
Review Article
J Endocr Disord. 2015; 2(1): 1019.
Fact or Fiction? Psychiatric and Neurocognitive Dysfunction in Patients with Primary Hyperparathyroidism
Dean Libet BS¹ and Campbell MJ²*
¹Department of Endocrine Disorders, Boston University School of Medicine, USA
²Department of Endocrine Disorders, University of California, USA
*Corresponding author: Michael J Campbell, Department of Endocrine Disorders, University of California, USA
Received: November 09, 2015; Accepted: December 15, 2015; Published: December 18, 2015
Abstract
Primary Hyperparathyroidism (PHPT) is a disease characterized by elevated serum calcium in the setting of an inappropriately non-suppressed parathyroid hormone level. Classically, patients with PHPT presented with the repercussions of longstanding bone turnover such as osteoporosis, fractures, and nephrolithiasis. Today, many patients with PHPT are discovered incidentally on routine biochemical testing and many lack the classic sequelae of the disease. Instead patients describe psychiatric and neurocognitive dysfunction that may or may not be attributable to their PHPT. In this review we examine the current literature on the non-classical manifestations of PHPT, as well as the evidence for and against early surgical intervention.
Keywords: Primary hyperparathyroidism; Neurocognitive dysfunction; Hypocalcaemia
Introduction
Primary Hyperparathyroidism (PHPT) is a disorder effecting 0.2-3% of the population [1] and is characterized by elevated serum calcium in the setting of an inappropriately non-suppressed Parathyroid Hormone (PTH) level. Classically, patients with PHPT presented with the repercussions of longstanding bone turnover such as osteoporosis, fractures, and nephrolithiasis [2]. Today, patients with PHPT are usually discovered on routine biochemical screening and many are considered “asymptomatic” because they lack the common repercussions of calcium dysregulation. Many of these “asymptomatic” patients suffer from vague psychiatric and neurocognitive symptoms including: depression, anxiety, fatigue, weakness, problems with word finding and sleep disturbances [3,4]. Such symptoms have long been associated with PHPT, however the mechanisms such psychiatric and neurocognitive dysfunction exclusive and the appropriate treatment remains a topic of debate [5,6]. The purpose of this review is to describe the current thoughts on the evaluation and management of patients with psychiatric and neurocognitive manifestations of PHPT.
Background
The parathyroid glands are located in the neck and typically lye adjacent to the thyroid. They are the chief regulators of calcium homeostasis in the body and function through the secretion of PTH. PTH is probably the most important regulator of calcium metabolism and works predominately by stimulating osteoclasts to resort bone, therefore increasing serum calcium and phosphorus levels. PTH also activates 1-a -hydroxylase in the kidney, which catalyzes the conversion of non-active 25-Hydroxy (25-OH) vitamin D to activated 1,25 dihydroxy (1,25-OH) vitamin D. This leads to increased absorption of calcium and phosphorus in the gut. Finally, PTH increases reabsorption of calcium and decreases reabsorption of phosphorus in the kidney (Figure 1).
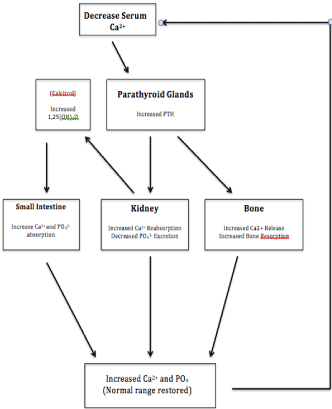
Figure 1: Physiology of calcium homeostasis in the body.
The secretion of PTH is regulated by a negative feedback mechanism. As serum calcium levels decrease the calcium receptors located on the parathyroid chief cells stimulate the parathyroid glands to increase PTH secretion. Conversely, as calcium levels rise, the calcium receptors shut down PTH secretion to maintain calcium levels at an appropriate set point. PHPT occurs as the result of the uncontrolled, autonomous secretion PTH that is not responsive to its usual negative feedback mechanism. In patients with PHPT, continued secretion of PTH despite an elevated calcium level leads to inappropriate removal of calcium from the bones and excretion via the urinary system. This can lead to osteoporosis and kidney stones (Figure 2).
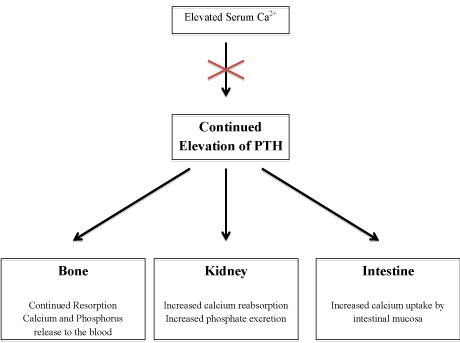
Figure 2: Pathophysiology of primary hyperparathyroidism.
In approximately 80-85% of patients, PHPT is the result of a single enlarged parathyroid adenoma that over secretes PTH. Approximately, 15% - 20% of patients have more than one enlarged parathyroid gland, which may be the result of multiple adenomas or four-gland hyperplasia [7]. Parathyroid cancer is rare and accounts for <1% of all PHPT.
Mechanisms of Neurocognitive Changes
The mechanisms of PHPT in psychiatric and neurocognitive dysfunction have yet to be fully elucidated. While elevated serum PTH and calcium levels are likely to play a major role in the clinical manifestation of PHPT, the neurocognitive deficits seen are likely the results of a complex interplay of multiple biochemical pathways. This is probably the reason that many researchers have found no correlation with calcium or PTH levels and the severity of neurologic symptoms [8,9].
Calcium
The effect of calcium on the nervous system is well established. Calcium plays a crucial role in nerve impulse conduction, regulation of neurotransmitter release, extracellular messaging and more. Hypocalcaemia can lead to seizures, tetany, paresthesias, irritability, and depression. Conversely, hypocalcaemia can result in somnolence, encephalopathy or coma [2]. Despite the large amount of information gathered on the neuronal actions of calcium, relatively little is known about the direct effects of extracellular calcium concentrations on neuronal and synaptic function. It is largely accepted that calcium in the Cerebral Spinal Fluid (CSF) is regulated independently from calcium in the bloodstream [10,5]. Although some researchers have reported a correlation between an increased concentration of serum calcium and high levels of calcium in the CSF, it is unclear whether this relationship is physiologic or secondary to the detrimental effect of calcium on the integrity of blood-brain barrier [11,12].
Using transcranial magnetic stimulation, Iacovelli et al., assessed changes in cortical exitability over the primary motor area in patients with hypocalcaemia. Patients with chronic hypocalcaemia and acute hypocalcaemia were compared with normal controls. While delivering stimulation during rest and sustained voluntary contraction of the target muscle, the authors measured changes in resting motor threshold, Motor Endplate Potential (MEP), and cortical silent periods. Their findings indicated that acute and chronic hypocalcaemia significantly decreased the MEP amplitude proportional to calcium levels. Furthermore, repeated experiments following Parathyroidectomy (PTX) showed normalized MEP facilitation in PHPT patients. This data indicates that calcium levels likely play a role in short-term synaptic plasticity. They hypothesize that ionized calcium alters synaptic plasticity either through altering the N-Methyl-D-Aspartate Receptor (NMDA) receptor or by depressing sodium current [5].
Using Single-Photon Emission Computed Tomography (SPECT), Mjaland et al., found reduced cerebral blood flow in PHPT patients, which improved in 13 of 14 patients following successful PTX. Eight of those 13 patients experienced pathological depression before surgery. Seven of those eight exhibited normalized depression scores following PTX [13]. Perrier et al., conducted a pilot study of six patients using fMRI to detect regional changes in cerebral oxygenation levels in patients with PHPT. The authors noted generalized improvement in cognitive functions and postoperative oxygenation changes in different cortical areas following PTX [14].
Parathyroid hormone
The role of PTH in neurological function remains largely unknown, however it has been hypothesized that PTH acts both directly and indirectly on the nervous system [2]. There are three types of PTH receptors and studies have demonstrated that both PTH Type 1 Receptor (PTH1R) and PTH Type 2 Receptors (PTH2R) are distributed in the central nervous system [15].
There is evidence, mostly in animal studies, that PTH directly increases dopamine turnover and neurotransmitter release, both of which are implicated in learning and memory [16]. There are also studies that implicate the role of PTH2R in behavior and anxiety [17]. The interaction between PTH and the blood brain barrier, however, remains a mystery.
PTH exerts a variety of secondary effects. One of these involves the production of Interleukin-6 (IL-6). Patients with PHPT have been shown to have elevated levels of IL-6. Similarly IL-6 levels can be increased in patients with depression, fatigue, sleep disorders [18,19].
Vitamin D
Another factor implicated in the neurological symptoms of PHPT is Vitamin D. Vitamin D deficiency has been associated with impaired cognitive functions and mood changes [20]. Unfortunately the interactions between Vitamin D and PTH are not completely understood. However, we do know that Vitamin D deficiency is more common in patients with PHPT and PTH plays a major role in the conversion of 25-OHvit D into its active form 1,25-OH vit D.
Rylander et al., demonstrated that vitamin D deficiency had a high prevalence in psychiatric patients [21]. A 2013 meta-analysis evaluated 14 observational studies and 3 interventional studies on low vitamin D levels and cognitive function. They found that low 25-OHvitD levels predicted lower executive function and processing speed [22].
Parathyroidectomy in patients with psychiatric and neurocognitive function
Assessment of patients with psychiatric and neurocognitive dysfunction from PHPT can be difficult for physicians, since such patients can be elderly with multiple chronic conditions. Such problems may be dismissed as menopause, stress or normal aging [6]. Non-classic symptoms broadly fall into two categories: 1) Psychiatric symptoms that can include depression, anxiety, and hallucinations and 2) Neurocognitive changes such as visual and spatial learning deficits and sleep disturbances [4].
Psychiatric symptoms
Changes in mood, fatigue and apathy are just some of the psychiatric manifestations that have been documented in association with PHPT since the early 1900s [2]. One of the difficulties in determining the link between PHPT and these symptoms has been a lack of appropriate tools to assess improvements in patient’s psychiatric symptoms before and after resolution of their PHPT. For the most part, researches have relied on psychiatric testing methods that have been validated in other branches of medicine, such as the Medical Outcomes Study Short Form 36 (SF-36) health survey. This questionnaire scores patients on 36 items on a scale from 1 to 6. Items range from general health, physical function, and body pain to mental health, social function, and emotional stability [23]. Despite its popularity, many have criticized the SF-36 for being too broad and generic. In response, alternative questionnaires have been used and developed. Pasieka et al., created The Parathyroid Assessment Score (PAS), which covers 13 categories as opposed the SF-36’s nine. The PAS was used in two separate studies with patients from three different countries and was found to have scores that correlated with the SF-36 [24].
Sheldon et al., prospectively evaluated 74 consecutive patients with PHPT undergoing PTX using the SF-36 both before and one year following successful surgery. The authors found that preoperatively patients with PHPT had lower scores in 5 of the 8 domains of the SF-36 when compared to the national norm. Post-parathyroidectomy patients had improvement in 7 of 8 domains and were comparable to the national norm [25]. Roman et al., conducted a prospective study of 41 patients both before and after parathyroidectomy and found significant improvement in multiple domains of the SF-36 including spatial learning, spatial working memory, and concentration, post PTX [26]. Babinska et al., reviewed the outcomes of 30 patients with PHPT who underwent PTX compared to non-PHPT patients as surgical controls. The control group was comprised of patients who qualified for surgery for either varicose veins of the lower limb, abdominal hernias, or gallbladder disease. The study group and controls were matched based on age, gender, BMI, and educational background. The authors found improvement in visual fluency and visual constructive abilities following PTX; however, they found no significant change in depression, impaired concentration, nonverbal learning, or direct memory [27]. Kahal et al., used the Hospital Anxiety and Depression scale, as well as, the Mood Rating Scale to compare 24 asymptomatic patients undergoing PTX with 23 controls undergoing hemithyroidectomy. They found that patients with PHPT had increased baseline depression that improved following surgery, however the authors found no significant change in anxiety symptoms [28].
Pasieka et al. assessed patients with PHPT who underwent PTX1 year and 10 years follow surgery and compared them to patients undergoing thyroidectomy, who served as controls. Using the PAS questionnaire, they found patients with PHPT had significantly worse quality of life scores at baseline. Patients who had a PTX (64%) showed sustained improvement that lasted at least ten years. Those undergoing thyroidectomy, showed no improvement on the PAS questionnaire following 10 years [29]. A similar case-control study, using Patient Health Questionnaire-9 (PHQ-9), found that PTX resulted in a greater improvement of depression symptoms compared to thyroid surgery or observation [30].
Unfortunately, not all studies show an improvement of psychiatric symptoms following parathyroidectomy. Bollerslev, et al., conducted a prospective study of 191 individuals with PHPT randomized into medical observation or PTX. Researchers used both the SF-36 and the Comprehensive Psychopathological Rating Scale (CPRS) to compare the two groups of patients between 1-2 years after surgery. Asymptomatic PHPT patients demonstrated lower Quality of Life (QOL) scores on the SF-36 when compared to controls at baseline. Two years following PTX, the authors found no clear improvement on either questionnaire [31]. Amstrup, et al., conducted a crosssectional study of 51 PHPT patients compared to population-based, age matched controls and found that patients reported consistently worse QOL scores (using the SF-36) 5 years after PTX [32]. Table 1 summarizes the major studies looking at the effects of PTX on psychiatric symptoms.
Author/Study
Year
Findings
Sheldon, et. al.
2007
Preoperative patients showed significant impairment in 5 of 8 domains. Postoperative patients significantly improved in 7 of 8 domains compared with preoperative scores.
2005
Preoperative PTX exhibited increase depression symptoms. Greater delays in spatial learning. Postoperative showed improvement in depression symptoms and indications that greater changes in PTH were correlated with improved learning efficiency.
Babinska, et al.
2012
Found impaired concentration, decreased nonverbal learning process, difficulties in using direct memory, verbal fluency and visual constructive abilities in PHPT patients before surgery. 1 year after surgery, there was significant improvement in direct memory, visual memory and visual-constructive abilities.
Kahal, et al.
2012
While adjusting for age and sex, postoperative PHPT patients showed significant improvement in neuropsychological symptoms, especially depression, compared with preoperative patients. Found no change in anxiety.
Pasieka, et al.
2009
Preoperative PHPT scored a mean of 318, which was decreased to 177 at 1 year post surgery (suggesting increased QOL).
Espiritu, et al.
2011
Found improvement in depression symptoms in postoperative patients compared to thyroid surgery control or observation groups.
Bollerslev, et al.
2007
Demonstrated lower QOL scores on SF-36, 2 years following PTX in PHPT patients.
Amstrup, et al.
2011
Patients consistently reported worse QOL scores on SF-36, 5 years post PTX.
PTX = parathyroidectomy
QOL = Quality of Life
PTH = parathyroid hormone
PHPT = primary hyperparathyroidism
SF-36 = Medical Outcomes Study Short Form 36
Table 1: Studies looking at the effects of parathyroid ectomy on psychiatric symptoms.
Neurocognitive symptoms
Neurocognitive manifestations of PHPT include deficits in attention, visual-spatial learning, memory, verbal fluency and sleep disturbances. The very nature of these symptoms presents an investigative challenge because of the difficulty in testing cognitive function [33].
Walker et al. evaluated cognition in 39 post-menopausal women with PHPT. Patients undergoing PTX were compared to a healthy control group of similar aged women using a variety of verbal and nonverbal tests. On baseline testing, post-menopausal women with mild PHPT had a weaker performance in cognitive testing, independent of anxiety or depression symptoms. Patients undergoing PTX had improvements in nonverbal abstraction, but also showed improvement in verbal memory, visual concentration/attention. Interestingly, there was no linear correlation with serum calcium or PTH levels with cognitive dysfunction [6].
Roman et al. used the Rey Auditory Verbal Learning Test and the Groton Maze Learning Test to assess verbal and visual learning before and after PTX. The authors were able to correlate a decrease in PTH with a decrease in both anxiety and cognitive errors. They concluded that PTH and anxiety play a role in neurocognitive function [34]. Benge, et al., attempted to identify subgroups of PHPT patients with cognitive impairment, that would benefit from PTX. Preoperatively many patients had a pattern of cognitive slowing, reductions in psychomotor speed, memory impairment and depression. After PTX, there was a trend for improvements on timed tests and depression, but a decline in memory. The authors found that older patients responded less well to surgical intervention, as did those with large changes in serum calcium and PTH [35].
PHPT has been associated with sleep disturbances, disruption of circadian rhythm, and autonomic nervous function in relation to sleep. Among the first to demonstrate a link between lower quality of sleep and PHPT was Jobern, et al., using the Comprehensive Psychopathological Rating Scale [36]. Walker et al., demonstrated similar results using the Epworth Sleepiness Scale and patient observation [37].
In a prospective randomized trial, Perrier et al. used a combination of methods to demonstrate that changes in total sleep time correlated with changes in PTH levels. The authors followed 18 patients and found that decreased serum PTH levels correlated with improved sleep and that sleepiness was associated with poorer cognitive performance. [38]. Table 2 summarizes the major studies evaluating the effects of PTX on neurocognitive function.
Author/Study
Year
Findings
Walker, et al.
2009
When evaluating post-menopausal women with PHPT, found weaker baseline performance in cognitive testing, independent of neuropsychological symptoms. Patients that underwent PTX, improved in nonverbal abstraction.
Roman, et al.
2011
Correlated a postoperative decrease in PTH with a decrease in anxiety and cognitive errors.
Benge, et al.
2009
Found a trend of improved results on timed tests and depression assessment in postoperative patients, but decline in memory.
Walker, et al.
2004
Using the Epworth Sleepiness Scale, found an association between PHPT and sleep disturbances/circadian rhythm.
Perrier, et al.
2009
Demonstrated that improved sleep and decreased daytime sleepiness correlated with decreased PTH levels.
PTX = parathyroidectomy
PTH = parathyroid hormone
PHPT = primary hyperparathyroidism
Table 2: Studies evaluating the effects of parathyroidectomy on neurocognitive function.
Recommendations for Treatment
In 2014, the Fourth International Workshop on PHPT met and a subgroup was commissioned to address key issues related to patients with asymptomatic PHPT. It was the consensus of the subgroup that recent data suggests that some patients with mild PHPT have neuropsychiatric and cognitive abnormalities and that a portion of these patients would improve after PTX. However, the group decided that it is not possible to predict which asymptomatic PHPT patients would benefits from surgery [39]. Thus they concluded that more evidence was necessary before recommending psychiatric symptoms and neurocognitive dysfunction as an indication for PTX. Other expert panels and communities have created their own guidelines for management of such patients and recommend surgery on a per patient basis, relying on physician discretion [40].
Conclusion
Although we have been investigating PHPT since the early 20th century, many questions remain unanswered. The psychiatric and neurocognitive symptoms associated with PHPT are well documented, but the mechanisms and ability to predict a durable cure following PTX remain elusive. While, current research suggests that some patients would benefit from PTX, a better understanding of this complex disease is necessary to guide physicians who care for patients who suffer from the neurocognitive and psychiatric manifestations of PHPT.
References
- Mihai, Radu, Sadler GP. “Pasieka’s Parathyroid Symptoms Scores Correlate with SF-36 Scores in Patients Undergoing Surgery for Primary Hyperparathyroidism.” World Journal of Surgery. 2008; 32: 807-814.
- Perrier, Nancy D, Weaver S, Gantela S, Rao DS. “Nonclassic, Extraskeletal Manifestations of Primary Hyperparathyroidism.” In Handbook of Parathyroid Diseases. 2012; 123-139.
- Walker, Marcella D, Mcmahon DJ, Inabnet WB, Lazar RM, Brown I, et al. “Neuropsychological Features in Primary Hyperparathyroidism: A Prospective Study.” The Journal of Clinical Endocrinology and Metabolism. 2009; 94: 1951-1958.
- Grant P, Velusamy A. What is the best way of assessing neurocognitive dysfunction in patients with primary hyperparathyroidism? J Clin Endocrinol Metab. 2014; 99: 49-55.
- Iacovelli, Elisa, Gilio F, Mascia ML, Scillitani A, Fucile S, et al. “Acute and Chronic Effects of Hypercalcemia on Cortical Excitability as Studied by 5 Hz Repetitive Transcranial Magnetic Stimulation.” The Journal of Physiology. 2011; 589: 1619-1626.
- Marcella WD, Mcmahon DJ, Inabnet WB, Lazar RM, Brown I, Vardy S, et al. “Neuropsychological Features in Primary Hyperparathyroidism: A Prospective Study.” The Journal of Clinical Endocrinology and Metabolism. 2009; 94: 1951-1958.
- Feder MK, Jessica, Sirrs S, Anderson D, Sharif J, Khan A, et al. “Primary Hyperparathyroidism: An Overview, Primary Hyperparathyroidism: An Overview.” International Journal of Endocrinology, International Journal of Endocrinology. 2011.
- Dotzenrath, Cornelia ME, Weyerbrock N, Vossough A, Verde PE, Ohmann C. “Neuropsychiatric and Cognitive Changes after Surgery for Primary Hyperparathyroidism.” World Journal of Surgery. 2006; 30: 680–685.
- Mittendorf, Elizabeth A, Wefel JS, Meyers CA, Doherty D, Shapiro SE, et al. “Improvement of Sleep Disturbance and Neurocognitive Function after Parathyroidectomy in Patients with Primary Hyperparathyroidism.” Endocrine Practice: Official Journal of the American College of Endocrinology and the American Association of Clinical Endocrinologists. 2007; 13: 338-344.
- Keep RF, Ulanski LJ, Xiang J, Ennis SR, Lorris Betz A. Blood-brain barrier mechanisms involved in brain calcium and potassium homeostasis. Brain Res. 1999; 815: 200-205.
- Murphy VAQR, Smith. “Homeostasis of Brain and Cerebrospinal Fluid Calcium Concentrations during Chronic Hypo- and Hypercalcemia.” Journal of Neurochemistry. 1986; 47: 1735-1741.
- Joborn CJ, Niklasson HF, Rastad J, Wide L, Agren H, Akerstrom G, et al. “Cerebrospinal Fluid Calcium, Parathyroid Hormone, and Monoamine and Purine Metabolites and the Blood-Brain Barrier Function in Primary Hyperparathyroidism.” Psychoneuroendocrinology. 1991; 16: 311-322.
- Mjaland OE. Normann E, Rynning HS, et al. “Regional Cerebral Blood Flow in Patients with Primary Hyperparathyroidism before and after Successful Parathyroidectomy.” The British Journal of Surgery. 2003; 90: 732–737.
- Nancy DP, Coker LH, Rorie KD, Burbank NS, Kirkland KA, Leah V. “Preliminary Report: Functional MRI of the Brain May Be the Ideal Tool for Evaluating Neuropsychologic and Sleep Complaints of Patients with Primary Hyperparathyroidism.” World Journal of Surgery. 2006; 30: 686-696.
- Wang T, Palkovits M, Rusnak M, Mezey E, Usdin TB. Distribution of parathyroid hormone-2 receptor-like immunoreactivity and messenger RNA in the rat nervous system. Neuroscience. 2000; 100: 629-649.
- Harvey S, Hayer S, Sloley BD. Parathyroid hormone-induced dopamine turnover in the rat medial basal hypothalamus. Peptides. 1993; 14: 269-274.
- Clementi GF, Prato DA, Cavaliere S, Benedetto AD, Leone F, Scapagnini U, et al. “Effects of Calcitonin, Parathyroid Hormone and Its Related Fragments on Acquisition of Active Avoidance Behavior.” Physiology & Behavior. 1984; 33: 913-916.
- Rohleder N, Aringer M, Boentert M. Role of interleukin-6 in stress, sleep, and fatigue. Ann N Y Acad Sci. 2012; 1261: 88-96.
- Schwalbe S, Ernst, Hansen K, Schmidt F, Schrezenmeier H, Marshall L, et al. “Acute Effects of Recombinant Human Interleukin-6 on Endocrine and Central Nervous Sleep Functions in Healthy Men.” The Journal of Clinical Endocrinology & Metabolism. 1998; 83: 1573-1579.
- Wilkins, Consuelo H, Sheline YI, Roe CM, Birge SJ, Morris JC. “Vitamin D Deficiency Is Associated with Low Mood and Worse Cognitive Performance in Older Adults.” The American Journal of Geriatric Psychiatry: Official Journal of the American Association for Geriatric Psychiatry. 2006; 14: 1032-1040.
- Rylander M, Verhulst S. Vitamin D insufficiency in psychiatric inpatients. J Psychiatr Pract. 2013; 19: 296-300.
- Annweiler, Cedric, Odasso MM, Llewellyn DJ, Devantoy SR, Duque G, et al. “Meta-Analysis of Memory and Executive Dysfunctions in Relation to Vitamin D.” Journal of Alzheimer’s Disease: JAD. 2013; 37: 147-171.
- Ware JE, Sherbourne CD. “The MOS 36-Item Short-Form Health Survey (SF-36). Conceptual Framework and Item Selection.” Medical Care. 1992; 30: 473-483.
- Pasieka, Janice L, Parsons LL, Demeure MJ, Wilson S, Malycha P, et al. “Patient-Based Surgical Outcome Tool Demonstrating Alleviation of Symptoms Following Parathyroidectomy in Patients with Primary Hyperparathyroidism.” World Journal of Surgery. 2002; 26: 942-949.
- Sheldon DG, Lee FT, Neil NJ, Ryan JA Jr. Surgical treatment of hyperparathyroidism improves health-related quality of life. Arch Surg. 2002; 137: 1022-1026.
- Sanziana AR, Sosa JA, Mayes L, Desmond E, Boudourakis L, Lin R, et al. “Parathyroidectomy Improves Neurocognitive Deficits in Patients with Primary Hyperparathyroidism.” Surgery. 2005; 138: 1121-1128.
- Babinska, Dominika, Stefaniak T, Oseka T, Babinska A, Nowak W, et al. “Evaluation of Selected Cognitive Functions before and after Surgery for Primary Hyperparathyroidism.” Langenbeck’s Archives of Surgery. 2012; 397: 825-831.
- Kahal, Hassan, Aye M, Rigby AS, Atkin SL. “The Effect of Parathyroidectomy on Neuropsychological Symptoms and Biochemical Parameters in Patients with Asymptomatic Primary Hyperparathyroidism.” Clinical Endocrinology. 2012; 76: 196-200.
- Pasieka JL, Parsons L, Jones J. The long-term benefit of parathyroidectomy in primary hyperparathyroidism: a 10-year prospective surgical outcome study. Surgery. 2009; 146: 1006-1013.
- Rachel PE, Kearns AE, Vickers KS, Grant C, Ryu E, Wermers RA. “Depression in Primary Hyperparathyroidism: Prevalence and Benefit of Surgery.” The Journal of Clinical Endocrinology and Metabolism. 2011; 96: 1737-1745.
- Bollerslev, Jens, Jansson S, Mollerup CL, Lundgren E, Varhaug JK, et al. “Medical Observation, Compared with Parathyroidectomy, for Asymptomatic Primary Hyperparathyroidism: A Prospective, Randomized Trial.” The Journal of Clinical Endocrinology and Metabolism. 2007; 92: 1687-1692.
- Kristine AA, Rejnmark L, Mosekilde L. “Patients with Surgically Cured Primary Hyperparathyroidism Have a Reduced Quality of Life Compared with Population-Based Healthy Sex-, Age-, and Season-Matched Controls.” European Journal of Endocrinology / European Federation of Endocrine Societies. 2011; 165: 753-760.
- Alex G, Morris L, Pasieka J, Perrier N. Nonclassical symptoms of primary hyperparathyroidism and their response to parathyroidectomy. Am Surg. 2013; 79: 337-343.
- Roman, Sanziana A, Sosa JA, Pietrzak RH, Snyder PJ, Daniel C, et al. “The Effects of Serum Calcium and Parathyroid Hormone Changes on Psychological and Cognitive Function in Patients Undergoing Parathyroidectomy for Primary Hyperparathyroidism.” Annals of Surgery. 2011; 253: 131-137.
- Jared FB, Perrier ND, Massman PJ, Meyers CA, Kayl AE, Wefel JS. “Cognitive and Affective Sequelae of Primary Hyperparathyroidism and Early Response to Parathyroidectomy.” Journal of the International Neuropsychological Society: JINS. 2009; 15: 1002-1011.
- Joborn C, Hetta J, Johansson H, Rastad J, Agren H, Akerstrom G, et al. Psychiatric morbidity in primary hyperparathyroidism. World J Surg. 1988; 12: 476-481.
- Walker, Paloyan R, Paloyan E, Gopalsami E. “Symptoms in Patients with Primary Hyperparathyroidism: Muscle Weakness or Sleepiness.” Endocrine Practice: Official Journal of the American College of Endocrinology and the American Association of Clinical Endocrinologists. 2004; 10: 404-408.
- Nancy DP, Balachandran D, Wefel JS, Jimenez C, Busaidy N, Morris GS, et al. “Prospective, Randomized, Controlled Trial of Parathyroidectomy versus Observation in Patients with ‘Asymptomatic’ Primary Hyperparathyroidism.” Surgery. 2009; 146: 1116-1122.
- Silverberg, Shonni J, Clarke BL, Peacock M, Bandeira F, Boutroy S, et al. “Current Issues in the Presentation of Asymptomatic Primary Hyperparathyroidism: Proceedings of the Fourth International Workshop.” The Journal of Clinical Endocrinology and Metabolism. 2014; 99: 3580-3594.
- Bilezikian, John P, Brandi ML, Eastell R, Silverberg SJ, Udelsman R, et al. “Guidelines for the Management of Asymptomatic Primary Hyperparathyroidism: Summary Statement from the Fourth International Workshop.” The Journal of Clinical Endocrinology and Metabolism. 2014; 99: 3561-3569.