
Case Report
J Endocr Disord. 2016; 3(1): 1023.
Live Birth after Controlled Ovarian Hyperstimulation (COH) by Luteinizing Hormone Free During IVF/ET Cycle in a Congenital Panhypopituitarism Woman
Shen SY, Hsieh CC, Chen CH* and Tzeng CR
Department of Obstetrics and Gynecology, Taipei Medical University Taiwan
*Corresponding author: Chi-Huang Chen, Center for Reproductive Medicine and Department of Obstetrics and Gynecology, College of Medicine and Taipei Medical University Hospital, No.252, Wusing St., Sinyi District, Taipei City 110, Taiwan
Received: June 08, 2016; Accepted: July 20, 2016; Published: July 25, 2016
Abstract
Introduction: To report a live birth in women with panhypopituitarism who received Growth Hormone (GH) and low dose human Chorionic Gonadotropin (hCG) with Follicle Stimulation Hormone (FSH) and Luteinizing Hormone (LH) resulted in shorter stimulation days.
Keywords: Controlled Ovarian Hyperstimulation (COH); Luteinizing hormone; IVF/ET; Congenital panhypopituitarism woman; HCG A 29-year-old woman with panhypopituitarism underwent novel ovulation induction with rFSH, growth hormone and low dose hCG achieving live birth. Lower costs and shorter stimulation days were observed compared with prior attempt at another medical center.
Conclusion: Ovulation induction in women with panhypopituitarism requires both Follicle Stimulation Hormone (FSH) and Luteinizing Hormone (LH) to achieve optimal follicular growth. Supplementation with GH and low dose hCG can shorten stimulation days at a much lower cost and subsequently a successfully live birth.
Keywords: Controlled Ovarian Hyperstimulation (COH); Luteinizing hormone; IVF/ET; Congenital panhypopituitarism woman; HCG
Introduction
World Health Organization (WHO) type I amenorrhea women are vulnerable to infertility due to a lack of endogenous gonadotropins, with endogenous gonadotropins necessary with respect to normal ovulation. These patients often are prescribed human Menopausal Gonadotropin (hMG) [1] in order to achieve adequate Ovulation Induction (OI). Poor response, however, can occur with inappropriate gonadotropin treatment. Other than women with type I amenorrhea, patients with panhypopituitarism, case study reported herein, renders inefficient anterior pituitary gland function and thus compromising normal prolactin activity. Taken together, panhypopituitarism is subject to multidisciplinary OI in contrast to WHO type I amenorrhea. Growth hormone may improve follicle growth in patients with panhypopituitarism, and adjuvant GH with hMG results in successful ovulation and pregnancy [2]. In choosing LH, Filicori et al. [3] used low-dose human Chorionic Gonadotropin (hCG) with a potency ratio of approximately 1:6-1:8 as an alternative, and showed no signs of premature luteinization.
The present case study reports a successful live birth in a patient with panhypopituitarism treated using a pilot protocol for OI for IVF/ET at a much lower financial cost.
Case Presentation
A woman, age 29, was aware of congenital pan-hypopituitarism when seeking treatment for her second IVF/ET. She was first diagnosed at the age 7. At that time she showed short stature, with MRI showing pituitary gland hypoplasia and low levels of whole serum hormone of the anterior pituitary gland except for normal prolactin level. Treatment at that time started with L-thyroxine (100 mg/day) and hydrocortisone (20 mg/day) without any resulting congenital abnormalities, visual problems, or anosmia. At age 14 years, she received GH replacement for 5 years, until the age of 18. At age 19 years, when 162 cm in height and 52 kg in weight, GH treatment was terminated. Cyclic ethinylestradiol (30 mg/day) and levonorgestrel (75 mg/day) was then prescribed, allowing menarche that year and then this treatment continued to maintain her period.
With respect to her prior pregnancy, she underwent one cycle of IVF/ET using GH/gonadotropins stimulation protocol, with this occurring prior to her 2nd IVF/ET attempt one year ago at another medical center. The 1st stimulation was applied for 22 days, during which 2 oocytes were retrieved and 2 embryos transferred, this occurring under a total dose of 48 amp of Pergoveris (7,200 IUFSH + 3,600 IULH) and 12 amp of growth hormone (120 IU). Biochemical pregnancy was achieved (Table 1). During the second IVF/ET cycle in our reproductive center, OI was started on period day 4 with daily subcutaneous injections of 225 IU rFSH (Gonal-F®; Merck Serono) for 11 days, 600 IU hCG (Fuji®, Tokyo, Japan) for first 8 days, then increased to 750 IU for 4 more days, and GH 4 IU for 10 days. Baseline serum FSH, LH, E2 were <0.3 mIU/ml. <0.07 mIU/ml and 14.6 pg/ml. Serum level of FSH, LH, E2 and progesterone levels on the day before ovulation triggering were 14.5 mIU/mL, <0.07 mIU/ mL, 4079.4 pg/ml, 1.51 ng/ml, respectively. Two follicles with a mean diameter of 16 mm and 12 follicles with a mean diameter of 15 mm were observed, with an endometrial thickness of 11mm. 13,000 IU of hCG (Ovidrel; Merck Serono) was administered on stimulation day 12 for final oocyte maturation, and oocyte pick-up was performed 36 hours later. Total dose of 2,475 IU FSH, 7,800 IU hCG, and 40 IU of GH were administered. Four oocytes were retrieved and 3 embryos transferred. Two weeks after embryo transfer, serum b-HCG level was 335 mIU/ml. At week 6, clinical pregnancy was confirmed via fetal heart activity. A healthy baby boy weighing 2,156gm was delivered by cesarean section for premature rupture of membrane at 35 weeks gestation. Comparisons of two cycles of IVF/ET are shown in (Table 1) and (Figure 1).
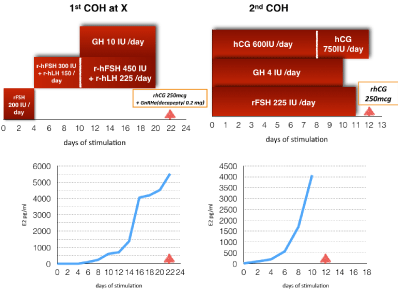
Figure 1: Comparison of two COH with and without hCG.
nohCG
hCG
FSH (IU)
7,800
2,475
LH (IU)
3,600
0
hCG (IU)
0
7800
GH (IU)
120
40
Stimulation days
22
12
Cost (USD)
4,646
1,388
No. of eggs retrieved
2
4
No. of MII oocytes
N/A
3
Fertilization rate (%)
N/A
75
No. of embryos transferred
2
3
No. of embryos frozen
0
0
No. of top quality embryos (D+3)
N/A
2 (8G1x2;6G3F30)
Delivery
Biochemical pregnancy
35+ weeks, PROM, C/S
Baby boy 2156 gm
Table 1: Compared with previous COH/IVF.
Conclusion
In early studies of hypo-gonadotropichypogonadism in women, it was shown that given FSH alone ovulation stimulation resulted in a much higher requirement of gonadotropin, slower follicular development, lower serum estrogen level, and fewer mature follicles achieved [4,5]. Co-treatment with LH can increase ovarian sensitivity to FSH, as was shown by Shoham et al [4]. A European multicenter study suggested daily r-hFSH plus daily r-hLH was effective in most hypogonadotropichypogonadism women for optimal follicular development and serum estrogen production. In a rat study, Mannaerts et al. [6] found in hypophysectomized female rats, (rFSH) increased ovarian weight and intraovarian Estradiol (E2) but not circulating E2 level in a dose-dependent manner. With the addition of hCG, both ovarian weight and circulating E2 were greatly augmented, providing a 3-fold increase in median plasma E2 concentration. The above work indicates that LH is necessary for stimulating follicular function. Filicori et al. [7] supplemented highly purified FSH with low dose hCG (50IU/day) in GnRHa down regulation patients during COH. Co-treatment with low dose hCG during COH enhanced FSH action, promoted follicle development, and shortened COH duration and reduced FSH doses thus lowering financial cost. The above lends support for the two cell two gonodotropin theory, and thus it has been suggested that LH for normal endogenous LH activity during COH may not be necessary. In contrast, findings from this case study indicated that LH activity is compulsory in early COH and a prerequisite for panhypopituitismvicitms. In particular, in our patient, with combination of hCG, she had 2,925 IU of rFSH, much lower than the dose she received in the previous stimulation cycle (7,800 IU rFSH) (Table 2). Financial costs were also less in the hCG co-treatment protocol, with further advantages of shorter stimulation days (12 days versus 22 days) and more oocytes retrieved (2 oocytes versus 4 oocytes).
40 IU GH was added based on clinical trials indicating that cotreatment with GH enhanced ovarian response to gonadotropins [8]. In women with hypogonadotropichypogonadism and growth hormone deficiency, administering adjuvant GH with gonadotropins has been shown to improve follicular development [8,9]. The role of GH for GH deficiency during COH remains controversial, except for a few studies that suggest that GH supplement may increase pregnancy rate [10].
For this patient, unexpectedly, lower oocytes were retrieved despite apparently normal E2 levels and follicular development. The reason for this suboptimal response is not fully understood. This patient has a long-standing history of hypogonadotropichypogonadism and, as such, is accustomed to a very low level of endogenous FSH and LH. Given this, the ovaries may exhibit an insufficient response to the hCG triggering maturation. This topic requires further research.
In conclusion, our experience highlights the combination of low dosage of GH, hCG and r-FSHin women with panhypopituitarism can effectively shorten the duration and thus significantly reduce the dose of gonadotropins. Compared to the use of hMG alone, it takes a longer stimulation period with concomitantly higher costs to achieve conception. Thus, the merit of the presented pilot protocol for COI for panhypopituitarism is that this approach achieves a effective COI at lower financial costs without compromising outcome.
Acknowledgement
SSY and CCH designed and conceived of the study and contributed critical reading of the manuscript and editing. HCC and TCR participated in performed literature review. All authors read and approved the final manuscript.
References
- Lewit N, Kol S. The low responder female IVF patient with hypogonadotropic hypogonadism: do not give up! Fertil Steril. 2000; 74: 401-402.
- Park JK, Murphy AA, Bordeaux BL, Dominguez CE, Session DR. Ovulation induction in a poor responder with panhypopituitarism: a case report and review of the literature. Gynecol Endocrinol. 2007; 23: 82-86.
- Filicori M, Cognigni GE, Gamberini E, Parmegiani L, Troilo E, Roset B. Efficacy of low-dose human chorionic gonadotropin alone to complete controlled ovarian stimulation. Fertil Steril. 2005; 84: 394-401.
- Shoham Z, Balen A, Patel A, Jacobs HS. Results of ovulation induction using human menopausal gonadotropin or purified follicle-stimulating hormone in hypogonadotropic hypogonadism patients. Fertil Steril. 1991; 56: 1048-1053.
- Schoot DC, Coelingh Bennink HJ, Mannaerts BM, Lamberts SW, Bouchard P, Fauser BC. Human recombinant follicle-stimulating hormone induces growth of preovulatory follicles without concomitant increase in androgen and estrogen biosynthesis in a woman with isolated gonadotropin deficiency. J Clin Endocrinol Metab. 1992; 74: 1471-1473.
- Mannaerts B, Uilenbroek J, Schot P, De Leeuw R. Folliculogenesis in hypophysectomized rats after treatment with recombinant human folliclestimulating hormone. Biol Reprod. 1994; 51: 72-81.
- Filicori M, Cognigni GE, Taraborrelli S, Spettoli D, Ciampaglia W, De Fatis CT et al. Luteinizing hormone activity supplementation enhances folliclestimulating hormone efficacy and improves ovulation induction outcome. J Clin Endocrinol Metab. 1999; 84: 2659-2663.
- Co treatment with growth hormone and gonadotropin for ovulation induction in hypogonadotropic patients: a prospective, randomized, placebo-controlled, dose-response study. European and Australian Multicenter Study. Fertil Steril. 1995; 64: 917-923.
- De Boer JA, Van der Meer M, Van der Veen EA, Schoemaker J. Growth Hormone (GH) substitution in hypogonadotropic, GH-deficient women decreases the follicle-stimulating hormone threshold for monofollicular growth. J Clin Endocrinol Metab. 1999; 84: 590-595.
- Salle A, Klein M, Pascal-Vigneron V, Dousset B, Leclere J, Weryha G. Successful pregnancy and birth after sequential co treatment with growth hormone and gonadotropins in a woman with panhypopituitarism: a new treatment protocol. Fertil Steril. 2000; 74: 1248-1250.