
Case Report
Austin ENT Open Access 2024; 4(1): 1014.
Extraocular Sebaceous Carcinoma of the Nasal Ala A Case Report
Ayyad K1,3*; El Azzouzi R2,3; Bouanani O2,3; Boulaadass M2,3; Essakalli L1,3
1Department of Otorhinolaryngology and Neck-Face surgery Hospital of Specialities Rabat, Morocco
2Department of Maxillofacial Surgery Hospital of Specialities Rabat, Morocco
3Faculty of Medecine and Pharmacy of Rabat, Mohammed V University Rabat, Morocco
*Corresponding author: Ayyad K Department of Otorhinolaryngology and Neck-Face Surgery, Mohammed V University, Rabat, Morocco. Tel: 212678123018 Email: Ayyad.ka@gmail.com
Received: April 08, 2024 Accepted: May 03, 2024 Published: May 10, 2024
Abstract
Sebaceous carcinoma is a rare and potentially aggressive cutaneous carcinoma that develops from the sebaceous glands of the skin. It can occur in the periocular or extraocular region. We report the case of an 87-year-old patient who presented with a skin lesion on the right nasal ala evolving over 1 year, yellowish in color, measuring 1 cm in size. Biopsy excision of the lesion with 1 cm safety margins revealed sebaceous carcinoma. There was no indication for adjuvant radiotherapy, and no recurrence was noted after 3 years of follow-up. Several risk factors have been implicated in this type of tumor, but the exact etiology remains unknown. Sebaceous carcinoma can be associated with Muir-Torre syndrome. Treatment is primarily surgical and involves Mohs micrographic surgery or wide local excision with adequate safety margins. The role of adjuvant radiotherapy in overall survival is uncertain. Regular and long-term surveillance is necessary. Screening for sebaceous carcinoma in at-risk populations remains controversial.
Keywords: Sebaceous carcinoma; Extraocular; Muir torre syndrome; Surgery
Introduction
Sebaceous carcinoma is a rare and potentially aggressive cutaneous carcinoma that develops from the sebaceous glands of the skin. It was initially described by Straatsma in 1956. While it most commonly develops in the periocular region, extraocular locations have been reported in the literature.The clinical presentation is nonspecific. Diagnosis is based on biopsy. The diagnosis of Muir-Torre syndrome should be considered when a sebaceous neoplasm is discovered.
We report a clinical case of an 87-year-old patient presenting with a skin lesion on the nasal wing, with biopsy excision revealing sebaceous carcinoma.
Clinical Observation
An 87-year-old patient, hypertensive and under treatment, with no personal or family history of colorectal cancer, presented for consultation regarding a skin lesion on the right nasal wing evolving over 1 year. The lesion was painless, progressively increasing in size, and without other associated signs. Clinical examination revealed nodular lesions with a yellowish color, located on the right nasal wing, measuring 1 cm in size (Figure 1). The rest of the facial skin examination was unremarkable, and lymph node areas were free. The patient underwent biopsy excision of the lesion with 1 cm safety margins (Figure 2). The final histopathological examination confirmed a diagnosis of sebaceous carcinoma, without intravascular tumor emboli and without perineural tumor sheathing. The patient did not have indications for adjuvant radiotherapy after multidisciplinary discussion. No recurrence was noted after 3 years of follow-up.
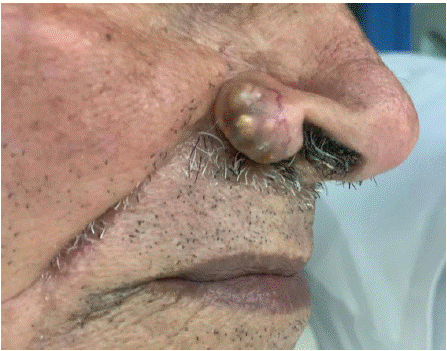
Figure 1: Lesion of the nasal ala.
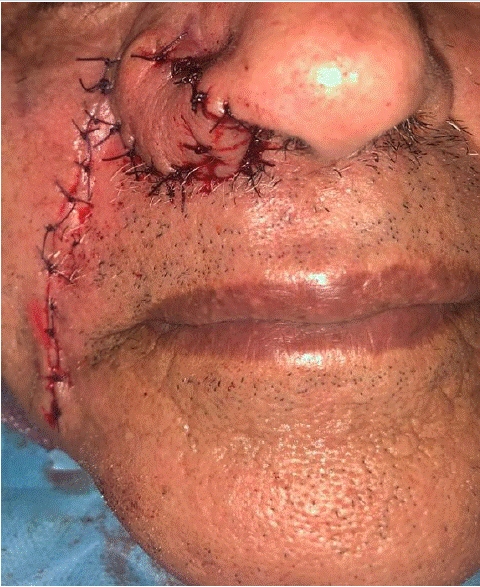
Figure 2: Clinical aspect after wide surgical excision and reconstruction.
Discussion
The incidence of sebaceous carcinoma is higher in men than in women. This incidence increases with age, with a peak observed in those over 80 years old [1]. Caucasians are affected in 80% of cases [2]. Risk factors have been implicated in the occurrence of sebaceous carcinoma. Pathogenic variants of DNA mismatch repair genes (MSH2, MSH6, MLH1) have been identified in 8 to 29% of patients. These variants are also found in patients with Lynch syndrome, and their presence increases the risk of developing other malignant conditions affecting the skin (keratoacanthoma) or internal organs (intestine and genito-urinary system). These conditions are grouped under the term Muir-Torre syndrome. Chronic exposure to ultraviolet radiation and immunosuppression (HIV, organ transplantation) are also risk factors for the development of this type of tumor. Viral involvement in sebaceous carcinoma has been suggested by some authors but is still under study [3].
Sebaceous carcinoma can develop either periocularly or extraocularly. The majority of sebaceous carcinomas are periocular tumors that most commonly originate from the Meibomian glands and Zeis glands. Extraocular localization primarily affects the head and neck due to the degree of photodamage and higher density of sebaceous glands compared to other sites [5].
The typical clinical appearance is that of a painless nodule with a pink or yellowish coloration. However, clinical characteristics can be quite varied, ranging from flesh-colored to red papules, plaques, or nodules, sometimes difficult to distinguish clinically from other more common types of skin neoplasms. The differential diagnosis may include benign skin lesions such as pyogenic granuloma or molluscum contagiosum, or skin cancers such as basal cell carcinomas, squamous cell carcinomas, or adnexal carcinomas.
A definitive diagnosis relies on biopsy with histological examination. For extraocular sebaceous carcinoma, the histological appearance is that of an infiltrating sebaceous neoplasm, uniformly nest or lobular, vacuolated within the dermis. On cytological examination, malignant cells are foamy, with scalloped nuclei, atypia, and mitoses. In most sebaceous carcinomas, dermal invasion is observed, rarely penetrating into the subcutaneous and muscular tissues. Positive immunohistochemical markers include nuclear factor XIIIa (AC-1A1), EMA (positive in squamous cell carcinoma), cytokeratin AE1 and AE3 (positive in squamous cell carcinoma), androgen receptor, adipophilin, and perilipin. Mainly negative markers are carcinoembryonic antigen, S100, HMB45, SOX10, CD5, GCDFP-15, D2-40, and Ber-EP4. Alternative markers used to identify extraocular sebaceous carcinoma include PGRMC1, ABHD5, SQS, CA15-3, CA19-9, CK8, and CK19. Immunohistochemistry is often used to establish a definitive diagnosis, but it is not necessary when histopathological results are typical. Staining for mismatch repair protein expression (MSH-2, MSH-6, and MLH-1) is generally performed for sebaceous neoplasms. The diagnosis of Muir-Torre syndrome-related sebaceous neoplasms is considered in the absence of nuclear staining for the respective gene products [7].
A comprehensive clinical examination, including examination of lymph node areas, is necessary.
The staging of extraocular sebaceous carcinoma is based on the TNM classification. Certain characteristics are considered high risk: thickness = 2 mm, Clark level = 4, perineural invasion, primary site at the ear or lips, poor or undifferentiated histology [8]. These characteristics are based on cutaneous squamous cell carcinoma, and their prognostic accuracy has been questioned by some authors [9].
Although Computed Tomography (CT), CT with Positron Emission Tomography (CT-PET), and Magnetic Resonance Imaging (MRI) are used in sebaceous carcinoma, there is currently no defined consensus. According to some authors, imaging is justified if lymph node involvement is detected on clinical examination, sentinel lymph node biopsy, or when distant metastases are suspected [5].
Genetic screening for Muir-Torre syndrome for extraocular sebaceous carcinoma is recommended when the Mayo score for Muir-Torre syndrome is 2 or higher [10]. This score combines 4 parameters: age at diagnosis of the first sebaceous neoplasm, number of sebaceous neoplasms, personal history of hereditary non-polyposis colorectal cancer (Lynch syndrome), family history of hereditary non-polyposis colorectal cancer.
The utility of sentinel lymph node biopsy is not well defined for extraocular sebaceous carcinomas unlike melanomas. According to a comparative study based on 1836 cases recorded in the SEER registry, the rate of regional metastases for extraocular sebaceous carcinomas is 1.4% [11]. Although rare, distant metastases have been reported in the literature. These involve the lung, liver, bone, and brain.
Treatment of sebaceous carcinoma is primarily surgical. It relies on Mohs micrographic surgery or wide local excision when Mohs surgery is not feasible. Wide local excision should be performed down to the fascial plane while maintaining a surgical safety margin of 1 cm. When immediate margin assessment is not available, staged excision may increase the likelihood of clear margins before reconstruction. Mohs micrographic surgery maximizes tissue preservation, allows for comprehensive margin assessment, and offers better recurrence-free survival compared to traditional wide local excisions [12].
The efficacy of radiotherapy as monotherapy has not yet been established. Radiotherapy may be considered by some authors for patients who are inoperable or have surgically unresectable tumors or nodal metastases. The role of adjuvant radiotherapy on overall survival and local and regional control is uncertain. Tumors treated with surgery and adjuvant radiotherapy in one study included locally advanced primary tumors with positive margins or perineural invasion [13].
For metastatic cases, conventional chemotherapy, immunotherapy, or targeted therapies, including anti-androgens, retinoid receptor ligands, and EGFR inhibitors, may be considered in management. Tumor profiling using next-generation sequencing or other methods can guide the selection of appropriate therapies.
Localized diseases and ocular tumors have better survival outcomes compared to regional diseases and extraocular tumors. Higher all-cause mortality has been observed in Black individuals, men, and those with extraocular tumors according to an analysis of SEER data [14]. The presence of metastasis at the time of diagnosis is the primary factor associated with reduced survival [11]. Clinical surveillance every 6 months is recommended during the first 3 years following treatment. Annual visits or visits at a frequency tailored to the patient may be considered thereafter. The benefit of lymph node ultrasound surveillance in the follow-up of extraocular sebaceous carcinoma remains uncertain compared to periocular sebaceous carcinoma [10]. According to literature data, the majority of recurrences occur within an average of 6 years [10].
Some studies recommend screening for sebaceous carcinoma in patients with Lynch syndrome and those who have undergone solid organ transplantation, as the risk is very high in these populations [15,16].
Conclusion
Sebaceous carcinoma is a rare and potentially aggressive malignant skin tumor. Several risk factors have been implicated, but the exact etiology remains unknown. The treatment of choice is surgical excision. Some societies recommend screening for sebaceous carcinoma in at-risk populations.
References
- Sargen MR, Mai Z-M, Engels EA, Goldstein AM, Tucker MA, Pfeiffer RM, et al. Ambient Ultraviolet Radiation and Sebaceous Carcinoma Incidence in the United States, 2000–2016. JNCI Cancer Spectrum. 2020; 4
- Dores GM, Curtis RE, Toro JR, Devesa SS, Fraumeni JF Jr. Incidence of cutaneous sebaceous carcinoma and risk of associated neoplasms: insight into Muir-Torre syndrome. Cancer. 2008; 113: 3372–81.
- Sargen MR, Starrett GJ, Engels EA, Cahoon EK, Tucker MA, Goldstein AM. Sebaceous carcinoma epidemiology and genetics: Emerging concepts and clinical implications for screening, prevention, and treatment. Clin Cancer Res. 2021; 27: 89-393.
- Rao NA, Hidayat AA, McLean IW, Zimmerman LE. Sebaceous carcinomas of the ocular adnexa: a clinicopathologic study of 104 cases, with five-year follow-up data. Hum Pathol. 1982; 13: 113–22.
- Knackstedt T, Samie FH. Sebaceous Carcinoma: A Review of the Scientific Literature. Curr Treat Options in Oncol. 2017; 18: 47.
- Nelson BR, Hamlet KR, Gillard M, Railan D, Johnson TM. Sebaceous carcinoma. J Am Acad Dermatol. 1995; 33: 1-15.
- Southey MC, Young MA, Whitty J, Mifsud S, Keilar M, Mead L, et al. Molecular pathologic analysis enhances the diagnosis and management of Muir-Torre syndrome and gives insight into its underlying molecular pathogenesis. Am J Surg Pathol. 2001; 25: 936–41.
- Edge S, Editor. American Joint Committee on Cancer. AJCC cancer staging manual. 7th ed. New York: Springer; 2010.
- Jambusaria-Pahlajani A, Kanetsky PA, Karia PS, Hwang WT, Gelfand JM, Whalen FM, et al. Evaluation of AJCC tumor staging for cutaneous squamous cell carcinoma and a proposed alternative tumor staging system. JAMA Dermatol. 2013; 149: 402–10.
- Owen JL, Kibbi N, Worley B, Kelm RC, Wang JV, Barker CA, et al. Sebaceous carcinoma: evidence-based clinical practice guidelines. Lancet Oncol. 2019; 20: e699–714.
- Tryggvason G, Bayon R, Pagedar NA. Epidemiology of sebaceous carcinoma of the head and neck: implications for lymph node management. Head Neck. 2012; 34: 1765–8.
- Hou J. Mohs micrographic surgery is associated with lower recurrence rate in patients with sebaceous carcinoma: an update of the Mayo Clinic experience over the past 2 decades. J Am Acad Dermatol. 2013; 68: AB163.
- Dasgupta T, Wilson LD, Yu JB. A retrospective review of 1349 cases of sebaceous carcinoma. Cancer. 2009; 115: 158–65.
- Tripathi R, Chen Z, Li L, Bordeaux JS. Incidence and survival of sebaceous carcinoma in the United States. J Am Acad Dermatol. 2016; 75: 1210–5.
- Ponti G, Ponz de Leon M. Muir-Torre syndrome. Lancet Oncol. 2005; 6: 980-7
- Acuna SA, Huang JW, Scott AL, Micic S, Daly C, Brezden-Masley C, et al. Cancer Screening Recommendations for Solid Organ Transplant Recipients: A Systematic Review of Clinical Practice Guidelines. Am J Transplant. 2017; 17: 103-14