
Case Report
Austin ENT Open Access. 2024; 4(1): 1013.
Mesenchymal Chondrosarcoma of the Maxillary Sinus: A Case Report
Zakaria El Hafi1,2*; Khalil Hjaouj1,2; Ayoub Bouteyine2,3; Razika Bencheikh1,2; Mohamed Anass Benbouzid1,2; Adelilah Oujilal1,2; Nadia Cherradi2,3; Leila Essakalli1,2
1Department ENT and Cervico-Facial Surgery, Hospital of Specialties/ CHU Ibn Sina Rabat, Morocco
2Faculty of Medicine and Pharmacy, Mohamed V University Rabat, Morocco
3Department of Cytopathology, Hospital of Specialties, CHU Ibn Sina Rabat, Morocco
*Corresponding author: Zakaria El Hafi Department of ENT and Cervico-Facial Surgery, Hospital of Specialties, CHU Ibn Sina Rabat, Morocco. Email: zakielhafi@gmail.com
Received: April 01, 2024 Accepted: May 01, 2024 Published: May 08, 2024
Abstract
head and neck Mesenchymal chondrosarcoma is a rare, highly aggressive entity. In this region, it has a strong affinity for the maxillofacial skeleton. Diagnosis is complex, often requiring molecular biology techniques. Treatment is essentially surgical, while adjuvant radiotherapy can reduce tumour mass when it extends beyond the limits of the bone, but has no real effect on prognosis. Chemotherapy, too, has shown no efficacy. Despite good excision with complete local control, close monitoring is necessary to detect any development of distant metastases, which is an indication of a poor prognosis.
Keywords: Mesenchymal chondrosarcoma; Maxillary sinus immunohistochemistry; A case report
Introduction
Mesenchymal chondrosarcoma is a rare, highly differentiated malignancy, a distinct entity from conventional or dedifferentiated chondrosarcoma and accounts for less than 2% of all chondrosarcomas and 0.1% of all head and neck malignancies [1]. Or it has a predilection for the maxillofacial skeleton, particularly in the mandible and maxilla [2].
It generally affects younger patients, with a poor prognosis.
The aim of this study is to illustrate the difficulties of diagnosis, given the non-specificity of the clinical, radiological and histopathological signs, necessitating recourse to molecular biology, and the therapeutic difficulties which, despite well-controlled surgical excision, remain unsatisfactory.
Case Report
Patient aged 18, with no previous history, who consulted for recurrent left unilateral epistaxis of low abundance associated with permanent ipsilateral nasal obstruction, anosmia, purulent rhinorrhea and headache.
The Examination revealed abolished nasal airflow on the left side and limited mouth opening. Endoscopy revealed a purplish budding mass fused to the upper surface of the inferior turbinate and the outer wall of the middle turbinate, not bleeding on contact and extending into the cavum.
The Blondeau CT scan revealed a voluminous lytic tissue process centered on the pterygoid process, irregular, hypodense in spontaneous contrast with hyperdense hematic areas, intensely and heterogeneously enhanced after contrast injection, containing areas of necrosis, measuring 74x57x48 mm (APxTxH). This process lysed the posterior and medial walls of the left maxillary sinus, the floor of the orbit, the floor of the sphenoidal sinus and the hard palate, with discreet endo-buccal extension. No calcifications were evident. It makes contact with the left internal carotid artery over less than 180°, with no parietal irregularities or endoluminal thrombosis.
Bilateral upper jugulo-carotid adenopathy’s were present, the largest measuring 12mm on the right and 10mm on the left, with a short axis (Figure 1).
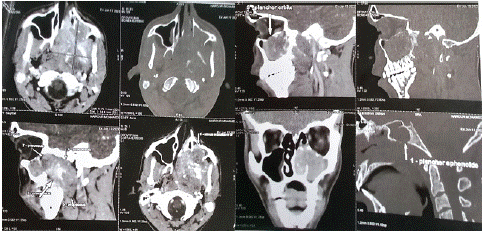
Figure 1: Nasosinus CT scan in axial and sagittal sections showing localization, locoregional extension and lytic appearance.
The Surgery began with a partial turbinectomy of the inferior turbinate and middle turbinate, exposing the extensive mass in the maxillary sinus. Several tumor fragments were sent for extemporaneous examination, and were found to be round-cell tumors requiring immunostaining. Next, the tumor was removed, filling the entire nasal cavity and sinus and invading the naso-sinusal wall and the posterior part of the hard palate. This was followed by an ethmoidectomy and a middle meatotomy.
The patient reported a marked improvement in clinical signs, with a return of his sense of smell and disappearance of rhinorrhea and headaches.
Histological examination revealed a biphasic tumor proliferation with a predominantly small, round basophilic cell contingent of high density and mitosis. The stroma is small, richly vascularized with hemangiopericytoma vessels. A second calcified chondroid tumor contingent is present in some areas. This led to the diagnosis of a mesenchymal chondrosarcoma (Figure 2).
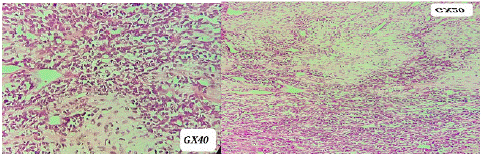
Figure 2: Pathological findings in favor of mesenchymal chondrosarcoma.
The patient was then referred to the Oncology Department, where he underwent radio chemotherapy.
Discussion
Chondrosarcoma is a malignant tumor of cartilage, bone and mesenchymal origin [2]. Mesenchymal chondrosarcoma is a rare subtype, accounting for less than 1% of total incidence. Its location in the head and neck region is uncommon, representing only 0.1% of carcinomas in this area, with an affinity for the bony facial mass [3].
Other less commonly encountered sites include the sinonasal tract, orbit and thyroid gland [4]. Areas affected in the sinonasal tract include, in decreasing order of frequency, the maxillary sinus, ethmoidal sinuses and nasal cavity [4]. Male predominance is noted between the fourth and seventh decades of life [5], although our patient is much younger than average. Lymph node and distant metastases are rare, accounting for 5.6% and 6.7% of cases respectively [6].
Clinical signs of mesenchymal chondrosarcoma lack specificity, and depend on location, making preoperative diagnosis difficult. Symptoms include facial deformity, maxillo-mandibular malocclusion and nasal obstruction. Characteristic of mesenchymal chondrosarcoma
is the tendency to erode and extend the bony margins in which it develops. For this 18-year-old, the main symptoms are nasal obstruction and epistaxis, making the diagnosis easy to confuse, particularly with a nasopharyngeal fibroma.
Mesenchymal chondrosarcoma is characterized by its hematogenous dissemination, so it frequently metastasizes to the lung and bone [7], and unlike conventional chondrosarcoma, the mesenchymal subtype shows locoregional and distant lymph node metastases.
Computed Tomography (CT) and Magnetic Resonance Imaging (MRI) are the two imaging tests essential for diagnosis, to rule out differential diagnoses, guide biopsy and, finally, staging.
CT usually shows a well-circumscribed mass of iso or hypodensity, with dotted, low-density calcifications and osteosclerosis of the margin [8]. Adjacent bone lysis is frequently found.
MRI can clarify soft-tissue extension, particularly to sensory and vital structures, and distinguishes between granulomatous tissue and recurrence in follow-up after surgery [9]. In contrast to conventional chondrosarcomas (characterized by extremely high signal intensity on T2-weighted images), non-calcified parts of mesenchymal chondrosarcomas show signal intensity less than or equal to that of gray matter on T1-weighted images, and are isointense to gray matter on T2-weighted images. T1-weighted images after gadolinium injection show inhomogeneous enhancement in calcified and non-calcified areas. Although our patient did not benefit from MRI, this technique could have provided valuable information.
Biopsy is performed endonasal, and definitive diagnosis is histological an immunohistochemical, it remains difficult and poses challenges in distinguishing between chondromas and chondrosarcomas, which are classified into three grades based on tissue density, nuclear differentiation and nuclear size25. The histological types include the mesenchymal variant, the most aggressive, occurring in two-thirds of cases before the age of 30 and presenting an advanced grade26. In small biopsy samples, when the cartilage component is absent, several small-cell neoplasms may enter into the differential diagnosis. These include hemangiopericytoma, synovial sarcoma, Ewing's sarcoma, PNET (primitive peripheral neuroectodermal tumor), olfactory neuroblastoma, rhabdomyosarcoma, small-cell osteosarcoma, anaplastic carcinoma (especially "oat" cell carcinoma), leukemic deposits (granulocytic sarcoma) and malignant lymphoma [3].
The main treatment is surgery, regardless of location, with very wide margins of excision. Radical surgery often results in significant aesthetic and/or functional abnormalities, necessitating complex reconstruction.
The efficacy of neoadjuvant modalities for mesenchymal chondrosarcoma is unproven. Several series report the use of preoperative radiotherapy to reduce tumor volume prior to radical resection [4]. Irradiation can reduce a bulky mass that extends beyond the bony limits of the maxillofacial skeleton into the soft tissues, with the hope of reducing extension by continuity or micro-metastases. However, even with radiotherapy-induced cytoreduction, the extent of planned en bloc resection should remain unchanged.
Neoadjuvant chemotherapy has not significantly improved tumor response in terms of tumor volume reduction, and its indication is limited to recurrences and metastases.
The post-treatment clinical course of patients with mesenchymal chondrosarcoma of the head and neck is variable. It ranges from complete tumor response and long-term survival to rapid local tumor progression with generalized metastases and death within a few months. Overall survival at 5 and 10 years is 55% and 27% respectively [3]. However, tumors of the head and neck appear to have a better outcome, particularly those associated with the facial skeleton. Vencio et al [10] reported 5- and 10-year survival rates for patients with jaw lesions of 82% and 56%, respectively. A recent study of mesenchymal chondrosarcoma of the sinonasal tract showed 5- and 10-year survival rates of 64% and 55%, respectively, with a mean survival of around 12 years, suggesting that early diagnosis of lesions in the head and neck may lead to better survival for mesenchymal chondrosarcoma.
Classical histological features of prognostic relevance, such as nuclear pleomorph (as a parameter of tumor classification) and tumor differentiation, are not informative in mesenchymal chondrosarcoma [9]. It has been suggested that proliferative activity, measured by specialized immunohistochemical staining, could be an important prognostic parameter.
Conclusion
Chondrosarcoma of the facial sinuses is a rare and aggressive tumor with a slow evolution. Clinical imaging is very important at all stages of diagnosis, treatment and monitoring. Surgical excision is an important factor in the success of treatment and in reducing the frequency of recurrences.
Early diagnosis of head and neck cancer appears to improve overall survival and quality of life.
Author Statements
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Informed Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images.
All data are available in the patient's medical file.
All authors approved final version of the manuscript.
All authors approved final version of the manuscript.
References
- Lichtenstein L, Bernstein D. Unusual benign and malignant chondroid tumors of bone. A survey of some mesenchymal cartilage tumors and malignant chondroblastic tumors, including a few multicentric ones, as well as many atypical benign chondroblastomas and chondromyxoid fibromas. Cancer. 1959; 12: 1142–57.
- Bertoni F, Picci P, Bacchini P, Capanna R, Innao V, Bacci G, et al. Mesenchymal chondrosarcoma of bone and soft tissues. Cancer. 1983; 52: 533–41.
- Nakashima Y, Unni KK, Shives TC, Swee RG, Dahlin DC. Mesenchymal chondrosarcoma of bone and soft tissue. A review of 111 cases. Cancer. 1986; 57: 2444–53.
- Knott PD, Gannon FH, Thompson LDR. Mesenchymal chondrosarcoma of the sinonasal tract: a clinicopathological study of 13 cases with a review of theliterature. Laryngoscope. 2003; 113: 783–90.
- Batsakis JG, Solomon AR, Rice DH. The pathology of the head and neck tumors: neoplasm of cartilage, bone and the notochord, part 7. head Neck Surg. 1980; 3: 43-57.
- Sei Young Lee, Young Chang Lim, Mee Hyun Song, Jae Yeon Seok, Won sang Lee, and Eun Chang Choi. Chondrosarcome of the head and neck. Yonsei Medical Journal. 2005; 46: 228-232.
- Pellitteri PK, Ferlito A, Fagan JJ, Suarez C, Devaney KO, Rinaldo A. Mesenchymal chondrosarcoma of thehead and neck. Oral Oncol. 2007; 43: 970-5.
- Gupta SR, Saran RK, Sharma P, Urs AB. A rare case of extraskeletal mesenchymal chondrosarcoma with dedifferentiation arising from the buccalspace in a young male. J Maxillofac Oral Surg. 2015; 14: 293-299.
- Yang BT, Wang ZC, Liu S, et al. CT and MRI diagnosis of chondrosarcoma in sinonasal and orbital region. Zhonghua Fang She Xue Za Zhi. 2006; 40: 572-576.
- Vencio EE, Reeve CM, Unni KK, Nascimento AG. Mesenchymal chondrosarcoma of the jaw bones: clinicopathologic study of 19 cases. Cancer 1998; 82: 2350–5.