
Case Report
J Endocr Disord. 2014;1(3): 1015.
Thyroxine in the Treatment of Chronic Hepatitis C Infection: A Case Report
Huy A Tran1*, Elizabeth A Ianna2, Tracey L Jones2, Glenn EM Reeves1
1Hunter Area Pathology Service, University of Newcastle, Australia
2Hepatitis C Service, Gastroenterology Department, Australia
*Corresponding author: Huy A Tran, Hunter Area Pathology Service, University of Newcastle, John Hunter Hospital, Locked Bag Number 1, Hunter Mail Region Centre, Newcastle, New South Wales 2310, Australia
Received: September 25, 2014; Accepted: November 18, 2014; Published: November 20, 2014
Abstract
Introduction: Chronic hepatitis C is a common and potentially fatal hepatic infection worldwide with an expected increase in incidence of complications including hepatoma, cirrhosis, portal hypertension and potential death. Current treatment regimen includes combination interferon-alpha, ribavirin and recently direct acting antiviral agents. Treated patients who are exposed to high endogenous thyroid hormone concentrations, commonly due to interferon-induced thyroiditis, achieve a better sustained virologic response. It is therefore plausible that thyroxine can be a potential adjuvant therapy in this setting with favorable outcomes.
Case Presentation: A 24 year-old woman with hypothyroidism and chronic hepatitis C, genotype 1, underwent routine treatment of interferon and ribavirin for 48 weeks. During the course of treatment and contrary to medical advice, she deliberately self-administered excessive thyroxine doses. Sustained virologic response was achieved at the completion of therapy despite her poor prognostic factors.
Conclusion: This is the first case report of self-treatment with high doses of thyroxine during treatment with Interferon and Ribavirin, which results in a sustained virologic response, averting potential hepatic related complications. This observation potentially offers an additional adjuvant option in the treatment of chronic hepatitis C infection.
Keywords: Thyroxine; Chronic hepatitis C; Ribavirin; Interferon; Virologic Response
Abbreviations
IFN-α: Interferon-alpha; RBV: Ribavirin; SVR: Sustained Virologic Response; fT4: Free Tetra-iodothyronine; fT3: Free Tri-iodothyronine; HCV: Hepatitis C Virus; BMI: Body Mass Index; RR: Reference Range; TSH: Thyrotropin; RNA: Ribonuclei Acid; IL-28B: Interleukin-28B
Introduction
Chronic hepatitis C is a worldwide epidemic with a prevalence of up to 8% [1]. Whilst the incidence has plateaued, HCV related complications are likely to continue to increase, especially cirrhosis, end-stage hepatic failure and hepatoma [2,3]. Current treatment regimen for this chronic infection includes pegylated-Interferon-alpha (IFN-α) and Ribavirin (RBV) [4]. With the addition of protease inhibitors, success rates of up to 85%, depending on the genotype, are achieved [5]. Previous reports indicated that exposure to high endogenous thyroid hormone concentrations in the presence of IFN-α and RBV contribute to a favorable treatment response [6,7]. The cause for this is uncertain and most data has been retrospective and anecdotal. Most responses are as a result of thyroiditis with a temporary exposure to supraphysiological levels of endogenous thyroid hormones [8]. The high endogenous concentrations of these hormones are thought to be virocidal and contribute to the relatively higher Sustained Virologic Response (SVR), indicating cure. We hereby report a first clinical case where unintentional and exogenous high dose of thyroxine supplements contributed to the eradication of chronic hepatitis C infection despite other unfavorable prognostic factors.
Case Presentation
A 24 year-old female Caucasian who was undergoing chronic hepatitis C treatment presented for an assessment of her thyroid status at the 12th week of treatment. She has a past medical history of intravenous drug abuse from which she contracted the viral infection, and reported primary hypothyroidism requiring regular thyroxine supplements. The cause of her hypothyroidism could not be ascertained. She has HCV genotype 1 and required 48 weeks of therapy with combination IFN-α and RBV. She has otherwise been well with no other health concern. Clinically she was obese with weight of 87.8kg, height of 1.58m, BMI~35kg/m2, blood pressure of 120/70 with prominent and multiple tattoos. There are no signs of chronic liver disease or portal hypertension. Her liver span was 16cm in the mid-clavicular line. No goiter was detected nor were there any signs of thyrotoxicosis. Laboratory investigations showed normal renal function and full blood count. Her alanine aminotransferase was 55 IU/L (Reference Range (RR), <50), alkaline phosphatase 128 IU/L (RR, <110), albumin 38gm/L (RR, 35-45). Her Thyrotropin (TSH) was <0.03 (RR: 0.4–4.0), fT4 32.8pmol/L (RR: 11.4–23.8), fT3 7.0pmol/L (RR: 3.3–6.2), consistent with over-replacement therapy. A thyroid ultrasound revealed a small thyroid with bilateral atrophic lobes. Her anti-thyroperoxidase and anti-thyroglobulin antibodies were undetectable. Her initial HCV Ribonucleic Acid (RNA) viral load was high at 6.5 log IU/mL. Her Interleukin-28B genotypes included RS12979860 CT and RS 8099917 GT. These constitute a poor predicted response rate of ~34.7% from the genetic contribution.
Because further information regarding the patient’s thyroid condition and the indication for thyroxine therapy could not be ascertained, the patient was advised to stop thyroxine pending a full revision of the diagnosis. However for unknown reasons, she declined medical advice and insisted on continuing with thyroxine. She completed the 48 weeks of ‘triple’ antiviral therapy including the prescribed thyroxine despite frequent reminders to stop. It was strongly suspected that she far exceeded the recommended amount. During this time, monthly thyroid function tests showed that her TSH levels were suppressed, with fT4 levels ranging between 27.8 and 47.7pmol/L and fT3 3.7 to 7.0pmol/L, Figure 1. She denied any thyrotoxic symptoms. Her HCV RNA was undetectable at 12 week therapy indicating an early viral response. Subsequently, her end of treatment and 24 week follow-up HCV RNA measurements were all undetected, consistent with cure. In spite of her unfavorable IL-28B genotype and other unfavorable prognostic factors, she managed to achieve SVR. This is consistent with our previous hypothesis that thyroid disease, especially the exposure to high fT4 concentration during treatment, carries a more favourable prognosis, above that of genetic propensity.
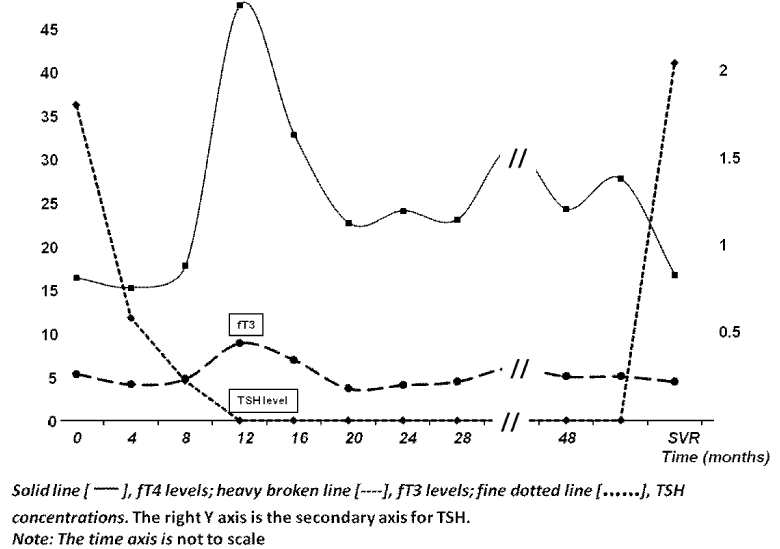
Figure 1: The fT4, fT3 and TSH profiles during the course of therapy. Note the similarity to the thyroid hormone profiles that occur in interferon-induced thyroiditis, see Figure 2.
Discussion
This is the first report regarding the use of exogenous thyroxine as ‘triple’ therapy together with IFN-α and RBV for chronic HCV infection. Previous reports suggested that the development of thyroid disease, particularly thyroiditis during treatment with combination IFN-α and RBV is a favorable prognostic factor for treatment outcome [6,7]. It is theorized that high dose of endogenous thyroxine is virocidal as well as synergizing with IFN to eradicate the virus [9,10]. This topic has been the subject of our previous publications [6-8]. This advantageous effect appears to be more powerful and more encompassing than other negative prognostic factors which are prominent in our case including genotype 1, high initial viral load and poor IL-28B genotypes [11]. Although not advisable, the ostensible and fortuitous exposure to high exogenous fT4 appears to confer a SVR advantage in this particular case, leading to the eradication of the virus. The fT4 profile mimics closely the naturally condition that occurs in interferon-induced thyroiditis where patients are exposure to similar and high concentrations of endogenous thyroid hormones, Figure 2 (adapted, with permission from reference 8).
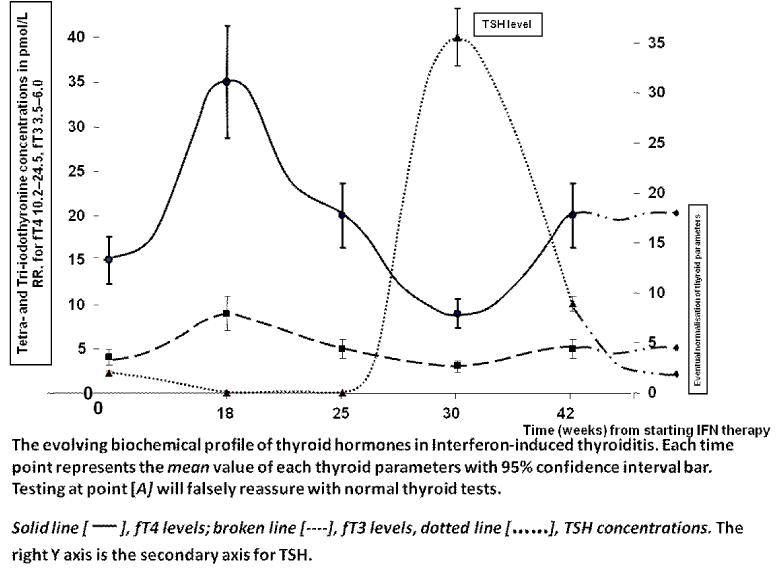
Figure 2: The evolving biochemical profile of thyroid parameters in Interferoninduced thyroiditis. Reproduced from reference 8, with permission.
This report is significant in that it involves the exogenous addition of thyroxine, albeit injudicious, and thereby opens up the possibility of its clinical application. Clearly further clinical investigations will be required to confirm this anecdotal finding. If the response rate to this ‘triple’ therapy is comparable to that of current routine combination IFN-α and RBV therapy, with or without protease inhibitor addition, it may be an improvement in treatment and more economical overall. It is critically important that this option be explored because eradication of HCV has been shown to reduce the incidence of hepatocellular carcinoma [12].
Conclusion
This is the first reported HCV infected case successfully treated using supraphysiological dose of thyroxine as part of a ‘triple’ combination therapy. This observation suggests that high thyroxine dose may be contributing to the successful treatment of HCV infection. Although clearly controversial, combination therapy including thyroxine is a relatively more economical option compared with protease inhibitor should it be proven to be equally or more effective adjunctive therapy.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief if required.
Authors’ Contributions
HAT conceived the study, participated in its design, assisted with data collection and statistical analysis, and coordinated and helped to draft the manuscript. GEMR contributed to the statistical and meta-analytical methods, and participated in the discussion and drafting of the manuscript. TLJ, EAI gathered, provided the data, and participated in the discussion and drafting of the manuscript. All authors read and approved the final revised manuscript.
Acknowledgment
We would like to sincere thank Geoffrey M Kellerman and Amanda J Caswell for their review and constructive comments of this manuscript.
References
- Williams IT, Bell BP, Kuhnert W, Alter MJ. Incidence and transmission patterns of acute hepatitis C in the United States, 1982-2006. Arch Intern Med. 2011; 171: 242-248.
- WHO website. Hepatitis C. 2014.
- Australian Government, Department of Health and Ageing. Third National Hepatitis C Strategy 2010–2013.
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: management of hepatitis C virus infection. J Hepatol. 2011; 55: 245-264.
- Ghany MG, Strader DB, Thomas DL, Seeff LB. American Association for the Study of Liver Diseases . Diagnosis, management, and treatment of hepatitis C: an update. Hepatology. 2009; 49: 1335-1374.
- Tran HA, Reeves GEM, Gibson R, Attia JR. Development of thyroid diseases in the treatment of chronic hepatitis C with a-interferon may be a good prognosticator in achieving a sustained virologic response: A meta-analysis. J Gastroenterol Hepatol, 2009; 24: 1163–1168.
- Tran HA, Jones TL, Gibson R, Reeves GEM. Thyroid disease is a favourable prognostic factor in achieving sustained virologic response in chronic hepatitis C undergoing combination therapy: A nested case control study. BMC Endocrine Disorders. 2011; 11: 10.
- Tran HA, Ianna EA, Jones TL, Reeves GE. The adjuvant role of thyroxine in the treatment of chronic hepatitis C infection. QJM. 2012; 105: 683-687.
- Lin HY, Thacore HR, Davis FB, Davis PJ. Thyroid hormone analogues potentiate the antiviral action of interferon-gamma by two mechanisms. J Cell Physiol. 1996; 167: 269-276.
- Lin HY, Thacore HR, Davis FB, Martino LJ, Davis PJ. Potentiation by thyroxine of interferon-gamma-induced HLA-DR expression is protein kinase A- and C-dependent. J Interferon Cytokine Res. 1996; 16: 17-24.
- Tran HA, Jones TL, Ianna EA, Gibson RA, Reeves GE. The reduced predictive value of interluekin 28b gene polymorphisms in a cohort of patients with thyroiditis developed during antiviral therapy for chronic hepatitis C: a preliminary study. Hepat Mon, 2012; 12: 6036.
- Morgan RL, Baack B, Smith BD, Yartel A, Pitasi M, Falck-Ytter Y. Eradication of hepatitis C virus infection and the development of hepatocellular carcinoma: a meta-analysis of observational studies. Ann Intern Med. 2013; 158: 329-337.