
Perspective
J Endocr Disord. 2017; 4(1): 1025.
Diabetic Nephropathy from RAAS to Autophagy: Old & New Players
Nakhoul N³, Nakhoul R³, Dahan I³, Farber E¹ and Nakhoul F1,3*
¹Department of Nephrology & Hypertension, Bar Ilan University, Israel
²Faculty of Medicine, Sezeged University, Hungary
³Metabolism and Diabetes Lab, Bar Ilan University, Israel
*Corresponding author: Nakhoul Farid, Department of Nephrology &Hypertension, Baruch Padeh Poriya Medical center, Lower Galilee, Faculty of Medicine to Zeged University, Bar Ilan University Ramat Gan, Israel
Received: March 29, 2017; Accepted: April 04, 2017; Published: April 07, 2017
Abbreviations
Hp: Haptoglobin; RAAS: Renin Angiotensin Aldosterone System; DM: Diabetes Mellitus; DN: Diabetic Nephropathy; ESRD: End Stage Kidney Disease; DKD: Diabetic Kidney Disease; SGL2 I: Sodium Glucose Transport 2 Inhibitor
Perspective
Diabetic Nephropathy (DN) is a leading cause of end-stage renal disease. Diabetic nephropathy occurs in up to half of patients with diabetes mellitus and currently accounts for over 45% of new cases of End Stage Renal Disease (ESRD). The classic description of Diabetic Kidney Disease (DKD) stages are glomerular hyper filtration, microalbuminuria, overt proteinuria, and a decline in the GFR, leading to ESRD and dialysis. The podocytes are primarily involved in diabetic nephropathy (Glomerular Theory), with basement membrane thickening, mesangial matrix expansion and proteinuria reflecting podocyte effusion. Proximal convolute tubule is also involved, with increased iron deposition, hypertrophy and increased number of mitochondria (Proximal tubule theory). Until recently, the use of RAAS blockers was the first-line in the treatment of early stages of DN and Blood pressure in patients with DKD. This was based on high quality randomized controlled trials throughout the range of type 2 diabetes and DKD. Despite of all the beneficial interventions available for patients with diabetes, including tight glucose control, tight blood pressure control, angiotensin converting enzyme inhibition, angiotensin II receptor or mineral corticoid receptor antagonism, diabetic nephropathy remains prevalent and is likely to progresses in most of these patients [1,2].
Recently different pathways were described as critical factors in the pathogenesis of DN such as the autophagy process. The intracellular degradation system may be important in maintaining podocyte homeostasis. However, the role of the autophagy-lysosome system especially in podocyte dysfunction under hyperglycemic stress is critical. Cells have several mechanisms to deal with stress and to maintain cellular homeostasis, such as the anti-oxidative stress response, and the Endoplasmic Reticulum (ER) stress response [3]. These findings allow us to hypothesize that diabetes can impair autophagy activity, making kidney cells, especially podocytes, vulnerable to diabetes-related metabolic stress and to consider autophagy as a new therapeutic target for DN [4-6]. Impaired autophagy may be involved in the pathogenesis of podocyte loss, leading to massive proteinuria with progression of DN to ESRD via increased activity of Mammalian Target of Rapamycin (mTORC1) system. Although current drug interventions mostly target a single risk factor, more substantial improvements of renal and cardiovascular outcomes can be expected when multiple factors are improved simultaneously [5,7,8].
The role of Mammalian Target of Rapamycin (mTOR) in the pathogenesis of diabetic nephropathy was studied by several investigators. mTOR signaling controls cellular growth, survival, and metabolism. mTOR is the catalytical subunit of 2 distinct complexes, mTOR Complexes1 and 2 ( mTORC1 and mTORC2). In some studies in diabetic mice, the mTOR inhibitor, Rapammun and Metformin were of protective effect against renal lesions [4,9].
Hyperglycemia is thought to promote oxidative stress during diabetic complications such as DN. Oxidative stress can be directly toxic to cells, causing damage to DNA, proteins and lipids. We had published that DM patients with Haptoglobin 2-2 had more DM micro and macrovascular complications than in DM patients with Haptoglobin 1-1. In Haptoglobin 2-2 DM patients, the use of vitamin E as anti-oxidant can be beneficial [10,11].
Recently different investigators had published new data regarding the protective effect of the selective vitamin D3/ VDR/klotho axis on DN. Increasing evidence demonstrates that the renoprotective action of Klotho is through inhibition of intrarenal RAAS. It has been shown that Klotho may inhibit intrarenal RAS by targeting Wnt/b-catenin signaling, decreasing glomerular fibrosis and progression of DN [12,13].
Klotho is a transmembrane protein, which serves as the cofactor for Fibroblast Growth Factor 23 (FGF-23) to bind to its cognate receptor and regulate phosphorus and vitamin D metabolism. The soluble form of klotho is reported to have antiaging properties which may be mediated via multiple systemic effects including regulation of insulin signaling and prevention of vascular calcium deposits, oxidative stress, and fibrosis. The kidney has the highest levels of klotho expression and is thought to be the major source of soluble klotho, which is released through proteolytic cleavage of the transmembrane form as well as alternative gene transcription. VDR is a well-established negative regulator of the RAAS. At cellular level, VDR directly suppresses renin gene transcription. These results seem to suggest that VDR may primarily target the local RAAS to confer cardiovascular and renal protection [13,14].
Recently calcitriol, the active compound of vitamin D, the 1,25-Dihydroxyvitamin D3(1,25 (OH)2D3, has been shown to function as a protective pathway during hyperglycemic state, functioning as negative endocrine regulator of the RAAS by suppressing the renin gene transcription. These so-called non-calcemic activities include regulation of renal and cardiovascular functions and modulation of immune response. The molecular basis for the broad functionalities of vitamin D is the expression of the Vitamin D Receptor (VDR) in virtually all tissues in the body and especially in podocytes. The activities of 1,25(OH)2D3 are mediated by the VDR, a member of the nuclear receptor super family. Relevant examples of these noncalcemic activities are regulation of the RAAS, and the Nuclear Factor B (NF-kB) pathway, two pathways involved in a broad range of pathological processes such as DN. Its well known today that klotho expression in the diabetic kidney is decreased in early stages. Our unpublished data in human and mice had shown decreased klotho and VDR expression in the glomeruli with decreased levels of the active vitamin D [14].
The recently introduced selective Sodium-Glucose Co transporter Type 2 (SGLT2) inhibitors improve glycemic control in an insulinindependent manner by blocking glucose reabsorption in the renal proximal tubule, thereby enhancing urinary glucose excretion. SGLT2 inhibitors exert multiple beneficial effects, including reductions in body weight and serum uric acid as well as BP lowering and attenuation of glomerular hyper filtration, SGL2 inhibitors ameliorate the autophagy process especially in the proximal tubular cells by reducing glucose toxic effect on mitochondrial bioenergetics. As the kidney relies on Oxidative Phosphorylation (OXPHOS) to provide the bulk requirements of ATP for tubular reabsorption, it is not surprising that mitochondrial homeostasis is strictly essential for an optimally functioning kidney, especially the PCT. Metformin an old anti-diabetic agent was found to be of great benefit in restoring autophagy in diabetic nephropathy [15-17].
The interaction between lysosomes of PCT during hyperglycemia/ DM and the axis vitamin D-Vitamin D Receptor, klotho and mTORC1-autophagy is shown in Figure 1.
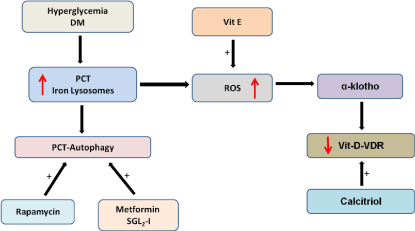
Figure 1: Schematic representation of the interaction between increased iron
deposition in the proximal convolute tubular cells (PCT) due to hyperglycemia
and klotho-vitamin D- Autophagy axis.
ROS: Reactive Oxygen Species, SGL22-1: Sodium Glucose Transport 2-
Inhibitor.
References
- Dugbartey GJ. Diabetic nephropathy: A potential savior with 'rotten-egg' smell. Pharmacol Rep. 2017; 69: 331-339.
- Doshi SM, Friedman AN. Diagnosis and Management of Type 2 Diabetic Kidney Disease. Clin J Am Soc Nephrol. 2017.
- Higgins GC and Coughlan MT. Mitochondrial dysfunction and mitophagy: the beginning and end to diabetic nephropathy? Br J Pharmacol. 2014; 171: 1917-1942.
- Yasuda-Yamahara M, Kume S, Tagawa A, Maegawa H, Uzu T. Emerging role of podocyte autophagy in the progression of diabetic nephropathy. Autophagy. 2015; 11: 2385-2386.
- Leventhal JS, Wyatt CM and Ross MJ. Recycling to discover something new: the role of autophagy in kidney disease. Kidney Int. 2017; 91: 4-6.
- Su J, Zhou L, Kong X, Yang X, Xiang X, Zhang Y. Endoplasmic reticulum is at the crossroads of autophagy, inflammation, and apoptosis signaling pathways and participates in the pathogenesis of diabetes mellitus. J Diabetes Res. 2013; 193461.
- Bhattacharjee N, Barma S, Konwar N, Dewanjee S, Manna P. Mechanistic insight of diabetic nephropathy and its pharmacotherapeutic targets: An update. Eur J Pharmacol. 2016; 791: 8-24.
- Tagawa A, Yasuda M, Kume S, Yamahara K, Nakazawa J, Chin-Kanasaki M. Impaired Podocyte Autophagy Exacerbates Proteinuria in Diabetic Nephropathy. Diabetes. 2016; 65: 755-767.
- Choi AM, Ryter SW, Levine B. Autophagy in human health and disease. N Engl J Med. 2013; 368: 651-662.
- Asleh R, Nakhoul FM, Miller-Lotan R, Awad H, Farbstein D, Levy NS. Poor lysosomal membrane integrity in proximal tubule cells of haptoglobin 2-2 genotype mice with diabetes mellitus. Free Radic Biol Med. 2012; 53: 779-786.
- Nakhoul FM, Miller-Lotan R, Awad H, Asleh R, Jad K, Nakhoul N. Pharmacogenomic effect of vitamin E on kidney structure and function in transgenic mice with the haptoglobin 2-2 genotype and diabetes mellitus. Am J Physiol Renal Physiol. 2009; 296: 830-838.
- Chokhandre MK, Mahmoud MI, Hakami T, Jafer M, Inamdar AS. Vitamin D & its analogues in type 2 diabetic nephropathy: a systematic review. J Diabetes Metab Disord. 2015; 14: 58.
- Drew DA, Katz R, Kritchevsky S, Ix J, Shlipak M, Gutierrez OM. Association between Soluble Klotho and Change in Kidney Function: The Health Aging and Body Composition Study. J Am Soc Nephrol. 2017.
- Nakhoul F, NN, Thaucho N, Farber E, Zhang H, Awawde M, Francis A, Dahan I. The Non Mineral Axis Klotho-Vitamin D in Diabetic Nephropathy: Review. Journal of Diabetes & Metabolism. 2015; 6.
- Bommel VEJ, Muskiet MH, Tonneijck L, Kramer MH, Nieuwdorp M, van Raalte DH. SGLT2 Inhibition in the Diabetic Kidney-From Mechanisms to Clinical Outcome. Clin J Am Soc Nephrol. 2017.
- Davies, MJ, Merton KW, Vijapurkar U, Balis DA, Desai M. Canagliflozin improves risk factors of metabolic syndrome in patients with type 2 diabetes mellitus and metabolic syndrome. Diabetes Metab Syndr Obes. 2017; 10: 47-55.
- Wang, XX, Levi J, Luo Y, Myakala K, Herman-Edelstein M, Qiu L, et al. SGLT2 Expression is increased in Human Diabetic Nephropathy: SGLT2 Inhibition Decreases Renal Lipid Accumulation, Inflammation and the Development of Nephropathy in Diabetic Mice. J Biol Chem. 2017.