrine gland, functionally connected to the hypothalamus via a small tube called the pituitary stalk. The anterior pituitary regulates several physiological processes and secretes up to nine hormones that regulate homeostasis. Follicle stimulating hormone drives stimulates the ovaries to produce eggs, and stimulates the testes to produce sperm. Luteinising hormone triggers ovulation, and stimulates the testes to produce testosterone. Negative feedback by circulating androgens (and estrogens) then regulates this pathway. Disorders of the pituitary gonadal axis results in androgen deficiency in men, or androgen excess or deficiency in women.
Olfactory sense
In mice, the olfactory sense is very important. Chemical signatures in rodents are detected primarily by two separate neural pathways. The first is the olfactory epithelium which projects directly into the main olfactory bulb – which detects a very wide range of volatile compounds. The Olfactory Mucosa (OM), located in the dorsal, posterior part of the nasal cavity, is a known target tissue for sex steroid hormone action. The second is the Vomeronasal Organ (VNO), which projects directly to the accessory olfactory bulb with distinct separate neuronal pathways. Sex steroids modulate the responsiveness of VNO neurons to biologically relevant odours. The VNO detects compounds that provide cues to the social and sexual status of other mice, and includes pheromone constituents.
The olfactory bulb itself is a sexually dimorphic brain region, similarly to the nuclei of the preoptic area in the anterior hypothalamus. Masculinisation of this area is controlled by high local estrogen concentrations, via local conversion from testosterone - reduced in females due to the binding capacity of locally expressed α-fetoprotein.
The Eye
Androgens influence the structural and functional characteristics of many occular tissues, with AR expression in the meibomian and lacrimal glands, conjunctiva and the cornea. Androgens are important in the glandular architecture and control of secretions such as meibum, mucus and tear output, and function in the pathophysiology regulating several immunological disorders.
AR expression is found in the nuclei of epithelial, stromal and endothelial cells of the cornea of both male and female mice [29]. AR is found in the lacrimal gland of MRI/lpr mice [30,31]. In a spontaneous keratoconus mouse model, the effects were seen solely in male mice, and only seen in testosterone treated female mice.
Cardiovascular tissue
Sex differences existsin cardiovascular disease, with two-fold higher incidence in males [32], with estrogens exerting a favourable effect lowering incidence in females [33]. However the exact role of testosterone and the AR remains complex. Androgens are a major risk factor, with testosterone replacement increasing risk [34], but conversely androgen deprivation therapy in patients undergoing treatment for prostate cancer also show increased risk [35].
Cardiac myocytes weakly express AR [36], as do endothelial cells and vascular smooth muscle [37], however the role of AR remains unclear, with androgens exerting hypertrophic effects on cardiac myocytes [38], with androgens also regulating the expression of Ca2+ channels [39].
Clues to the role of AR come from the ARKO mouse. AR regulates physiological cardiac growth and modulates cardiac adaptive hypertrophy and fibrosis during angiotensin II induced cardiac remodelling [40].
The effects of testosterone on vasculature are controversial. Androgens may increase atherosclerosis [41], but other studies have shown lowered testosterone also to be associated with risk [42]. The ARKO mouse shows exaggerated angiotensin II-induced medial thickening in the coronary artery and aorta, and in Apo-E deficient mice AR loss accelerates atherosclerosis [43]. The AR also promotes angiogenesis via VEGF-related mechanisms [44]. Hypertension also shows sex differences, with reduced testosterone levels associated with high blood pressure and with testosterone improving vascular stiffness [45].
Immune system and Bone Marrow
Sexual dimorphism exists in the immune system and its associated diseases. Females havedisproportional predisposition to autoimmune diseases [46] e.g. autoimmuno glomerulonephritis [47], rheumatoid arthritis [48], lupus [49], scleroderma [50], Sjogrens syndrome [51], and Grave’s disease [50]. Estrogens tend to promote a heightened risk of autoimmunity whilst androgens exert a more suppressive effect.
The Bone marrow
Historically, androgens were the main agents used for stimulating erythropoiesis, and used for improving granulocyte and platelet counts in anaemic patients [52]. The bone marrow itself is a strongly AR-expressing tissue, in the stromal cells, megakaryocytes, and bone marrow endothelial cells, as well as several immunological precursor cell types [53].
The Thymus
The thymus “educates” and matures the T-lymphocytes, creating an environment where thymocytes develop and is important in selecting and eliminating “self” responding thymocytes. The full repertoire of T-cells are built up quickly in neonatal and preadolescence, and soon after puberty it begins to atrophy and involution follows sexual maturity, which can be reversed by castration [54]. Androgen treatment to suppress autoimmune disease requires the presence of a thymus. As well as thymus size, androgens modulate the cell cycle composition. AR expression is reported in the thymus but the exact cell type is debated, as thymocytes express AR at certain developmental stages. High AR levels have been found in immature thymocytes (CD4/8) [55], but other studies in hypogonadal men treated with androgens show an increased level of peripheral CD8+ cells [56].
The Spleen
Multiple Sclerosis (MS) is a chronic demyelinating disease of the CNS, which has an unknown pathology. Sex hormones are thought to be critical modulators in susceptibility and prognosis, with the disease being more prominent in women, but being more common in men with increasing age with a tendancy to be more severe in older men, as a consequence of reducing testosterone levels [57]. Mouse models of autoimmune encephalomyelitis (EAE) show increasing severity in castrated males and a reduced severity in testosterone treated females [58,59]. Interestingly, the administration of testosterone to castrated males only benefitted the younger males, whereas the older males developed acute and severe EAE and an impaired T cell function. One hypothesis, includes the possibility that Tes>DHT conversion may be a more active in younger males, whereas in later life the Tes>E2 becomes more prominent, reducing the effects. Testosterone has been shown to diminish the secretion of TNF, IFN, and to reduce anti-inflammatory responses, and to supress the activity of regulatory CD4+CD25+ T cells in the spleen, a pathway blocked by anti-androgens [60].
Bone
Historically, androgens are considered as bone anabolic agents - important for growth, maintenance, and maintaining bone mineral density, with osteoporosis being more prevalent in women than men. In the 1940s, Albright and Reifenstein were among the first to refer to the antiosteoporotic and anabolic properties of androgens.
Skeleton
Steroid effects on the pubertal growth spurt are well known, as are the effects on bone during this time. The cartilaginous epiphyseal plate in the metaphysis at each end of a long bone is found in children and adolescents. In adults, who have stopped growing, the plate is replaced by an epiphyseal line. In puberty increasing levels of estrogen, in both females and males, leads to increased apoptosis of chondrocytes in the epiphyseal plate. Depletion of chondrocytes due to apoptosis leads to less ossification and growth slows down and later stops when the entire cartilage has become replaced by bone.
ERα levels elevate during matrix maturation but decline during mineralization. ERβ levels remain low but constant. AR levels appear low during the proliferation stage and then increases throughout differentiation, peaking at the mineralization stage [61]. Osteoblasts are mononucleate cells that are responsible for bone formation. Osteoblasts arise from pluripotent osteoprogenitor cells located in the deeper layer of periosteum and the bone marrow – a strongly androgen responsive tissue.
In contrast to other tissues where the AR is downregulated by androgens, the bone osteoblasts show an upregulation of AR with androgen [62]. Additionally, the moderate expression of a 5a-reductase isoform in the bone aids in the conversion of testosterone to the more potent DHT, and amplifies the androgenic signal [63].
Craniofacial bones
Facial masculinity is a marker for pubertal testosterone exposure. Sexual dimorphisms may develop prenatally, but become exaggerated as male testosterone levels rise during puberty and act on pre-existing differences, e.g. jaw width, cheekbone prominence, eyebrow ridges, chin, nose, remodelling and lengthening of facial bone contours. Epidemiological studies show predominance in certain forms of craniosynostosis (78% male) [64]. Levels of AR RNA and protein in facial bones are relatively similar between the sexes, with the higher levels of circulating testosterone responsible for the growth in males. Androgens are important in the craniofacial development of several mammals e.g. primates, mice and rats. In primates, the face plays an important role in social cognition, choosing a mate and in behaviour. Primate brains have structures specializing in perceiving facial expressions of emotions and intentions. Features that contribute to perceived facial dominance, such as strong jaws or broad cheek bones may indicate superior physical strength, increased fecundity, and fitness of the male. However, interestingly, several studies have indicated that females do not show an absolute preference for these facial features, as several of these physical attributes also correlated with lack of warmth, lack of trust, too dominant and anti-social behaviour. This may explain why, in humans, strong testosterone driven facial features have not been evolutionary over-selected.
Finger length
Manning [65] recently showed that testosterone before birth stimulates prenatal growth of the 4th finger (4D) while oestrogen promotes the growth of the 2nd finger (2D). The 2D:4D ratio may then be a reliable method of evaluating testosterone exposure during sexually dimorphic development, but is not a reliable method of evaluating or predicting male health, fertility and performance.
Muscle
Skeletal muscle
Androgens have an anabolic activity towards skeletal muscles, playing an important role during development, adaptation, and homeostasis. ARKO mice have a reduced muscle mass and contractile strength [66]. Anti-androgenic treatment for prostate cancer or hyperplasia causes reduction in muscle size and strength [67]. Treating female laboratory animals or elderly men with low serum testosterone with testosterone increases lean body weight, although via poorly understood mechanisms. Although, therapeutically used for muscle wasting diseases, anabolic (androgenic) steroids are widely abused for “body building” and sports. Muscle increase can arise from either myoblast proliferation or net increase of protein content, and androgens can affect muscle by these two pathways. Androgens also promote the differentiation of mesenchymal multipotent cells down the myogenic lineage. Ornithine decarboxylase, the rate limiting enzyme in polyamine biosynthesis, is upregulated by the androgen receptor in skeletal muscle and regulates myoblast proliferation and differentiation [68].
AR ablation within myocytes not only reduces lean body mass but also muscle strength and fibrilar organisation [69], and conversely AR overexpression increases body mass [70], albeit with profound disruptions in myofibrilar ultrastructure [71]. It is further complicated by the fact that androgen can exert a genomic (transcriptional) and non-genomic (via EGFR/G-coupled receptor) action upon target cells.
Smooth muscle
Smooth muscle is found in many tissues e.g. male and female reproductive tracts, uterus, gastrointestinal tract, vascular and lymphatic system, respiratory tract and the eye. Their physiological structure and function are very similar between tissues, although their stimulatory signals differ enormously.
Airway smooth muscle cells express various steroid receptors, including the AR. Airway diseases such as asthma, chronic obstructive pulmonary disease and pulmonary fibrosis exhibit sex differences in their distribution and severity. Testosterone and estradiol have a mitogenic effect on airway smooth muscle cells through a pathway involving non-genomic MAPK and PI3K pathways [72].
Skin, Hair follicles, Mammary Glands and Adipose Tissues
Skin
In the skin, hair growth and sebum production are highly androgen regulated. The AR is expressed in both genital and non-genital skin regions, within the stratum basale cells including fibroblasts, sweat glands, smooth muscle, endothelial, sebaceous, and hair follicle root cells [73]. In genital skin, AR is expressed within spinous and granular cell layers and in genital and areolar melanocytes [74,75], where androgens affect regional skin pigmentation at puberty.
Oil produced by the sebaceous glands (sebum) in the skin, lubricates and water proofs the skin and hair. In humans, the glands are predominantly found in the scalp and on the face, but are ubiquitously found in most mammals. Although sebaceous gland development is not governed by androgen action, sebum production itself is androgen controlled. Humans with androgen insensitivity syndrome show no sebum production, whilst patients with 5-reductase deficiency showed normal sebum production [8], indicating an androgen requirement but not necessarily DHT.
Circulating androgens affect several cutaneous structures. Skin may be regarded as a peripheral organ that locally synthesises significant amounts of androgens. For example, sebocytes and dermal papilla cells have the capacity to convert the adrenal precursor androgens DHEA-S and androstenedione into more potent androgens such as testosterone (& DHT) [76,77], with skin-specific 5a-reductase isoforms [78]. Both androstenedione and testosterone have the capacity to stimulate sebum secretion in humans.
The hair follicles
Human hair follicles are strongly hormonally influenced. Hair forms part of the secondary sexual characteristics e.g. pubic hair in both sexes and beard and body hair in men. Beard hairs, arise from fine uncoloured vellus hairs devoid of a medulla, which become increasingly coarse, strongly pigmented and medullated during puberty in response to the androgen surge [79]. In both sexes, pubic hair follicles also undergo this transition [80]. AR is expressed in many medulla hair follicles cells and dermal papilla cells. The human hair keratin-7 gene, expressed in hair follicle trichocytes, contains strong AREs in its gene promoter, and is expressed strongly in beard and sexual hair follicles, but not expressed in terminal scalp follicles [81]. The dermal papilla is considered to be the main site of androgen action, with circulating androgens from the blood activating the production of paracrine factors for target cells e.g. keratinocytes. In culture, hair follicle cells from androgen sensitive areas all contain AR and are responsive to androgens, however, only the cells from scalp or beard hair actively convert testosterone to DHT [82-84].
Treatment of women with idiopathic hirsutism with antiandrogens drastically reduces abnormal facial and body hair growth [79]. AR null humans do not develop sexual hairs [80], whilst 5a-reductase deficient males develop a female-like distribution pattern of body hair [85].
Human hair follicles cannot be regarded as uniformly androgen dependent, e.g. the eyelashes are unresponsive, whereas in human males, the scalp follicles may show regression of terminal hairs back to vellus fine hairs, leading to male pattern baldness. This represents the apparent paradoxical nature of the hair follicle, in terms of two follicles inches away from each other e.g. on the scalp may respond differently to the same circulating androgens via unknown mechanisms [85].
Mammary glands
The mammary gland represents a sexually dimorphic tissue. In females the mammary gland fully develops to maturation under the dominant control of E2, but in males there is little or no growth, due to the apparent dominance of testosterone. AR has been observed to be expressed strongly in certain mammary gland cell types, and AR expression is frequently expressed in breast cancer, and can be exploited as a therapeutic avenue. In females, androgenic precursors are as high as in males, but testosterone levels are lower and fluctuate with the estrous cycle where testosterone is produced in the ovaries.
Adipose tissue
Androgens are important regulators of fat mass and distribution in mammals. Male and female body fat patterns are very different, and the adipose tissue itself may be a strong source of peripheral conversion of adrenal metabolites and produce androgens and estrogens. Men accumulate fat around the abdomen and women accumulate fat around the gluteofemoral regions. Testosterone inhibits fat deposition in both sexes and low plasma testosterone may be associated with increased fat deposits [102].
Gastrointestinal Tract (and auxiliary organs)
Salivary glands
The salivary glands in mammals consist of three major glands, the parotid gland, mandibular ramus, the submandibular glands located beneath the lower jaws, and the sublingual glands located beneath the tongue. Sexual dimorphism exists in the salivary gland, and the glandular epithelial cells are known to express high levels of steroid hormone receptors, including AR [86], ERβ [87], and PR [88] to a lesser extent. In rodents the male submandibular gland is larger and has a more complex morphology, with the granular convoluted tubules being much more developed. Masculinization is caused by circulating androgens [89]. The watery saliva produced, contains mucins, proteins, salts and various steroid hormones, at higher concentrations in the male gland. In an androgen independent state the development of the gland is feminine by default, castration of male mice leads to a female-like salivary glandphenotype, that may be reversed by androgen treatment [90] and the treatment of female mice with testosterone induces a male-like salivary gland pattern [91]. Testosterone extensively influences gene expression in the male submandibular gland influencing over 1300 genes [92].
Sjögren’s Syndrome (SS) is a chronic autoimmune disease, occurring commonly in women (9-1 ratio at 40-50 years) and is characterized by focal adenitis, serum antibodies, dysfunction and atrophy of the acinar cells of the salivary and lacrimal glands, leading to diminished secretory capacity and oral and lacrimal dryness [93]. SS patients are often affected by dental caries and oral candidosis [94]. Androgen replacement therapy can reduce the inflammation in salivary (and lacrimal) glands of female SS mice [95].
The Small intestine
The AR mRNA is expressed at relatively high levels in the rumen, reticulum, omasum and duodenum, but AR protein expression is low. The small intestine epithelium is influenced by androgens [96,97], and may alter the intestinal electrolyte fluid balance and modulate glucose transport. Androgens promote growth in the small intestine and stimulate Ca2+ absorption. Androgens act on the basolateral membrane of the enterocytes and activate second messenger molecules to modulate ion channel function. Gender differences have been reported in the absorption of ions e.g. testosterone and E2 increase Ca2+ transport in the duodenum, and testosterone can control the expression and activity of the vasoactive intestinal peptide [98], which functions within the intestine, controlling e.g. glucose uptake, electrolyte balance, smooth muscle relaxation and pancreatic secretions.
Reproductive and maturational nutritive needs are examples for situations in which the changes in circulatory concentrations of steroid hormones would need to affect GI tract functions, for example changes in hormone levels during pregnancy would lead to increased uptake of Ca2+ from the gut for foetal bone development.
The Small intestine
The AR promotes β-catenin nuclear import, in an APC independent manner [99-101]. Additionally the reduction in the AR CAG repeat sequence is correlated with a higher incidence of colon carcinoma and colon neoplasia occurs more commonly in men [90].
Vocal Chord/Fold
The effect of hormones on the human larynx is a well-known phenomenon, especially for human males. The physiological change during puberty affects male vocal chords, leading to deepening of the voice. However, the larynx also undergoes hormonally-induced changes during maturation, for example the change in voice pattern in both sexes occurs in the elderly [103], but also during the menstrual cycle and pregnancy in females [104]. In primates and monkeys, males respond strongly to the pitch changes in the female voice at the time of peak fertility.
A direct testosterone effect on the vocal chords has long been assumed, but steroid receptor expression and activity within the tissue has proven difficult to demonstrate. Recently, Voelter et al, have shown androgen receptor expression in the basal and intermediate layer of the stratified epithelium [105].
Conclusion
Androgens are regarded as being the male hormone, involved in virilisation, and estrogens as being the female involved in the estrous cycle. Physiological evidence from mouse model systems have shown that this is too simplistic. Testosterone conversion to estrogen is vitally important in the development of the male, and androgens have an important role in the females. Steroid hormones have more far-reaching effects on the whole body, than merely the reproductive organs (see Figure 3). An apparent increased risk of male neurological disorders and increased female risk of autoimmune disorders are evident from the literature (see Table 1). Although, possibly linked to reproduction via complex evolutionary pathways, the target organs for androgens are multiple and widespread.

Figure 3: Diagrammatic representation of the major androgen responsive tissues in the human body (outside the reproductive tissue which are not included).
References
- Ciana P, Di Luccio G, Belcredito S, Pollio G, Vegeto E, Tatangelo L, et al. Engineering of a mouse for the in vivo profiling of estrogen receptor activity. Mol Endocrinol. 2001; 15: 1104-1113.
- Ciana P, Raviscioni M, Mussi P, Vegeto E, Que I, Parker MG, et al. In vivo imaging of transcriptionally active estrogen receptors. Nat Med. 2003; 9: 82-86.
- Pihlajamaa P, Zhang FP, Saarinen L, Mikkonen L, Hautaniemi S, Jänne OA. The phytoestrogen genistein is a tissue-specific androgen receptor modulator. Endocrinology. 2011; 152: 4395-4405.
- Dart DA, Waxman J, Aboagye EO, Bevan CL. Visualising androgen receptor activity in male and female mice. PLoS One. 2013; 8: e71694.
- Houten SM, Volle DH, Cummins CL, Mangelsdorf DJ, Auwerx J. In vivo imaging of farnesoid X receptor activity reveals the ileum as the primary bile acid signaling tissue. Mol Endocrinol. 2007; 21: 1312-1323.
- Dart DA, Spencer-Dene B, Gamble SC, Waxman J, Bevan CL. Manipulating prohibitin levels provides evidence for an in vivo role in androgen regulation of prostate tumours. Endocr Relat Cancer. 2009; 16: 1157-1169.
- McEwen BS, Lieberburg I, Chaptal C, Krey LC. Aromatization: important for sexual differentiation of the neonatal rat brain. Horm Behav. 1977; 9: 249-263.
- Imperato-McGinley J, Gautier T, Cai LQ, Yee B, Epstein J, Pochi P. The androgen control of sebum production. Studies of subjects with dihydrotestosterone deficiency and complete androgen insensitivity. J Clin Endocrinol Metab. 1993; 76: 524-528.
- Grumbach MM, Auchus RJ. Estrogen: consequences and implications of human mutations in synthesis and action. J Clin Endocrinol Metab. 1999; 84: 4677-4694.
- Sumner BE, Fink G. Testosterone as well as estrogen increases serotonin2A receptor mRNA and binding site densities in the male rat brain. Brain Res Mol Brain Res. 1998; 59: 205-214.
- Baum MJ, Vreeburg JT. Copulation in castrated male rats following combined treatment with estradiol and dihydrotestosterone. Science. 1973; 182: 283-285.
- Portillo W, Díaz NF, Cabrera EA, Fernández-Guasti A, Paredes RG. Comparative analysis of immunoreactive cells for androgen receptors and oestrogen receptor alpha in copulating and non-copulating male rats. J Neuroendocrinol. 2006; 18: 168-176.
- Rissman EF. Roles of oestrogen receptors alpha and beta in behavioural neuroendocrinology: beyond Yin/Yang. J Neuroendocrinol. 2008; 20: 873-879.
- Guivarc'h D, Vernier P, Vincent JD. Sex steroid hormones change the differential distribution of the isoforms of the D2 dopamine receptor messenger RNA in the rat brain. Neuroscience. 1995; 69: 159-166.
- Pike CJ, Carroll JC, Rosario ER, Barron AM. Protective actions of sex steroid hormones in Alzheimer's disease. Front Neuroendocrinol. 2009; 30: 239-258.
- Handa RJ, Burgess LH, Kerr JE, O'Keefe JA. Gonadal steroid hormone receptors and sex differences in the hypothalamo-pituitary-adrenal axis. Horm Behav. 1994; 28: 464-476.
- Handa RJ, Nunley KM, Lorens SA, Louie JP, McGivern RF, Bollnow MR. Androgen regulation of adrenocorticotropin and corticosterone secretion in the male rat following novelty and foot shock stressors. Physiol Behav. 1994; 55: 117-124.
- Daan S, Damassa D, Pittendrigh CS, Smith ER. An effect of castration and testosterone replacement on a circadian pacemaker in mice (Mus musculus). Proc Natl Acad Sci U S A. 1975; 72: 3744-3747.
- Karatsoreos IN, Wang A, Sasanian J, Silver R. A role for androgens in regulating circadian behavior and the suprachiasmatic nucleus. Endocrinology. 2007; 148: 5487-5495.
- Karatsoreos IN, Butler MP, Lesauter J, Silver R. Androgens modulate structure and function of the suprachiasmatic nucleus brain clock. Endocrinology. 2011; 152: 1970-1978.
- Meredith JM, Turek FW, Levine JE. Effects of gonadotropin-releasing hormone pulse frequency modulation on the reproductive axis of photoinhibited male Siberian hamsters. Biol Reprod. 1998; 59: 813-819.
- Bittman EL, Ehrlich DA, Ogdahl JL, Jetton AE. Photoperiod and testosterone regulate androgen receptor immunostaining in the Siberian hamster brain. Biol Reprod. 2003; 69: 876-884.
- Johnson RT, Schneider A, DonCarlos LL, Breedlove SM, Jordan CL. Astrocytes in the rat medial amygdala are responsive to adult androgens. J Comp Neurol. 2012; 520: 2531-2544.
- Bos PA, Terburg D, van Honk J. Testosterone decreases trust in socially naive humans. Proc Natl Acad Sci U S A. 2010; 107: 9991-9995.
- Bos PA, Hermans EJ, Ramsey NF, van Honk J. The neural mechanisms by which testosterone acts on interpersonal trust. Neuroimage. 2012; 61: 730-737.
- Sobue G, Hashizume Y, Mukai E, Hirayama M, Mitsuma T, Takahashi A. X-linked recessive bulbospinal neuronopathy. A clinicopathological study. Brain. 1989; 112 : 209-232.
- Sobue G. X-linked recessive bulbospinal neuronopathy (SBMA). Nagoya J Med Sci. 1995; 58: 95-106.
- Fischbeck KH. Kennedy disease. J Inherit Metab Dis. 1997; 20: 152-158.
- Tachibana M, Kobayashi Y, Kasukabe T, Kawajiri K, Matsushima Y. Expression of androgen receptor in mouse eye tissues. Invest Ophthalmol Vis Sci. 2000; 41: 64-66.
- Ono M, Rocha FJ, Sullivan DA. Immunocytochemical location and hormonal control of androgen receptors in lacrimal tissues of the female MRL/Mp-lpr/lpr mouse model of Sjögren's syndrome. Exp Eye Res. 1995; 61: 659-666.
- Rocha FJ, Kelleher RS, Edwards JA, Pena JD, Ono M, Sullivan DA. Binding characteristics, immunocytochemical location and hormonal regulation of androgen receptors in lacrimal tissue. Adv Exp Med Biol. 1994; 350: 157-160.
- Kalin MF, Zumoff B. Sex hormones and coronary disease: a review of the clinical studies. Steroids. 1990; 55: 330-352.
- Malhotra A, Buttrick P, Scheuer J. Effects of sex hormones on development of physiological and pathological cardiac hypertrophy in male and female rats. Am J Physiol. 1990; 259: H866-871.
- Calof OM, Singh AB, Lee ML, Kenny AM, Urban RJ, Tenover JL, et al. Adverse events associated with testosterone replacement in middle-aged and older men: a meta-analysis of randomized, placebo-controlled trials. J Gerontol A Biol Sci Med Sci. 2005; 60: 1451-1457.
- Martín-Merino E, Johansson S, Morris T, García Rodríguez LA. Androgen deprivation therapy and the risk of coronary heart disease and heart failure in patients with prostate cancer: a nested case-control study in UK primary care. Drug Saf. 2011; 34: 1061-1077.
- McGill HC Jr, Anselmo VC, Buchanan JM, Sheridan PJ. The heart is a target organ for androgen. Science. 1980; 207: 775-777.
- Horwitz KB, Horwitz LD. Canine vascular tissues are targets for androgens, estrogens, progestins, and glucocorticoids. J Clin Invest. 1982; 69: 750-758.
- Marsh JD, Lehmann MH, Ritchie RH, Gwathmey JK, Green GE, Schiebinger RJ. Androgen receptors mediate hypertrophy in cardiac myocytes. Circulation. 1998; 98: 256-261.
- Golden KL, Marsh JD, Jiang Y, Brown T, Moulden J. Gonadectomy of adult male rats reduces contractility of isolated cardiac myocytes. Am J Physiol Endocrinol Metab. 2003; 285: E449-453.
- Ikeda Y, Aihara K, Yoshida S, Sato T, Yagi S, Iwase T, et al. Androgen-androgen receptor system protects against angiotensin II-induced vascular remodeling. Endocrinology. 2009; 150: 2857-2864.
- Adams MR, Williams JK, Kaplan JR. Effects of androgens on coronary artery atherosclerosis and atherosclerosis-related impairment of vascular responsiveness. Arterioscler Thromb Vasc Biol. 1995; 15: 562-570.
- Muller M, van den Beld AW, Bots ML, Grobbee DE, Lamberts SW, van der Schouw YT. Endogenous sex hormones and progression of carotid atherosclerosis in elderly men. Circulation. 2004; 109: 2074-2079.
- Bourghardt J, Wilhelmson AS, Alexanderson C, De Gendt K, Verhoeven G, Krettek A, et al. Androgen receptor-dependent and independent atheroprotection by testosterone in male mice. Endocrinology. 2010; 151: 5428-5437.
- Cai J, Hong Y, Weng C, Tan C, Imperato-McGinley J, Zhu YS. Androgen stimulates endothelial cell proliferation via an androgen receptor/VEGF/cyclin A-mediated mechanism. Am J Physiol Heart Circ Physiol. 2011; 300: H1210-1221.
- Yaron M, Greenman Y, Rosenfeld JB, Izkhakov E, Limor R, Osher E, et al. Effect of testosterone replacement therapy on arterial stiffness in older hypogonadal men. Eur J Endocrinol. 2009; 160: 839-846.
- Dubois EL, Tuffanelli DL. Clinical manifestations of systemic lupus erythematosus. computer analysis of 520 cases. Jama. 1964; 190: 104-111.
- Ichii O, Konno A, Sasaki N, Endoh D, Hashimoto Y, Kon Y. Onset of autoimmune glomerulonephritis derived from the telomeric region of MRL-chromosome 1 is associated with the male sex hormone in mice. Lupus. 2009; 18: 491-500.
- Glynn LE. Experimental model of rheumatoid arthritis. J Belge Rhumatol Med Phys. 1967; 22: 201-203.
- Inman RD. Immunologic sex differences and the female predominance in systemic lupus erythematosus. Arthritis Rheum. 1978; 21: 849-852.
- Rodnan GP, Myerowitz RL, Justh GO. Morphologic changes in the digital arteries of patients with progressive systemic sclerosis (scleroderma) and Raynaud phenomenon. Medicine (Baltimore). 1980; 59: 393-408.
- Whaley K, Buchanan WW. Recent advances in Sjogren's syndrome. Mod Trends Rheumatol. 1971; 2: 139-157.
- Besa EC. Hematologic effects of androgens revisited: an alternative therapy in various hematologic conditions. Semin Hematol. 1994; 31: 134-145.
- Smithson G, Couse JF, Lubahn DB, Korach KS, Kincade PW. The role of estrogen receptors and androgen receptors in sex steroid regulation of B lymphopoiesis. J Immunol. 1998; 161: 27-34.
- Henderson J. On the relationship of the thymus to the sexual organs: I. The influence of castration on the thymus. J Physiol. 1904; 31: 222-229.
- Viselli SM, Olsen NJ, Shults K, Steizer G, Kovacs WJ. Immunochemical and flow cytometric analysis of androgen receptor expression in thymocytes. Mol Cell Endocrinol. 1995; 109: 19-26.
- Bizzarro A, Valentini G, Di Martino G, DaPonte A, De Bellis A, Iacono G. Influence of testosterone therapy on clinical and immunological features of autoimmune diseases associated with Klinefelter's syndrome. J Clin Endocrinol Metab. 1987; 64: 32-36.
- Birk K, Ford C, Smeltzer S, Ryan D, Miller R, Rudick RA. The clinical course of multiple sclerosis during pregnancy and the puerperium. Arch Neurol. 1990; 47: 738-742.
- Voskuhl RR, Pitchekian-Halabi H, MacKenzie-Graham A, McFarland HF, Raine CS. Gender differences in autoimmune demyelination in the mouse: implications for multiple sclerosis. Ann Neurol. 1996; 39: 724-733.
- Bebo BF Jr, Vandenbark AA, Offner H. Male SJL mice do not relapse after induction of EAE with PLP 139-151. J Neurosci Res. 1996; 45: 680-689.
- Matejuk A, Hopke C, Vandenbark AA, Hurn PD, Offner H. Middle-age male mice have increased severity of experimental autoimmune encephalomyelitis and are unresponsive to testosterone therapy. J Immunol. 2005; 174: 2387-2395.
- Wiren KM, Chapman Evans A, Zhang XW. Osteoblast differentiation influences androgen and estrogen receptor-alpha and -beta expression. J Endocrinol. 2002; 175: 683-694.
- Wiren KM, Zhang X, Chang C, Keenan E, Orwoll ES. Transcriptional up-regulation of the human androgen receptor by androgen in bone cells. Endocrinology. 1997; 138: 2291-2300.
- Windahl SH, Andersson N, Börjesson AE, Swanson C, Svensson J, Movérare-Skrtic S, et al. Reduced bone mass and muscle strength in male 5a-reductase type 1 inactivated mice. PLoS One. 2011; 6: e21402.
- Shillito J Jr, Matson DD. Craniosynostosis: a review of 519 surgical patients. Pediatrics. 1968; 41: 829-853.
- Manning JT. Digit Ratio: A Pointer to Fertility, Behaviour and Health: Rutgers University Press, New Jersey. 2002.
- MacLean HE, Chiu WS, Notini AJ, Axell AM, Davey RA, McManus JF, et al. Impaired skeletal muscle development and function in male, but not female, genomic androgen receptor knockout mice. FASEB J. 2008; 22: 2676-2689.
- Basaria S, Lieb J, Tang AM, DeWeese T, Carducci M, Eisenberger M, et al. Long-term effects of androgen deprivation therapy in prostate cancer patients. Clin Endocrinol (Oxf). 2002; 56: 779-786.
- Lee NK, Skinner JP, Zajac JD, MacLean HE. Ornithine decarboxylase is upregulated by the androgen receptor in skeletal muscle and regulates myoblast proliferation. Am J Physiol Endocrinol Metab. 2011; 301: E172-179.
- Ophoff J, Van Proeyen K, Callewaert F, De Gendt K, De Bock K, Vanden Bosch A, et al. Androgen signaling in myocytes contributes to the maintenance of muscle mass and fiber type regulation but not to muscle strength or fatigue. Endocrinology. 2009; 150: 3558-3566.
- Niel L, Shah AH, Lewis GA, Mo K, Chatterjee D, Fernando SM, et al. Sexual differentiation of the spinal nucleus of the bulbocavernosus is not mediated solely by androgen receptors in muscle fibers. Endocrinology. 2009; 150: 3207-3213.
- Musa M, Fernando SM, Chatterjee D, Monks DA. Subcellular effects of myocyte-specific androgen receptor overexpression in mice. J Endocrinol. 2011; 210: 93-104.
- Stamatiou R, Paraskeva E, Papagianni M, Molyvdas PA, Hatziefthimiou A. The mitogenic effect of testosterone and 17ß- estradiol on airway smooth muscle cells. Steroids. 2011; 76: 400-408.
- Liang T, Hoyer S, Yu R, Soltani K, Lorincz AL, Hiipakka RA, et al. Immunocytochemical localization of androgen receptors in human skin using monoclonal antibodies against the androgen receptor. J Invest Dermatol. 1993; 100: 663-666.
- Bläuer M, Vaalasti A, Pauli SL, Ylikomi T, Joensuu T, Tuohimaa P. Location of androgen receptor in human skin. J Invest Dermatol. 1991; 97: 264-268.
- Tadokoro T, Itami S, Hosokawa K, Terashi H, Takayasu S. Human genital melanocytes as androgen target cells. J Invest Dermatol. 1997; 109: 513-517.
- Hoffmann R, Rot A, Niiyama S, Billich A. Steroid sulfatase in the human hair follicle concentrates in the dermal papilla. J Invest Dermatol. 2001; 117: 1342-1348.
- Fritsch M, Orfanos CE, Zouboulis CC. Sebocytes are the key regulators of androgen homeostasis in human skin. J Invest Dermatol. 2001; 116: 793-800.
- Chen W, Zouboulis CC, Fritsch M, Blume-Peytavi U, Kodelja V, Goerdt S, et al. Evidence of heterogeneity and quantitative differences of the type 1 5alpha-reductase expression in cultured human skin cells--evidence of its presence in melanocytes. J Invest Dermatol. 1998; 110: 84-89.
- Ebling FJ. The biology of hair. Dermatol Clin. 1987; 5: 467-481.
- Randall VA. Androgens and human hair growth. Clin Endocrinol (Oxf). 1994; 40: 439-457.
- Jave-Suarez LF, Langbein L, Winter H, Praetzel S, Rogers MA, Schweizer J. Androgen regulation of the human hair follicle: the type I hair keratin hHa7 is a direct target gene in trichocytes. J Invest Dermatol. 2004; 122: 555-564.
- Randall VA, Thornton MJ, Hamada K, Redfern CP, Nutbrown M, Ebling FJ, et al. Androgens and the hair follicle. Cultured human dermal papilla cells as a model system. Ann N Y Acad Sci. 1991; 642: 355-375.
- Randall VA, Thornton MJ, Hamada K, Messenger AG. Androgen action in cultured dermal papilla cells from human hair follicles. Skin Pharmacol. 1994; 7: 20-26.
- Thornton MJ, Hamada K, Laing I, Messenger AG, Randall VA. Metabolism of testosterone by cultured dermal papilla cells from human beard, pubic, and scalp hair follicles. Ann N Y Acad Sci. 1991; 642: 452-453.
- Randall VA, Hibberts NA, Thornton MJ, Hamada K, Merrick AE, Kato S, et al. The hair follicle: a paradoxical androgen target organ. Horm Res. 2000; 54: 243-250.
- Laine M, Bläuer M, Ylikomi T, Tuohimaa P, Aitasalo K, Happonen RP, et al. Immunohistochemical demonstration of androgen receptors in human salivary glands. Arch Oral Biol. 1993; 38: 299-302.
- Välimaa H, Savolainen S, Soukka T, Silvoniemi P, Mäkelä S, Kujari H, et al. Estrogen receptor-beta is the predominant estrogen receptor subtype in human oral epithelium and salivary glands. J Endocrinol. 2004; 180: 55-62.
- Ozono S, Onozuka M, Sato K, Ito Y. Immunohistochemical localization of estradiol, progesterone, and progesterone receptor in human salivary glands and salivary adenoid cystic carcinomas. Cell Struct Funct. 1992; 17: 169-175.
- Sawada K, Noumura T. Sexually dimorphic duct system of the submandibular gland in mouse with testicular feminization mutation (Tfm/Y). Acta Anat (Basel). 1992; 143: 241-245.
- Hosoi K, Kobayashi S, Ueha T. Influence of androgens on beta-glucuronidase activity in liver, kidney and submandibular salivary gland of the mouse. Arch Oral Biol. 1978; 23: 905-909.
- Kim YK, Ogita Z. Sexual dimorphism of SDS peptide patterns from the submaxillary glands of mice. J Exp Zool. 1981; 218: 447-453.
- Treister NS, Richards SM, Suzuki T, Jensen RV, Sullivan DA. Influence of androgens on gene expression in the BALB/c mouse submandibular gland. J Dent Res. 2005; 84: 1187-1192.
- Vitali C, Bombardieri S, Jonsson R, Moutsopoulos HM, Alexander EL, Carsons SE, et al. Classification criteria for Sjögren's syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis. 2002; 61: 554-558.
- Soto-Rojas AE, Villa AR, Sifuentes-Osornio J, Alarcón-Segovia D, Kraus A. Oral candidiasis and Sjögren's syndrome. J Rheumatol. 1998; 25: 911-915.
- Sullivan DA, Ariga H, Vendramini AC, Rocha FJ, Ono M, Sato EH. Androgen-induced suppression of autoimmune disease in lacrimal glands of mouse models of Sjögren's syndrome. Adv Exp Med Biol. 1994; 350: 683-690.
- Carriere RM. The influence of thyroid and testicular hormones on the epithelium of crypts of Lieberkühn in the rat's intestine. Anat Rec. 1966; 156: 423-431.
- Tuohimaa P, Niemi M. The effect of testosterone on cell renewal and mitotic cycles in sex accessory glands of castrated mice. Acta Endocrinol (Copenh). 1968; 58: 696-704.
- Carmena MJ, Recio MN, Prieto JC. Influence of castration and testosterone treatment on the vasoactive intestinal peptide receptor/effector system in rat prostatic epithelial cells. Biochim Biophys Acta. 1988; 969: 86-90.
- Truica CI, Byers S, Gelmann EP. Beta-catenin affects androgen receptor transcriptional activity and ligand specificity. Cancer Res. 2000; 60: 4709-4713.
- Mulholland DJ, Cheng H, Reid K, Rennie PS, Nelson CC. The androgen receptor can promote beta-catenin nuclear translocation independently of adenomatous polyposis coli. J Biol Chem. 2002; 277: 17933-17943.
- Yang F, Li X, Sharma M, Sasaki CY, Longo DL, Lim B, et al. Linking beta-catenin to androgen-signaling pathway. J Biol Chem. 2002; 277: 11336-11344.
- Blouin K, Nadeau M, Perreault M, Veilleux A, Drolet R, Marceau P, et al. Effects of androgens on adipocyte differentiation and adipose tissue explant metabolism in men and women. Clin Endocrinol (Oxf). 2010; 72: 176-188.
- Hirano M, Kurita S, Sakaguchi S. Ageing of the vibratory tissue of human vocal folds. Acta Otolaryngol. 1989; 107: 428-433.
- Amir O, Biron-Shental T. The impact of hormonal fluctuations on female vocal folds. Curr Opin Otolaryngol Head Neck Surg. 2004; 12: 180-184.
- Voelter Ch, Kleinsasser N, Joa P, Nowack I, Martínez R, Hagen R, et al. Detection of hormone receptors in the human vocal fold. Eur Arch Otorhinolaryngol. 2008; 265: 1239-1244.
- Baron-Cohen S, Lombardo MV, Auyeung B, Ashwin E, Chakrabarti B, Knickmeyer R. Why are autism spectrum conditions more prevalent in males? PLoS Biol. 2011; 9: e1001081.
- Wooten GF, Currie LJ, Bovbjerg VE, Lee JK, Patrie J. Are men at greater risk for Parkinson's disease than women? J Neurol Neurosurg Psychiatry. 2004; 75: 637-639.
- Schmidt R, Kienbacher E, Benke T, Dal-Bianco P, Delazer M, Ladurner G, et al. [Sex differences in Alzheimer's disease]. Neuropsychiatr. 2008; 22: 1-15.
- Nolen-Hoeksema S, Girgus JS. The emergence of gender differences in depression during adolescence. Psychol Bull. 1994; 115: 424-443.
- Ochoa S, Usall J, Cobo J, Labad X, Kulkarni J. Gender differences in schizophrenia and first-episode psychosis: a comprehensive literature review. Schizophr Res Treatment. 2012; 2012: 916198.
- Weng MY, Huang YT, Liu MF, Lu TH. Incidence and mortality of treated primary Sjogren's syndrome in Taiwan: a population-based study. J Rheumatol. 2011; 38: 706-708.
- Gabriel R. Sex ratio in glomerulonephritis. Lancet. 1979; 2: 51.
- Kvien TK, Uhlig T, ødegård S, Heiberg MS. Epidemiological aspects of rheumatoid arthritis: the sex ratio. Ann N Y Acad Sci. 2006; 1069: 212-222.
- Lockshin MD. Sex ratio and rheumatic disease. Autoimmun Rev. 2002; 1: 162-167.
- Weckerle CE, Niewold TB. The unexplained female predominance of systemic lupus erythematosus: clues from genetic and cytokine studies. Clin Rev Allergy Immunol. 2011; 40: 42-49.
- PausJenssen ES, Cockcroft DW. Sex differences in asthma, atopy, and airway hyperresponsiveness in a university population. Ann Allergy Asthma Immunol. 2003; 91: 34-37.
- Olson AL, Swigris JJ, Lezotte DC, Norris JM, Wilson CG, Brown KK. Mortality from pulmonary fibrosis increased in the United States from 1992 to 2003. Am J Respir Crit Care Med. 2007; 176: 277-284.
- Gottlieb SH. Does sex matter? Diabetes, heart disease, and gender. Diabetes Forecast. 2005; 58: 33-35.
- Guggenbuhl P. Osteoporosis in males and females: Is there really a difference? Joint Bone Spine. 2009; 76: 595-601.
- Boyadjiev SA. International Craniosynostosis Consortium. Genetic analysis of non-syndromic craniosynostosis. Orthod Craniofac Res. 2007; 10: 129-137.
- Disanto G, Ramagopalan SV. On the sex ratio of multiple sclerosis. Mult Scler. 2013; 19: 3-4.
- Murphy G, Devesa SS, Cross AJ, Inskip PD, McGlynn KA, Cook MB. Sex disparities in colorectal cancer incidence by anatomic subsite, race and age. Int J Cancer. 2011; 128: 1668-1675.
- Ota R, Fujiki K, Nakayasu K. [Estimation of patient visit rate and incidence of keratoconus in the 23 wards of Tokyo]. Nihon Ganka Gakkai Zasshi. 2002; 106: 365-372.