
Review Article
J Endocr Disord. 2022; 8(1): 1048.
In Children with 46, XY DSD; HCG Testing is not always the Best Answer for Testicular Function Assessment
Khater D* and Raafat S
Associate Professor of Pediatric Endocrine and Diabetes at Faculty of Medicine, Alexandria University, Egypt
*Corresponding author: Doaa Khater, Associate Professor of Pediatric Endocrine and Diabetes at Faculty of Medicine, Alexandria University, Egypt
Received: August 23, 2022; Accepted: September 24, 2022; Published: October 01, 2022
Abstract
Hormonal levels are the hallmark for the assessment of testicular function in XY DSD (Disorders of sex development). Traditionally, it has relied on testosterone level increment after hCG (human Chorionic Gonadotropin) stimulation testing. More recently role of Sertoli cell hormones is more emphasized.
Objectives: Evaluating the role of serum anti-mullerian hormone and inhibin B on function of the pre-pubertal testis without the need for hCG stimulation test.
Method: The study was conducted in the Endocrinology Clinic in Alexandria University Children’s Hospital. All patients who present with XY DSD were tested for Testosterone (T), Dihydrotestosterone (DHT), Follicle Stimulating Hormone (FSH), Luteinizing Hormone (LH), Anti-Mullerian Hormone (AMH), inhibin B. All cases had hCG stimulation test.
Results: The hCG stimulation test was done for 32 cases. There was significant positive correlation between serum testosterone levels before and after hCG stimulation test (p <0.001). Similarly, significant correlation was identified between basal AMH and testosterone increment after hCG stimulation (p <0.001) and between basal levels of AMH and inhibin (MCp= 0.025).
Conclusion: Single measurement of basal AMH and/or inhibin B can detect the presence and function of testes by a reliable non-invasive way. Basal AMH assessment is an important tool to distinguish between cryptorchidism and anorchia. hCG test is needed in the work-up of patients with inconclusive results.
Keywords: Testosterone; Anti-mullerian hormone; Inhibin B; Testis
List of Abbreviation
AMH: Antimullerian Hormone; DHT: Dihydrotestosterone; DSD: Disorders of Sex Development; FSH: Follicle Stimulating Hormone; hCG: human Chorionic Gonadotropin; T: Testosterone; LH: Luteinizing Hormone; LCH: Leydig Cell Hypoplasia; PAIS: Partial Androgen Insensitivity Syndrome; TRS: Testicular Regression Syndrome
Introduction
The classification of 46, XY DSD includes defects in testicular development, testosterone biosynthetic defects or its action defect [1-4].
Traditionally, the standard endocrinological evaluation of 46, XY DSD cases is based on measurement of testosterone, dihydrotestosterone and androstenedione and their ratios either in mini-puberty or after human Chorionic Gonadotropin (hCG) stimulation which reflects the activity of the testicular Leydig cells. Although it leads to etiological diagnosis of XY DSD cases such as testosterone biosynthetic defects, 5-alpha reductase deficiency or abnormal androgen receptor activity, however, it is cumbersome and needs multiple sampling [5]. More recently, there has been growing evidence of the value of Sertoli cell function assessment as it secretes hormones like Anti-Mullerian Hormone (AMH) and inhibin B. Mullerian ducts have completely disappeared in the male 10 weeks after conception, but testes continue to churn out high amounts of AMH throughout childhood, when basal testosterone and gonadotropin levels have little clinical use. This makes AMH an appealing biomarker for pediatric endocrinologists, not to mention that prior gonadotropin stimulation is not required [6].
Moreover, it can be used in the evaluation of boys with nonpalpable gonads [7]. Inhibin B is another useful marker of normal testicular tissue; therefore, basal inhibin B measurement can be used as a reliable tool to assess both the existence and function of the testes and spermatogenesis afterwards [8-10].
However, the value of AMH and inhibin Bin evaluating cases with XY DSD has been challenged [2,11]. We report our experience of the role of serum AMH and inhibin Bin assessing the function of the pre-pubertal testis without the need for hCG stimulation test.
Methods
All cases of 46, XY DSD who were referred to Endocrinology Clinic in Alexandria University Children’s Hospital, Egypt for evaluation of atypical genitalia during the period from 1/1/2017- 31/12/2019 were included in the study. After informed consent and explanation of the study objectives, peripheral blood was collected for karyotyping, basal serum Testosterone (T), Dihydrotestosterone (DHT), Follicle Stimulating Hormone (FSH), luteinizing Hormone (LH), Antimullarian Hormone (AMH) and inhibin B [12]. All patients had a Human Chorionic Gonadotropin stimulation test using human chorionic gonadotropin (1500 IU/day) intra-muscular injections for three consecutive days. Venous samples were taken before the test and 24 hours after the third injection, then T/DHT ratio was calculated. We considered functioning testicular tissue when testosterone increment was more than twice the baseline value or absolute testosterone concentration above the upper limit of normal pre-pubertal range [1,13]. Patients with T/ DHT ratio >25 were diagnosed as 5 alpha reductase deficiency and below this value were diagnosed as androgen insensitivity syndrome [14]. Infants in the period of mini-puberty, testosterone and dihydrotestosterone were measured without stimulation and the T/ DHT ratio was calculated. The results of serum hormones were compared with normal reference ranges according to age [15].
Data were analyzed using IBM SPSS software package version 20.0. (Armonk, NY: IBM Corp) [16]. The research has been complied with all the relevant national regulations, institutional policies and in accordance the tenets of the Helsinki Declaration, and has been approved by the Ethics Committee of the College of Medicine, Alexandria University. Written informed consents were obtained from all patients’ legal guardians
Results
Forty cases were identified with XY DSD. Their age of presentation ranged between zero day to 13 years with a mean of 2.75 ± 3.45
Basal levels of FSH were within normal reference range for age and sex in 35 cases (87.5%) and higher than reference range in 5. Serum LH levels were within reference range for age in all cases. Basal AMH levels were within normal reference range for age in 29 cases (72.5%), 9 cases had low level of AMH and only 2 had high basal AMH. For basal inhibin B, 16 cases (40%) had normal serum level for age, 15 cases had high levels (37.5%), and 9 cases showed low level (22.5%) (Figure 1).
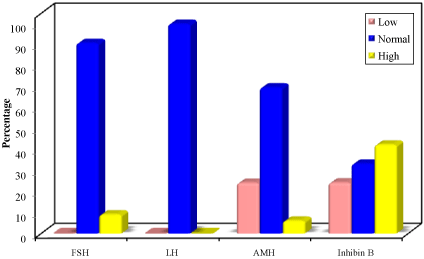
Figure 1: Distribution of the studied cases according to basal FSH, LH, AMH
and inhibin B levels for age (n =40).
FSH: Follicle Stimulating Hormone; LH= Luteinizing Hormone; AMH= Anti-
Mullerian Hormone
The hCG stimulation test was done for 32 cases (8 cases were in mini-puberty period that does not need hCG testing). The relationship between serum testosterone level before and after hCG stimulation testis shown in (Table 1).
Testosterone level (ng/ml)
Before
After
Delta testosterone #
Min. – Max.
0.01 – 3.30
0.05 – 9.72
0.0 – 7.78
0.50 ± 0.82
4.03 ± 2.28
3.72 ± 1.97
Median
0.1
4.93
2.77
P
<0.001*
#: delta (after – before)
p: p value for Wilcoxon signed ranks test for comparing between before and after stimulation.
*: Statistically significant at p = 0.05
SD= Standard Deviation
hCG= Human Chorionic Gonadotropin
Table 1: Comparison between serum testosterone level before and after hCG stimulation (n=32).
After hCG stimulation test, adequate testosterone response (increment) was observed in 26 cases (81.2%). While, the remaining 6 cases had inadequate response. The testosterone/ DHT ratio was calculated in each case. That ratio was exceeding 25 in 14 cases who were diagnosed as 5 alpha reductase deficiency. Furthermore, such ratio couldn’t be assessed in one case.
Table 2 shows the correlation between basal inhibin B, AMH, FSH and testosterone response after hCG stimulation test.
Response of testosterone
Test of sig.
P
No Response (n = 6)
Response (n = 26)
No.
%
No.
%
FSH
Low
0
0
0
0
Χ2=16.246*
FEp=0.005*
Normal
2
33.3
26
100
High
4
66.7
0
0
Inhibin B
Low
5
83.3
4
15.4
Χ2=6.789*
MCp=0.003*
Normal
1
16.7
11
42.3
High
0
0
11
42.3
AMH
Low
6
100
3
11.6
Χ2=13.812*
MCp=0.001*
Normal
0
0
22
84.6
High
0
0
1
3.8
c2, p:c2 and p values for Chi square test
MC: Monte Carlo for Chi square test
FE: Fisher Exact for Chi square test
*: Statistically significant at p = 0.05
FSH= Follicle Stimulating Hormone
LH= Luteinizing Hormone
AMH= Anti-Mullerian Hormone
Table 2: Relationship between testosterone response after hCG stimulation with basal FSH, inhibin B and AMH (n = 32).
Similarly, there was a significant correlation between basal AMH and testosterone increment after hCG stimulation i.e. delta change of serum testosterone (p <0.001) (Figure2).
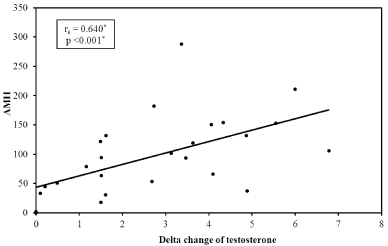
Figure 2: Correlation between Delta change of testosterone and serum AMH.
AMH: Anti-Mullerian Hormone
A positive correlation was found between basal level of AMH and inhibin (MCp= 0.003) (Table 3).
Inhibin B
Test of sig.
P
AMH
Low
Normal
High
(n= 9)
(n= 16)
(n= 15)
No.
%
No.
%
No.
%
Low
6
66.7
2
12.5
1
7.1
c2=12.180*
MCp=0.003*
Normal
3
33.3
14
87.5
12
78.6
High
0
0
0
0
2
14.3
Min. – Max.
0.01 – 163.1
17.73 - 171.10
30.70 - 333.0
H=6.886
0.074
Mean ± SD.
60.33 ± 51.61
90.65 ± 62.18
145.04 ± 98.96
Median
48.2
76.8
122
Sig. between stages
p1=0.312, p2=0.003* p3=0.170
rs(p)
0.513*(0.004*)
AMH= Anti-Mullerian Hormone
c2, p: c2 and p values for Chi square test
MC: Monte Carlo for Chi square test
H,p: H and p values for Kruskal Wallis test, Significance between groups was done using Mann Whitney test
rs: Spearman coefficient
*: Statistically significant at p = 0.05
Table 3: Relationship between serum levels of Inhibin B and AMH.
Discussion
In the current study, both AMH and inhibin B levels were generally lower than normal reference ranges for age in cases with primary gonadal failure, and they were normal in Partial Androgen Insensitivity Syndrome (PAIS), and 5a-reductase deficiency. AMH was higher than reference ranges in 2 cases with androgen insensitivity syndrome while, Inhibin B was higher in 15cases. Despite these variations, the results showed a positive correlation between mean basal levels of AMH and inhibin B which suggest a discriminatory value of the determination of AMH and inhibin B levels in the diagnosis of XY DSD cases.
Several previous reports [1,2,10,17] have shown that AMH and inhibin B are low in dysgenetic testes, undetectable in patients with anorchia and normal or high in other causes of XY DSD (androgen biosynthesis or action defect, AIS, 5a-reductase deficiency and Leydig cell hypoplasia). A study in Denmark showed that the median serum level of inhibin B hormone in patients with vanishing testes was markedly lower than those in cases of bilateral cryptorchidism [18].
A significant correlation between AMH and inhibin B was demonstrated in older patients 1-13year-old in former studies, however, they were not correlated for 0.5-1 year-old patients with XY DSD [2, 19,20].
Our study found a strong correlation between basal AMH, inhibin B, and FSH levels and hCG induced testosterone increment (p <0.001).
It worth mentioning that the need for hCG stimulation test in the work-up is controversial. Adding to that, there is an extensive range of regimens for hCG stimulation test performance and the definition of an adequate testosterone response to hCG stimulation is also unclear and may depend on the regimen and the age of the child [15]. Our data showed that a low AMH correlates well with a low hCG-stimulated level in most of the cases, however a normal AMH might not predict a normal HCG-stimulated testosterone value as in the cases with LCH. Similar findings were reported by Ahmed SF et al [1] while other investigators found that assessment of serum AMH confirms the testicular function without the need for hCG stimulation tests [17]. In our study, 32 cases -beyond the period of mini-pubertyhad hCG stimulation test. Post stimulation testosterone level showed increment after stimulation in most of them (81.2%). There was a positive relation between serum testosterone level before and after hCG stimulation test indicating an adequate leydig cell function. Patients who did not show adequate post stimulation increment of testosterone (Leydig cell dysfunction) were 2 cases with studied Testicular Regression Syndrome (TRS), 2 with gonadal dysgenesis, 1 with Leydig cell hypoplasia and 1 with 5 alpha reductase deficiency. Post stimulation inadequate testosterone response with lower level of AMH and inhibin B in the case with 5 alpha reductase deficiency is attributed to associated cryptorchidism.
The studied Testicular Regression Syndrome (TRS) cases had very low basal levels of AMH and inhibin B. Stoppa-Vaucher S et al [21] concluded that undetectable AMH level and the absence of Müllerian structures on pelvic ultrasound strongly suggests a diagnosis of bilateral anorchia as early as 3 days of age. Weintraub A et al [22] reported that assessment of AMH can distinguish between cryptorchidism and anorchia. We can conclude that a single measurement of basal AMH and/or inhibin B is highly confirmative of the presence and function of testes. Therefore, basal AMH and inhibin B might substitute the need for
Serum FSH levels, were within normal reference ranges for age in all cases except 3cases with TRS and 2patients with gonadal dysgensis. These cases had high FSH levels with low AMH and inhibin B levels. Assessment of basal FSH levels in patients with XY DSD might elucidate their etiology. High FSH with a low AMH and inhibin B can discriminate cases of defective testicular development from other causes of XY DSD. Moreover, the inverse relationship between circulating FSH and inhibin B levels observed in the current study adds to the current evidence that pre-pubertal Sertoli cells in humans can inhibit FSH release [18,23,24].
On the contrary, basal LH levels were within the normal reference ranges in all cases. Cases with primary gonadal failure showed normal LH levels despite high serum FSH levels. Previous report of 395 cases of under-masculinized 46XY males suggests that FSH is a more useful indicator of testicular dysfunction in the pre-pubertal age group being an indirect measure of Sertoli cell function.
The limitations of this study include a single center experience and a small number of patients.
In conclusion, our study confirmed that a single measurement of basal AMH and/or inhibin B can detect the presence and function of testes. Basal AMH assessment is an important tool to distinguish between cryptorchidism and anorchia. Basal FSH level is an indirect indicator of the Sertoli cell function. Future prospective studies on larger number of patientsmay decide on the real need for hCG testing in the work-up of these patients.
Author Contributions
All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
Competing Interests
The funding organization played no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the report for publication.
References
- Ahmed SF, Achermann JC, Arlt W, Balen A, Conway G, Edwards Z, et al. Society for Endocrinology UK guidance on the initial evaluation of an infant or an adolescent with a suspected disorder of sex development (revised 2015). Clin Endocrinol (Oxf). 2016; 84: 771-88.
- Hafez M, El Dayem SM, El Mougy F, Atef A, Kandil M, Galal A, et al. The role of anti-Mullerian and inhibin B hormones in the evaluation of 46, XY disorders of sex development. J Pediatr Endocrinol Metab. 2014; 27: 891-9.
- Donohoue PA. XY DSD. In: BehrmanRE, KliegmanRM, StantonBF, SchorNF, St. GameIIIJW, editorsNelson textbook of pediatrics. 20th ed. Vol. 46. Philadelphia: Saunders; 2016: 2751-9.
- Erdogan S, Kara C, Uçaktürk A, Aydin M. Etiological classification and clinical assessment of children and adolescents with disorders of sex development. J Clin Res Pediatr Endocrinol. 2011; 3: 77-83.
- Lee PA, Nordenström A, Houk CP, Ahmed SF, Auchus R, Baratz A, et al. Global Disorders of Sex Development Update since 2006: Perceptions, approach and care. Horm Res Paediatr. 2016; 85: 158-80.
- Josso N, Rey RA. What does AMH tell us in pediatric disorders of sex development? Front Endocrinol (Lausanne). 2020; 11: 619.
- Baetens D, Mladenov W, Delle Chiaie B, Menten B, Desloovere A, Iotova V, et al. Extensive clinical, hormonal and genetic screening in a large consecutive series of 46, XY neonates and infants with atypical sexual development. Orphanet J Rare Dis. 2014; 9: 209.
- Iliadou PK, Tsametis C, Kaprara A, Papadimas I, Goulis DG. The Sertoli cell: novel clinical potentiality. Hormones (Athens). 2015; 14: 504-14.
- Moradi M, Alemi M, Moradi A, Izadi B, Parhodah F, Torkaman Asadi F. Does inhibin B help us to confidently refuse diagnostic testicular biopsy in azoospermia?. Iran J Reprod Med. 2012; 10: 243-8.
- Grinspon RP, Loreti N, Braslavsky D, Bedecarrás P, Ambao V, Gottlieb S, et al. Sertoli cell markers in the diagnosis of paediatric male hypogonadism. J Pediatr Endocrinol Metab. 2012; 25: 3-11.
- Hughes IA, Nihoul-Fékété C, Thomas B, Cohen-Kettenis PT. Consequences of the ESPE/LWPES guidelines for diagnosis and treatment of disorders of sex development. Best Pract Res Clin Endocrinol Metab. 2007; 21: 351-65.
- Wang C, Wu J, Zong C, Xu J, Ju H. Chemiluminescent immunoassay and its applications. Chin J Anal Chem. 2012; 40: 3-10.
- Ng KL, Ahmed SF, Hughes IA. Pituitary-gonadal axis in male under masculinisation. Arch Dis Child. 2000; 82: 54-8.
- Phelan N, Williams EL, Cardamone S, Lee M, Creighton SM, Rumsby G, et al. Screening for mutations in 17 β-hydroxysteroid dehydrogenase and androgen receptor in women presenting with partially virilised 46, XY disorders of sex development. Eur J Endocrinol. 2015; 172: 745-51.
- Andersson AM, Toppari J, Haavisto AM, Petersen JH, Simell T, Simell O, et al. Longitudinal reproductive hormone profiles in infants: peak of inhibin B levels in infant boys exceeds levels in adult men. J Clin Endocrinol Metab. 1998; 83: 675-81.
- Kirkpatrick LA, Feeney BC. A simple guide to IBM SPSS Statistics for version 20.0. student ed. Wadsworth, Belmont, CA: Cengage Learning; 2013.
- Grinspon RP, Rey RA. New perspectives in the diagnosis of pediatric male hypogonadism: the importance of AMH as a Sertoli cell marker. Arq Bras Endocrinol Metab. 2011; 55: 512-9.
- Thorup J, Petersen BL, Kvist K, Cortes D. Bilateral vanished testes diagnosed with a single blood sample showing very high gonadotropins (folliclestimulating hormone and luteinizing hormone) and very low inhibin B. Scand J Urol Nephrol. 2011; 45: 425-31.
- Cortes D, Clasen-Linde E, Hutson JM, LiR, Thorup J. The Sertoli cell hormones inhibin-B and anti Müllerian hormone have different patterns of secretion in prepubertal cryptorchid boys. J Pediatr Surg. 2016; 51: 475-80.
- Kubini K, Zachmann M, Albers N, Hiort O, Bettendorf M, Wölfle J, et al. Basal inhibin B and the testosterone response to human chorionic gonadotropin correlate in prepubertal boys. J Clin Endocrinol Metab. 2000; 85: 134-8.
- Stoppa-Vaucher S, Djemli A, Van Vliet G. Undetectable AMH at 3 days of age: a clue to bilateral anorchia. Clin Biochem. 2010; 43: 1373-4.
- Weintraub A, Eldar-Geva T. Anti-Mullerian hormone (AMH) determinations in the pediatric and adolescent endocrinepractice. Pediatr Endocrinol Rev. 2017; 14: 364-70.
- Juniarto AZ, van der Zwan YG, Santosa A, Ariani MD, Eggers S, Hersmus R, et al. Hormonal evaluation in relation to phenotype and genotype in 286 patients with a disorder of sex development from Indonesia. Clin Endocrinol (Oxf). 2016; 85: 247-57.
- Raivio T, Dunkel L. Inverse relationship between serum inhibin B and FSH levels in prepubertal boys with cryptorchidism. Pediatr Res. 1999; 46: 496- 500.